Abstract
Clinical stroke induces inflammatory processes leading to cerebral injury. IL-10 expression is elevated during major CNS diseases and limits inflammation in the brain. Recent evidence demonstrated that absence of B-cells led to larger infarct volumes and increased numbers of activated T-cells, monocytes and microglial cells in the brain, thus implicating a regulatory role of B-cell subpopulations in limiting CNS damage from stroke. The aim of this study was to determine whether the IL-10-producing regulatory B-cell subset can limit CNS inflammation and reduce infarct volume following ischemic stroke in B-cell deficient (µMT−/−) mice. Five million IL-10-producing B-cells were obtained from IL-10-GFP reporter mice and transferred i.v. to µMT−/− mice. After 24 h following this transfer, recipients were subjected to 60 min of middle cerebral artery occlusion (MCAO) followed by 48 hours of reperfusion. Compared to vehicle-treated controls, the IL-10+ B-cell-replenished µMT−/− mice had reduced infarct volume and fewer infiltrating activated T-cells and monocytes in the affected brain hemisphere. These effects in CNS were accompanied by significant increases in regulatory T-cells and expression of the co-inhibitory receptor, PD-1, with a significant reduction in the proinflammatory milieu in the periphery. These novel observations provide the first proof of both immunoregulatory and protective functions of IL-10-secreting B-cells in MCAO that potentially could impart significant benefit for stroke patients in the clinic.
Keywords: MCAO, inflammatory cells, regulatory B-cells, IL-10
Introduction
Stroke is the most frequent cause of permanent disability and the third leading cause of death in adults worldwide (Donnan et al., 2008). The devastating neuropathological impact of ischemic stroke in experimental and clinical stroke is augmented by systemic activation of inflammatory immune cells that infiltrate the developing lesion (Campanella et al., 2002; Offner et al., 2006a; Yilmaz et al., 2006; Gee et al., 2007; Ajmo et al., 2008). In a clinical scenario, the susceptibility of stroke patients and the subsequent prognosis are influenced by systemic inflammatory processes (Emsley and Hopkins, 2008; McColl et al., 2009), with poorer outcomes upon systemic inflammation (Baird et al., 2002; Elkind et al., 2004; McColl et al., 2007). Experimentally, inhibiting the inflammatory responses elicited by time-dependent recruitment and activation of inflammatory cells decreases infarct size and neurological deficit (Wang, 2005; Yilmaz and Granger, 2008). Albeit anti-inflammatory approaches have proven successful in animal models (Zhang et al., 1995; Prestigiacomo et al., 1999; Zhang et al., 2003), attempts to translate this into the clinic have been unsuccessful (Investigators, 2001; Becker, 2002). A comprehensive understanding of the heterogeneous and time-dependent recruitment of inflammatory cells following focal cerebral ischemia is prerequisite for developing effective therapeutic interventions. With tissue plasminogen activator (tPA), which must be provided within 4.5 hours after stroke onset, being the only globally approved treatment, there is a need for an efficacious treatment that can be administered beyond the time frame of few hours post-stroke.
There is renewed interest in the protective role of regulatory T- and B-cells that can suppress inflammation and limit central nervous system (CNS) damage induced by infiltrating pro-inflammatory cells (Frenkel et al., 2005; Hurn et al., 2007; Liesz et al., 2009; Carter et al., 2011; Ren et al., 2011c). Our recent studies demonstrated that B-cell-deficient µMT−/− mice developed larger infarct volumes, higher mortality and more severe functional deficits (Ren et al., 2011c). MCAO-induced changes were prevented in µMT−/−mice after transfer of highly purified wild-type (WT) B-cells, but not IL-10-deficient B-cells, thus implicating IL-10-secreting B-cells as a major regulatory cell type in stroke (Ren et al., 2011c; Offner and Hurn, 2012). Thus, the aim of the present study was to investigate the effect of B-cells, enriched for IL-10 production, on stroke outcome. For this purpose, we took advantage of the IL-10 GFP reporter mice to obtain B-cells with the ability to produce copious amounts of IL-10 after 48 hours of LPS stimulation and evaluated the stroke outcome by replenishing B-cell-deficient mice with this IL-10 enriched B-cell population 24 hours prior to MCAO. Our results clearly demonstrate the beneficial role of this regulatory B-cell population by a significant decrease in the infarct volumes in the recipient mice. Proinflammatory responses in the recipient mice were not only inhibited in the brains but also in the periphery. The results clearly establish that transferred B-cells led to the retention of T-cells and monocytes in the periphery thus attenuating their infiltration into the CNS, implicating their peripheral regulatory role.
Materials and Methods
Animals
µMT−/−mice containing no B-cells because of targeted disruption of the membrane exon of the immunoglobulin mu chain gene, originally obtained from Jackson Laboratories (Bar Harbor, ME), were bred at the Animal Resource Facility at the Portland Veteran Affairs Medical Center. IL-10 transcriptional reporter mice were obtained from Dr. Christopher Karp, Division of Molecular Immunology, University of Cincinnati College of Medicine, Cincinnati, Ohio. The generation and characterization of these mice has been described by (Madan et al., 2009). The IL10-GFP reporter mice have a floxed neomycin-IRES eGFP cassette (Mohrs et al., 2001) inserted between the endogenous stop site and the poly(A) site of Il10 to help track IL-10 producing cells in vivo. The mice designated as Vert-X are homozygous, develop normally and are viable and fertile without any obvious phenotype. All mice (on a C57BL/6J background) were used at 7–8 weeks of age and were housed in the Animal Resource Facility at the Portland Veterans Affairs Medical Center and at Oregon Health & Science University in accordance with institutional guidelines. The study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by Portland Veteran Affairs Medical Center and Oregon Health and Science University Animal Care and Use Committees.
Cell sorting and Adoptive transfer of B-cells
Male IL-10 GFP reporter mice served as donors of B-cells. Splenic CD19+ B-cells were purified using paramagnetic bead-conjugated antibodies (Abs) from the CD19 cell isolation kit and subsequently separated by AutoMACS (Miltenyi Biotec, Auburn, CA). The positive fraction of the cells thus separated were CD19+ B-cells with a purity of ≥ 95%. CD19+ B-cells were suspended in RPMI 1640 medium with 2% Fetal Bovine Serum (FBS) and cultured in the presence of 1 µg/mL of lipopolysaccharide (LPS, E. coli strain K12) for 48 hours. After 48 hours of culture, B-cells were harvested from culture plates, washed free of LPS and viable cells were counted using a hemocytometer with trypan blue exclusion method. 5×106 purified IL-10-GFP+ B-cells from the donor mice were suspended in 100 µL RPMI 1640 medium and were transferred intravenously (i.v.) into µMT−/− mice (experimental group). Each µMT−/− mouse either received 5×106/100 µL purified IL-10-GFP+ B-cells or 100 µL RPMI 1640 medium (control group).
Middle Cerebral Artery Occlusion (MCAO) Model
Transient focal cerebral ischemia was induced in male µMT−/− mice for 60 min as previously described (Chen et al., 2012) by reversible right MCAO under isoflurane anesthesia followed by 48 hours of reperfusion. The surgeon was blinded to treatment group. Head and body temperature were controlled at 36.5 ± 1.0°C throughout MCAO surgery with warm water pads and a heating lamp. Occlusion and reperfusion were verified in each animal by laser Doppler flowmetry (LDF) (Model DRT4, Moor Instruments Ltd., Oxford, England). Occlusion was accomplished by introducing a 6-0 nylon monofilament (ETHICON, Inc., Somerville, NJ, USA) with a heat-blunted silicone-coated tip (230–250 µm diameter) through the right external carotid artery and internal carotid artery to the origin of the middle cerebral artery. Adequacy of artery occlusion was confirmed by monitoring cortical blood flow at the onset of the occlusion with a LDF probe affixed to the skull. Animals were excluded if intra-ischemic LDF was greater than 25% pre-ischemic baseline. After the occlusion, the incision was closed with 6-0 surgical sutures (ETHICON, Inc., Somerville, NJ, USA). Then each animal was awakened during occlusion and was placed in a separate cage with a warm water pad and heating lamp. At the end of the 60 min ischemic period, mice were briefly re-anesthetized, the laser Doppler probe was repositioned over the same site on the skull, and the occluding filament was withdrawn for reperfusion. Mice were then allowed to recover.
Neurological deficit score
Neurological function was evaluated at baseline (before MCAO), just before reperfusion, and at 24 h and 48 h of reperfusion using a 0 to 5 point scale neurological deficit score (Chen et al., 2012) as follows: 0, no neurological dysfunction; 1, failure to extend left forelimb fully when lifted by tail; 2, circling to the contralateral side; 3, falling to the left; 4, no spontaneous movement or in a comatose state; 5, death.
Infarct Volume Analysis
Mice were euthanized and brains collected at 48 hours of reperfusion for 2,3,5-triphenyltetrazolium chloride (TTC) histology and then digital image analysis of infarct volume as previously published (Chen et al., 2012). Images were analyzed in a blinded fashion using Sigma Scan Pro 5.0 Software (Systat, Inc., Point Richmond, CA). To control for edema, infarct volume (cortex, striatum, and hemisphere) was determined by subtraction of the ipsilateral noninfarcted hemispheric volume from the contralateral hemispheric volume. This value was then divided by the contralateral hemispheric volume and multiplied by 100 to yield the total infarction volume as a percent of the contralateral region.
Isolation of leukocytes from spleen and brain
Spleens from individual control and B-cell recipient µMT−/− mice were removed and a single-cell suspension was prepared by passing the tissue through a 100 µm nylon mesh (BD Falcon, Bedford, MA). The cells were washed using RPMI 1640. Red cells were lysed using 1× red cell lysis buffer (eBioscience, Inc., San Diego, CA) and incubated for 3 min. Cells were then washed twice with RPMI 1640, counted, and resuspended in stimulation medium (RPMI, containing 10% FBS, 1% sodium pyruvate, 1% L-glutamine, 0.4% βME). The brain was divided into the ischemic (right) and nonischemic (left) hemisphere, dissociated mechanically through a 100 µm nylon mesh screen, resuspended in 80% percoll (GE Healthcare, Pittsburgh, PA) overlaid with 40% percoll and subjected to density gradient centrifugation for 30 min at 1600 rpm, according to a method described previously (Campanella et al., 2002). Inflammatory cells were removed from the interface for further analysis. Cells were then washed twice with RPMI 1640, counted, and resuspended in stimulation medium. Cells from individual brain hemispheres were used for flow cytometry.
Analysis of cell populations by fluorescence-activated cell sorting (FACS)
All antibodies were purchased from BD Biosciences (San Jose, CA) or eBioscience, Inc. (San Diego, CA) as published (Offner et al., 2006a; Offner et al., 2006b). Four-color (FITC, PE, APC and PerCP/PECy7) fluorescence flow cytometry analyses were performed to determine the phenotypes of splenocytes, and brain leukocytes, as previously published (Offner et al., 2006a). Single-cell suspensions were washed with staining medium (PBS containing 0.1% NaN3 and 1% bovine serum albumin (Sigma, Illinois) and incubated with the combinations of the following monoclonal antibodies: CD3 (17A2), CD4 (GK1.5), CD8 (53–6.7), CD11b (M1/70), CD45 (Ly-5), CD19 (1D3), MHCII (2G9), CD69 (H1.2F3), CD44 (IM7), and PD-1 (RPM-1) for 10 min at 4°C. One mL of staining buffer was added to wash the cells. Propidium iodide (PI) was added to identify dead cells. The FoxP3 staining kit was used according to the manufacturer’s protocol (eBioscience) and as previously described (Zhang et al., 2010). FACS data acquisition was performed on FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and data were analyzed using FCS express software (De Novo Software, Los Angeles, CA).
Intracellular Staining
Intracellular staining was visualized using a published immunofluorescence protocol (Subramanian et al., 2011). Briefly, 2×106 cells/mL were resuspended in complete medium (RPMI 1640 containing 10% fetal calf serum, 1 mM/L pyruvate, 200 µg/mL penicillin, 200 U/mL streptomycin, 4 mM/L L-glutamine, and 5×10−5 mol/L 2-β-ME) with PMA (50 ng/mL), ionomycin (500 ng/mL), and Brefeldin A (10 µg/mL; Sigma-Aldrich) for 4 hours. For intracellular IL-10 detection, a modification was followed for the immunofluorescence staining protocol (Yanaba et al., 2008). Briefly, isolated leukocytes or purified cells were resuspended (2×106 cells/mL) in complete medium and cultured with LPS (10 µg/mL) in addition to PMA (50 ng/mL), ionomycin (500 ng/mL), and Brefeldin A (10 µg/mL) (all reagents are from Sigma-Aldrich) for 4 hours. Fc receptors were blocked with anti-FcR mAb (2.3G2; BD PharMingen) before cell-surface staining, fixed and permeabilized with the Fixation/Permeabilization buffer (eBioscience) according to the manufacturer’s instructions. Permeabilized cells were washed with 1× Permeabilization Buffer (eBioscience) and were stained with tumor necrosis factor-α (MP6-XT22; BD Pharmingen), Interferon-γ (XMG1.2; eBioscience), or APC-conjugated anti-IL-10 mAb (JES5-16E3; eBioscience). Isotype matched mAb served as negative controls to demonstrate specificity and to establish background TNF-α, IFN-γ and IL-10-staining levels.
Statistical Analysis
Data are presented as mean ± SEM. Differences in regional infarct volumes were determined with Student’s t-test. Functional outcomes for neurological deficit scores were analyzed by Mann-Whitney U test. Statistical significance was p≤0.05. Statistical analyses were performed using SigmaStat Statistical Software, Version 9.01 (Systat Software Inc., Chicago, IL, USA). Differences between percentages of cellular subtypes for FACS analysis were analyzed with Student’s t-test. Brain leukocyte counts were analyzed by one-way analysis of variance (ANOVA) followed by a Neuman-Kuels post hoc test. The criterion for statistical significance was p ≤ 0.05. All values are reported as mean ± SD. Significant differences are denoted as *p ≤ 0.05; **p≤ 0.01; ***p≤0.001.
Results
Exclusions relative to MCAO
B-cell deficient animals that received IL-10-GFP+ B-cells (n=26 total) or RPMI (n=30 total) had a similar number (IL-10-GFP+ B-cells, n=6; RPMI, n=8) and percentage (IL-10-GFP+ B-cells, 23%; RPMI, 27%) of mice excluded relative to MCAO. In the IL-10-GFP+ B-cell group, five animals (19%) were excluded due to peri-operative death (n=1, 4%), failure to occlude (n=1, 4%), or met the mean ischemic LDF exclusion criteria of greater than 25% of baseline LDF (n=3, 12%). One animal was euthanized due to tearing of the right external carotid artery during surgical manipulations (4%). In the RPMI group, eight animals (27%) were excluded due to peri-operative death (n=3, 10%), subarachnoid hemorrhage (n=1, 3%), or met the mean ischemic LDF exclusion criteria of greater than 25% of baseline LDF (n=4, 13%). At initiation of reperfusion, all experimental groups reperfused to greater than 50% pre-ischemic baseline (data not shown). For studies evaluating infarct volume, 13 mice were included in the RPMI group while 10 mice were included in the IL-10-GFP+ B-cell treated group. For studies evaluating immunology, 9 mice were included in the RPMI group while 10 mice were included in the IL-10-GFP+ B-cell treated group.
Adoptive transfer of IL-10-GFP+ B-cells to µMT−/− mice reduces ischemic infarct size
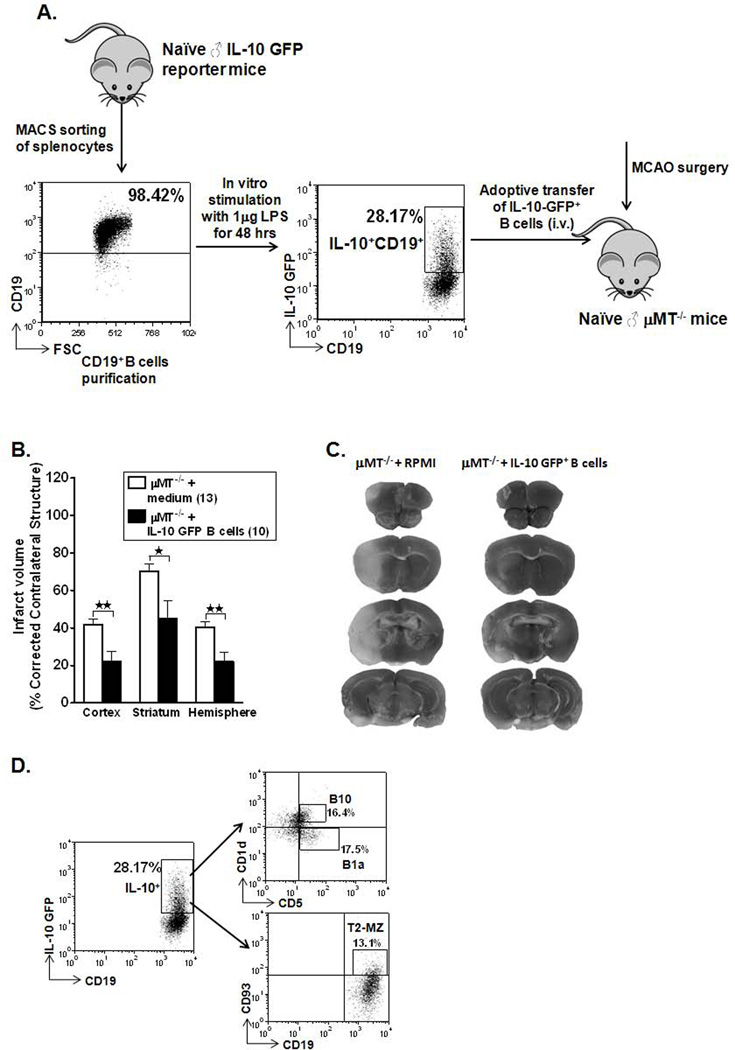
As demonstrated in our studies (Ren et al., 2011c), adoptive transfer of WT CD19+ B-cells improved ischemic outcomes in B-cell-deficient mice, but the transfer of B-cells from IL-10−/− donors did not. Hence, we hypothesized for the current study that the protective ability of the B-cells is mediated via its IL-10 production. To address this hypothesis directly, B-cells were purified from spleens of IL-10 GFP reporter mice by AUTOMACS sorting and cultured for 48 hours in presence of LPS as illustrated in Figure 1A). After LPS stimulation, B-cells were washed free of LPS and 5 million B-cells, enriched in IL-10 production, were suspended in 100 µL of RPMI 1640 medium and transferred to B-cell-deficient recipient mice via i.v. injections, 1 day before MCAO. One hundred µL RPMI medium was injected (i.v.) in µMT−/− mice that served as controls for the experiment. As shown in Figure 1B and 1C, B-cell deficient animals that received IL-10-GFP+ B-cells (n=10) exhibited significantly reduced cortical (p=0.002), striatal (p=0.014) and total hemisphere (p=0.002) infarct volumes after 60 min MCAO followed by 48 hours of reperfusion compared to no cell transfer (RPMI) controls (n=13). There were no significant differences in neurological deficit scores before initiation of MCAO or at 24 h and 48 h reperfusion between RPMI and IL-10-GFP+ B-cell treated groups (data not shown).
Figure 1. Adoptive transfer of IL-10-GFP+ B-cells to µMT−/− mice reduces ischemic infarct size and experimental stroke outcome.
A. Schematic representation of enrichment of IL-10+ GFP+ CD19+ B-cells from spleens of IL-10 GFP reporter mice, cultured in the presence of 1µg/ml LPS for 48 hours and adoptive transfer of 5 million IL-10-GFP+ B-cells into µMT−/− mice, via i.v. injections. B. Intravenous transfer of 5 million IL-10-GFP+ B-cells reduced infarct volume in µMT−/− B-cell deficient mice 48 hours following 60 minutes of middle cerebral artery occlusion (MCAO) compared to intravenous transfer of RPMI vehicle (no cells). *p≤0.05; **p≤0.01 (Student t-test). C. Representative 2,3,5 triphenyltetetrazolium chloride stained cerebral sections 48 hours following 60 minutes of MCAO. Localization of the ischemic lesion differed between µMT−/− mice receiving intravenous IL-10-GFP+ B-cells or RPMI vehicle (no cells). D. Purified B-cells obtained from IL-10 GFP reporter mice were further characterized for regulatory B-cell sub-populations (B10, B1a & T2-MZ) after 48 hours of culture in the presence of LPS by flow cytometry.
Characterization of IL-10-enriched B cells
Purified B-cells were further quantified by FACS analysis for the content of regulatory B-cell subpopulations present after 48 hours of culture in presence of LPS before transfer into µMT−/− recipient mice; 16.4 % of the IL-10+ B-cells were the classic B10 cells (CD1dhiCD5+CD19+), 17.5% were B1a cell (CD1d−CD5+CD19+) and 13.1% were T2-MZ cells (CD93+CD23+CD19+) (Fig. 1D). Thus, the three best-characterized Breg sub-populations were equally distributed. These same Breg populations were found to be present at very low percentages in the IL-10− B-cell-fraction (data not shown). Thus, we conclude that IL-10+ B-cells are potent immunoregulators and transfer of these cells into B-cell deficient mice leads to significantly smaller infarct volumes, post-experimental stroke.
IL-10-GFP+ B-cells reduce cerebral inflammatory cell infiltration post-MCAO
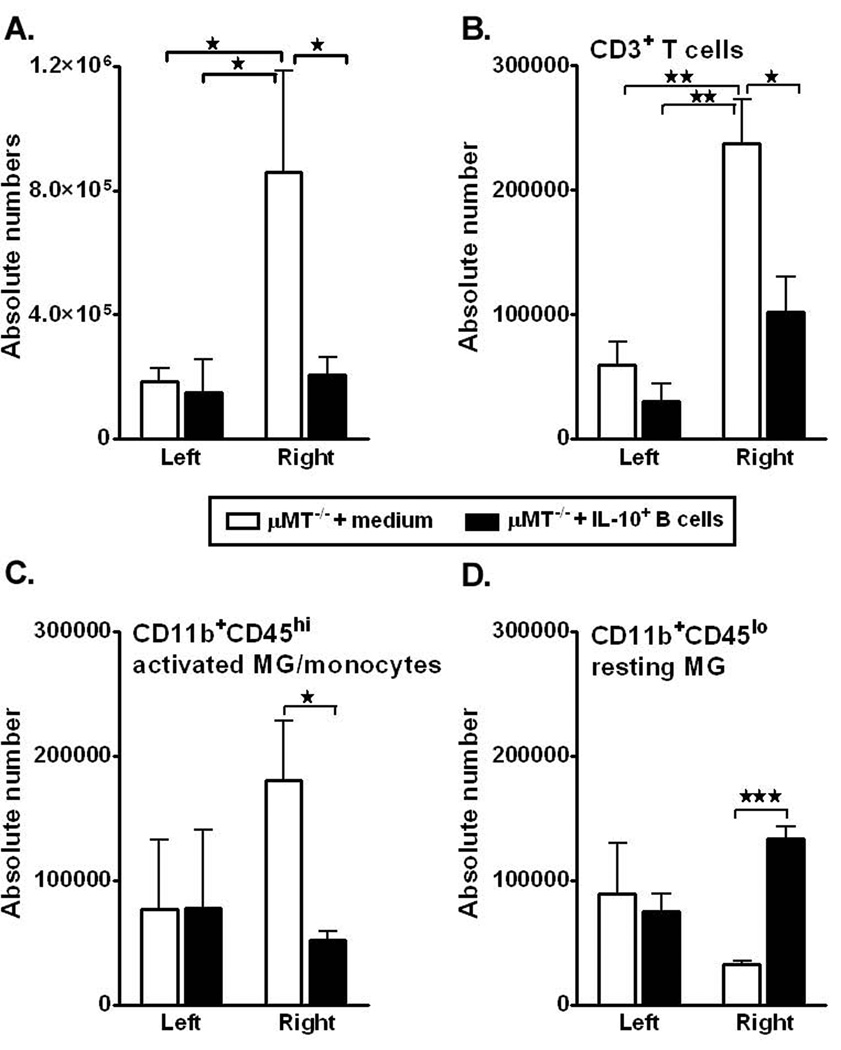
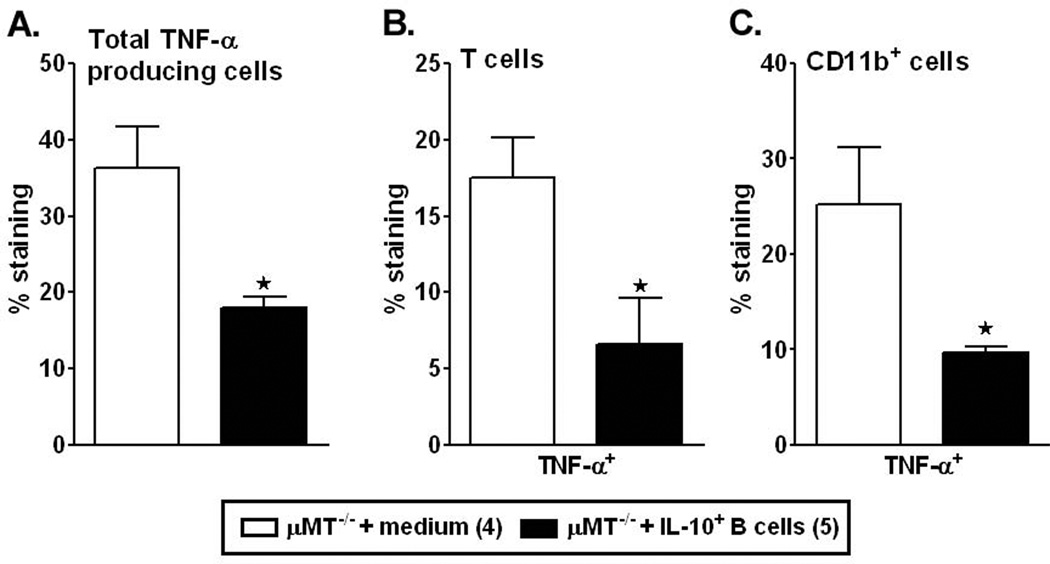
Post-ischemia, brain inflammatory responses are characterized by a rapid activation of resident cells (mainly microglial cells), followed by the infiltration of circulating inflammatory cells, including granulocytes (neutrophils), T-cells, monocytes/macrophages, and other cells in the ischemic brain region, as demonstrated in animal models (Schilling et al., 2003; Tanaka et al., 2003; Kriz, 2006; Amantea et al., 2009). Also, our earlier study demonstrated that lack of B-cells resulted in significantly greater accumulation of leukocyte subsets in the affected hemisphere of MCAO-treated µMT−/− mice after 48 hours of reperfusion as compared to the WT mice (Ren et al., 2011c). Hence, to determine whether transfer of IL-10+ B-cells altered the activation or infiltrating immune cell populations in the brain, leukocytes were isolated from the non-ischemic (left) and ischemic (right) hemispheres of brain from both control and IL-10-GFP+ B-cell treated µMT−/− recipient mice. Enumeration of absolute cell numbers of live leukocytes obtained from each of the hemispheres (Fig. 2A) demonstrated a significant reduction in the B-cell-transferred group as compared to the medium-treated group (p=0.01). To determine whether transfer of IL-10-GFP+ B-cells influenced the leukocyte composition vs. controls, numbers of CD3+ T-cells, activated microglia/infiltrating macrophages (CD11b+CD45high) and resting/resident microglia (CD11b+CD45low) were evaluated by flow cytometry. After 48 hours of reperfusion, the absolute numbers of infiltrating CD3+ T-cells (Fig. 2B, p=0.018) and CD11b+CD45high (Fig. 2C, p=0.048) subpopulations were significantly reduced in the MCAO-induced B-cell-transferred group as compared to the control µMT−/− mice. Subsequently, the absolute numbers of the resting microglia (CD11b+CD45low, Fig. 2D) were significantly higher (p=0.001) in the B-cell-transferred group, indicating comparative less inflammation in the ischemic hemisphere. Transfer of IL-10+ B-cells further led to significant reductions in percentages of not only the total TNF-α production by leukocytes (Fig. 3A, TNF-α+CD45+; p=0.030), but also by CD3+ T-cells (Fig. 3B, p= 0.050) and CD11b+ subpopulations (Fig. 3C, p= 0.041), in the ischemic hemisphere of brains as compared to the ischemic hemisphere of control recipient mice, further confirming the reduction in inflammatory milieu due to the regulatory effects of the IL-10-GFP+ B-cells. Similar to the findings in our earlier studies (Ren et al., 2011c), transferred non- IL-10-GFP+ B-cells could not be found in either left (non-ischemic) or right (ischemic) hemispheres of the brains of the experimental group (not shown), strongly indicating peripheral regulation of the immune system leading to alleviated inflammatory responses in the brains.
Figure 2. IL-10 GFP+ B-cells reduce cerebral cell infiltration post-MCAO.
Forty-eight hours after MCAO, mononuclear cells were isolated from brains of RPMI and IL-10 GFP+ B-cell recipient mice and were analyzed for A. Total cell count via hemocytometer. Values represent mean numbers (±SEM) of indicated cell subsets from 10–11 mice per group, from 3 separate experiments. B. CD3+ T-cells. C. CD11b+CD45high activated microglia (MG)/monocytes, and D.CD11b+CD45low resting MG obtained from the non-ischemic (left) and ischemic (right) hemispheres of µMT−/− recipient mice. B–D, Values represent mean numbers (±SEM) of indicated cell subsets, gated on live leukocytes (by PI exclusion), from 4–5 mice per group, from at least 2 separate experiments. Statistical analysis was performed with ANOVA followed by Neuman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤ 0.01; ***p≤ 0.001).
Figure 3. IL-10-GFP+ B-cells reduce cerebral inflammatory responses post-MCAO.
Determinations of: A. total TNF-α production by CD45+ cells; B. TNF-α+CD3+ T-cells; and C. TNF-α+CD11b+ cells quantified in the nonischemic (left) and ischemic (right) hemispheres from µMT−/− mice transferred with medium or IL-10-GFP+ B-cells after 48 hours of MCAO. Values represent mean numbers (±SEM) of indicated cell subsets from 4–5 mice of each group, from at least 2 separate experiments. Statistical analysis was performed with ANOVA followed by Neuman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05).
IL-10-GFP+ B-cells rescue MCAO-induced splenic atrophy in recipient µMT−/− mice
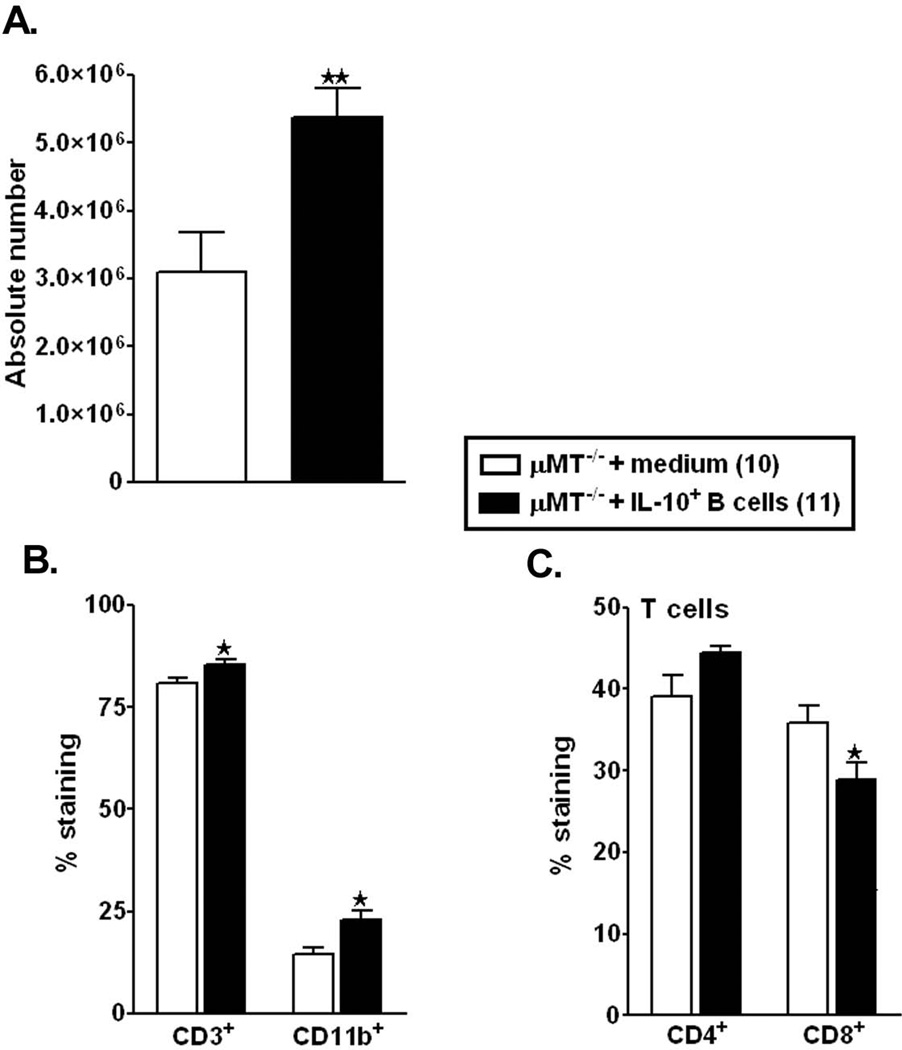
Since the µMT−/− mice that were the recipients of IL-10-GFP+ B-cells had an improved stroke outcome as well as reduced brain inflammation, we next sought to assess the protective effects of IL-10-GFP+ B-cell transfers on peripheral immune responses. Stroke-induced splenic atrophy is an established phenomenon (Offner et al., 2006b). Hence, the cell numbers in the spleen were evaluated in post-ischemic RPMI- and B-cell-transferred µMT−/− mice. As expected and previously demonstrated (Offner et al., 2006b), MCAO resulted in large reductions in spleen cell numbers in RPMI-treated µMT−/− mice. The reduction in spleen counts was from ~38–40 million spleen cells/naïve µMT−/− mouse (data not shown) to ~4 million spleens cells/ µMT−/− mouse after 60mins MCAO and 48 reperfusion, accounting to ~90% reduction in the total splenocytes (Fig. 4A). Interestingly, the viable cell counts were significantly increased in spleens of µMT−/− with B-cell transfers as compared to the RPMI-treated µMT−/− mice (p=0.005) 48 hours following MCAO (Fig. 4A). Given the partial restoration of splenic cell numbers in the IL-10+ B-cell-treated group, we further evaluated specific surviving splenic cell types. B-cells comprise ~60% of the normal spleens, but in their absence, the major remaining populations of T-cells and monocytes were enumerated. There was a significant increase in the percentage of both T-cells (CD3+; p=0.050) and monocytes (CD11b+; p= 0.026) in the spleens of µMT−/− mice treated with IL-10-GFP+ B-cells (Fig. 4B). Upon further characterizing the CD3+ T-cells population, there was a trend towards increased CD4+ T-cells (p=0.069) and a significant decrease in the CD8+ T-cells (p=0.050) in the spleens (Fig. 4C). After 48 hours of reperfusion, 1% of the transferred GFP+ B-cells could be detected in the spleens of the µMT−/− recipient mice (data not shown), indicating that the increase in the cell numbers is not contributed by the transferred cells, but due to the influence of the transferred cells on the splenic constitution.
Figure 4. IL-10-GFP+ B-cells rescue MCAO-induced splenic atrophy in recipient µMT−/− mice.
Forty-eight hours after MCAO, mononuclear cells were isolated from spleens of RPMI or IL-10-GFP+ B-cell recipient µMT−/− mice and were analyzed for: A. Total cell count via hemocytometer. Values represent mean numbers (±SEM) of indicated cell subsets from 10–11 mice in each group, from at least 3 separate experiments; B. CD3+ T-cells and CD11b+ monocytes; and C. CD4+ and CD8+ T-cell populations. Values represent mean numbers (±SEM) of indicated cell subsets, gated on live leukocytes (by PI exclusion), from 4–5 mice of each group, from 2 separate experiments. Statistical analysis was performed with Student’s t-test to compare between RPMI and IL-10-GFP+ B-cell recipient mice. Significant differences between sample means are indicated (*p≤0.05).
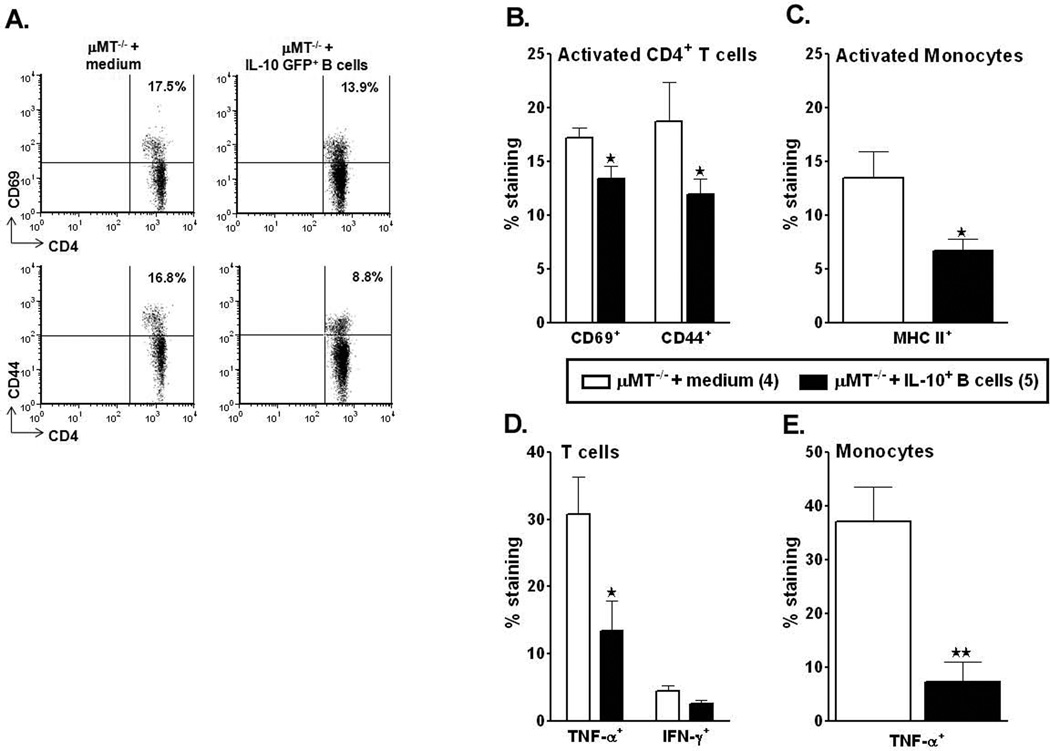
IL-10-GFP+ B-cells inhibit activation and suppress enhanced pro-inflammatory states of the T-cells and monocytes in the periphery of recipient mice
To further evaluate the possible regulatory effects of the potent IL-10-producing B-cells on T-cells and monocytes, activation states and inflammatory factors produced by these cell types were quantified after 60 min of MCAO and 48 hours of reperfusion. Thus, to correlate the reduced infarct volumes and reduced splenic atrophy to the activation states of T-cells in the spleens which might be influencing the splenic milieu, we evaluated the percent expression of CD44 and CD69 by CD4+ T-cells in the spleens of control vs. B-cell-replenished mice. CD44 is a memory T-cell marker that is involved in T-cell activation, binding to selectins on the endothelium during transmigration across the blood-brain barrier and release of cytokines in brain to amplify the inflammatory response; and CD69, an ‘activation marker’ that is rapidly induced on mature T-cells after stimulation through the TCR. As shown in Figure 5A and 5B, splenocytes from RPMI-treated µMT−/− mice after MCAO had increased percentages of CD44+ and CD69+ CD4+ T-cells. Transfer of IL-10 GFP+ B-cells prevented this increase, resulting in a significant decrease in CD44 and CD69 expression by CD4+ T-cells (p=0.049 and 0.033, respectively) compared to RPMI-treated mice. Similarly, the expression of Major Histocompatibility Complex class II on monocytes was significantly reduced (p=0.038) in the B-cell-transferred µMT−/− mice (Fig. 5C). To further evaluate possible regulatory effects of the transferred IL-10-GFP+ B-cells on the cytokine production abilities of T-cells and monocytes post-MCAO, inflammatory factors were quantified in ex vivo activated cells from the spleens. As shown in Figure 5D and 5E, the percentage of CD3+ T-cells and CD11b+ monocytes-secreting TNF-α was significantly decreased in the B-cell-replenished mice (p=0.035 and 0.005) as compared to the RPMI-treated µMT−/− recipients. Although the production of IFN-γ by CD3+ T-cells was also dampened in the B-cell recipients, this decrease was not significant (p=0.063) as compared to the RPMI-treated control mice. These results indicate a dampening role played by the IL-10+ B-cells in limiting both the activation states and proinflammatory responses in the periphery after MCAO.
Figure 5. IL-10-GFP+ B-cells inhibit activation and suppress enhanced pro-inflammatory states of the T-cells and monocytes in the periphery of recipient mice.
Forty-eight hours after MCAO, mononuclear cells were isolated from spleens of RPMI or IL-10-GFP+ B-cell recipient µMT−/− mice and were analyzed for: A. and B. expression of activation markers, CD69 and CD44 on gated CD4+ T-cells; C. expression of MHC class II by CD11b+ monocytes, D. TNF-α+ and IFN-γ+ CD3+ T-cells and E. TNF-α+CD11b+ monocytes. Values represent mean numbers (±SEM) of indicated cell subsets from 4–5 mice of each group, from 2 separate experiments. Statistical analysis was performed with Student's t-test to compare between RPMI and IL-10-GFP+ B-cell recipient mice. Significant differences between sample means are indicated (*p≤0.05 and **p≤0.01).
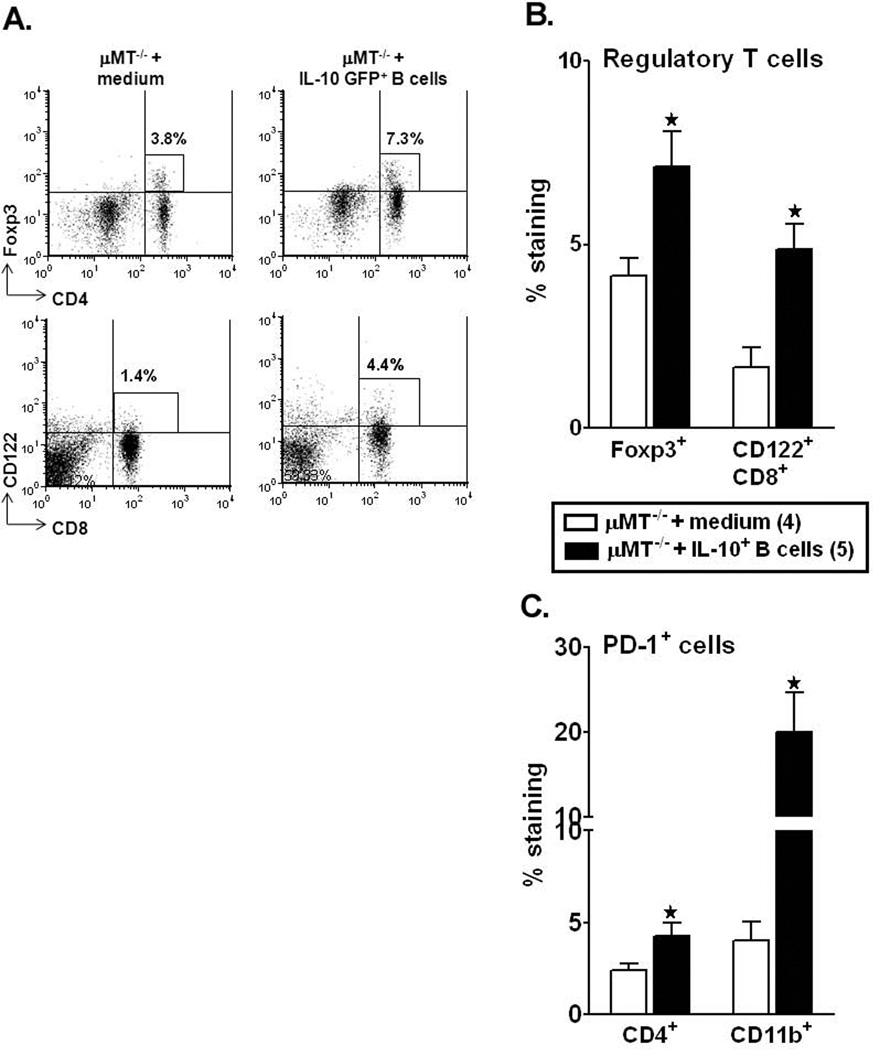
IL-10+ regulatory B-cells enhance regulatory T-cell populations and PD-1 expression in the periphery of recipient mice after MCAO
Our earlier studies demonstrate that MCAO induced a 3 fold increase in the percentage of Foxp3+CD4+ Tregs in the spleens as compared to sham or naïve mice (Offner et al., 2006b). Assessment of percent of the Foxp3+CD4+ T-cell sub-population in the µMT−/− recipients demonstrated a significant increase (p=0.026) in the IL-10+ B-cell-recipients as compared to control mice (Fig. 6A and B). Recently CD8+CD122+ regulatory T-cells have been identified (Rifa'i et al., 2004). CD8+CD122+ Tregs are naturally occurring Treg that effectively suppress the proliferation and IFN-γ production of both CD8+ and CD4+ target cells by virtue of their IL-10 production (Rifa'i et al., 2008). We found a significant increase in the percentage of CD8+CD122+ Tregs in the B-cell recipients vs. controls (p=0.047, Fig. 6A and B). To further assess known regulatory factors in the MCAO-protected IL-10-GFP+ B-cell recipients, we evaluated the expression of PD-1 on T-cells and monocytes. Our recent work (Ren et al., 2011a) has implicated PD-1 signaling in limiting CNS inflammation in MCAO. Interestingly, there was a significant increase in percentage of PD-1 expression on CD4+ T-cells (p=0.049) and CD11b+ monocytes (p=0.031), thus confirming that the IL-10-GFP+ B-cells play a protective role in the induction of regulatory T-cells, which in turn could contribute to immunomodulation in the recipient mice after stroke.
Figure 6. Regulatory IL-10-GFP+ B-cells enhance regulatory T-cell populations and PD-1 expression in the periphery of recipient mice after MCAO.
Splenocytes from RPMI and IL-10-GFP+ B-cell transferred µMT−/− recipient mice were harvested 48 hours after MCAO and assessed for expression of: A and B. FoxP3+CD4+ T-cells and CD8+CD122+ T-cells; C. PD-1-expressing CD4+ T-cells and CD11b+ monocytes. Data are representative of 2 independent experiments with spleens processed from 4–5 individual mice (mean ± SEM). Significant differences between the groups (* p≤0.05) were determined using Student’s t-test.
Discussion
Ischemic stroke induces neurological deficits in almost third of the patients, leading to increased mortality and long-term functional disability (Castillo et al., 1997; Davalos et al., 1999). Although underlying mechanisms have not been completely unraveled, that ischemia evokes inflammatory responses has been characterized. Brain inflammatory responses post-ischemia are characterized by a rapid activation of resident cells (mainly microglial cells), followed by the infiltration of circulating inflammatory cells in the ischemic brain region, as demonstrated in animal models (Schilling et al., 2003; Kriz, 2006; Amantea et al., 2009) and in stroke patients (Lindsberg et al., 1996; Buck et al., 2008). The outcome of post-ischemic inflammatory processes mainly depends on the imbalance between activation of pro-inflammatory cytokine cascade and induction of anti-inflammatory cytokines and antioxidant mechanisms (Wang et al., 2007). With the anti-inflammatory mechanisms being considered as a plausible therapeutic target in stroke, a major challenge is to understand how to enhance the early immunoregulation that limits CNS inflammation while preventing excessive systemic suppression. It is now clear that human stroke creates not just a single organ insult, but a complex interaction between two great physiological systems: the CNS and the peripheral immune system. Our earlier reports demonstrated that that focal cerebral ischemia results in dynamic and widespread activation of inflammatory cytokines, chemokines, and chemokine receptors in the peripheral immune system (Offner et al., 2006a). Also, we recently demonstrated that total B-cell populations can limit infarct volume and functional neurological deficits as well as inhibit activation and recruitment of inflammatory T-cells, macrophages, and microglia into the CNS infarct after experimental stroke in mice. These regulatory activities were associated with increased percentages of IL-10-secreting CD19+ B-cells. The current study provides the first definitive proof of this concept, demonstrating directly the capability of IL-10-producing B-cells to ameliorate infarct volumes and improve peripheral immunosuppression upon transfer into µMT−/− mice.
Regulatory B-cells (Bregs) are known to mediate protection against other inflammatory CNS conditions (Mann et al., 2007; Yanaba et al., 2008; Matsushita et al., 2010) and ability of Bregs to limit CNS injury is associated with anti-inflammatory effects of IL-10 (Fillatreau et al., 2002), hence referred to as B10s. Recent studies in collagen-induced arthritis have identified the transitional 2 marginal-zone precursor (T2-MZP) cells to exert immunosuppressive functions in vivo as well as in vitro (Evans et al., 2007; Blair et al., 2009). Also, it has come to our attention that another subset of B-cells known as B1a (CD1d−CD5+CD19+ cells) are efficient IL-10 producers (DiLillo et al., 2010). It has been speculated that B-cells conditioned in an inflammatory environment acquire a regulatory phenotype (Gray et al., 2007; Yanaba et al., 2009). In the context of stroke immunology, IL-10, an anti-inflammatory cytokine, acts by inhibiting IL-1β and TNF-α and also by suppressing cytokine receptor expression and receptor activation. It is synthesized in the CNS and is upregulated in experimental stroke (Strle et al., 2001). Moreover, the inhibitory effects of IL-10 on pro-inflammatory cytokine production have already been documented in various peripheral inflammatory models including endotoxemia, pancreatitis, and hepatitis (Moore et al., 2001). Both exogenous administration (Spera et al., 1998) and gene transfer of IL-10 (Ooboshi et al., 2005) in cerebral ischemia models appear to have beneficial effects. Other studies also demonstrate that induction of mucosal tolerance by IL-10-producing MOG (myelin oligodendrocyte glycoprotein)-reactive T-cells (Frenkel et al., 2005) or by transgenic overexpression of IL-10 (de Bilbao et al., 2009) reduced infarct volume. Patients with acute ischemic stroke have elevated numbers of peripheral blood mononuclear cells secreting IL-10 (Pelidou et al., 1999) and elevated concentrations of IL-10 in cerebrospinal fluid (Tarkowski et al., 1997). Furthermore, subjects with low IL-10 levels have an increased risk of stroke (van Exel et al., 2002). Hence, as part of our strategy to develop novel therapeutic immunomodulators, we employed the IL-10 GFP reporter mice in the present study to obtain splenic B-cells. It has been reported that in vivo administration of LPS to these mice results in the dominance of B-cells among IL-10-expressing cells in lymphoid tissues (Madan et al., 2009). Unlike the aforementioned study, in vivo administration of LPS was avoided to prevent systemic inflammation in the donor mice. Instead, we cultured B-cells, obtained from naïve IL-10 GFP reporter mice, with LPS for 48 h in vitro. Our study demonstrated that upon 48 h stimulation with LPS, ~ 30% of B-cells were able to produce IL-10, unlike the 7% produced by B-cells obtained from naïve mice as shown in our previous study (Chen et al., 2012). Not only were the purified B-cells enriched for IL-10 production but upon characterizing the IL-10+ fraction, we found an equivalent distribution among Breg subsets (i.e., B10, T2-MZ and B1a, Fig. 1D). This finding offers a promising aspect because it suggests that all 3 B regulatory sub-types render immunomodulatory effects in the recipients, rather than a dominant contribution of only one of the subsets. Future studies entailing transfers of specific Breg subsets would be able to discern the contribution of each of these subsets in experimental stroke. Thus, our present study conclusively demonstrates that the transfer of these IL-10-enriched B-cells inhibits the expected severe stroke outcome in the B-cell-deficient mice.
Previous work has directed our working hypothesis that evolving cerebral ischemic injury elicits a cycle of injury from brain-to-spleen-to-brain (Offner et al., 2009). The evolving brain injury “signals” for splenic activation which leads to apoptosis and drastic loss of immune cells. The activated spleen also releases cells into the blood, followed by trafficking across the microvasculature into the brain. Thus, upon evaluating the splenic responses in the IL-10+ B-cell-recipient mice, we here demonstrated that MCAO-induced splenic inflammatory responses and activated states of T-cells and monocytes were attenuated significantly (Figures 4A–C and 5A–D). When the splenic regulatory T-cell subsets were evaluated, we also found a significant increase in FoxP3+ Treg cells and the recently identified CD122+CD8+ regulatory subpopulations in the IL-10+ B-cell recipient mice (Fig. 6A–B). While one of our initial studies demonstrated that splenic atrophy in experimental stroke is accompanied by increased FoxP3+ regulatory T-cells (Offner et al., 2006b), our recent study using conditional Treg-deficient mice failed to implicate CD4+CD25+FoxP3+Tregs as having protective activity against MCAO (Ren et al., 2011). More recent studies indicate that regulatory T-cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO (Stubbe et al., 2013). Thus, although our previous study (Ren et al., 2011b) did not demonstrate larger infarct volumes in Treg deficient mice, it did not exclude the contribution of other regulatory cells. CD8+CD122+ regulatory T-cells have been characterized to produce IL-10 to suppress IFN-γ production and proliferation of CD8+ T-cells (Endharti et al., 2005; Rifa'i et al., 2008) to elicit their immune-regulatory activity. While, the percentage of CD8+CD122+ Treg population was significantly increased in B-cell-replenished recipient mice, there was essentially no IL-10 detected (data not shown). This could be attributed to the low IL-10 production by immune cells in the B-cell-deficient mice as demonstrated in (Ren et al., 2011c), thus, indicating other means of regulation by the CD8+CD122+ population, i.e. either by cell-to-cell interaction or via the production of other immunomodulatory products. While the percentage of CD8+ T-cells was significantly lower in the spleens of B-cell-recipients, preliminary results indicate a relative increase in CD8+CD122+ population in ischemic hemispheres of IL-10+ B-cell recipients (data not shown; n=2 for RPMI recipients and n=4 for B-cell recipients, in 2 separate experiments), suggesting a plausible trafficking of these regulatory T-cells to the ischemic half of the brain. This is a potentially important correlation emphasizing the brain-to-spleen-to-brain cycle of immunomodulation. The MCAO-protected IL-10+ B-cell recipient mice also exhibited a significant increase in expression of the co-inhibitory receptor PD-1 on CD4+ T-cells and CD11b+ monocytes in the spleens, which our previous studies (Ren et al., 2011a) found to be a critical factor for limiting functional and histological damage after MCAO.
In summary, the current study conclusively demonstrates the beneficial effects of IL-10+ B-cells in B-cell-deficient mice in controlling experimental stroke outcome. Improvement in stroke outcome was attributed to the significant reduction in brain infiltrating cells as well as their proinflammatory states. Our study also demonstrated a novel connection between the ischemic lesion in brain and evolving inflammatory changes in distant peripheral immune cell populations. Reduction in infarct volumes promoted reduction in ischemia-related splenic atrophy accompanied by lower proinflammatory and activated states of the splenic T-cells and monocytes. Not only could the IL-10+ B-cells attenuate proinflammatory responses, but other regulatory T-cell subsets were found to be significantly upregulated. Thus, our novel observations are the first to implicate the IL-10 producing capacity of B-cells as a major regulatory mediator in stroke.
Acknowledgements
The authors wish to thank Melissa Barber for assistance with manuscript preparation. This work was supported by NIH/NINDS 1RO1 NS075887. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest - The authors declare no competing financial interests.
References
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, Tress BM, Colman PG, Jerums G, Chambers BR, Davis SM. The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci. 2002;9:618–626. doi: 10.1054/jocn.2002.1081. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18(Suppl 2):s18–s22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, Ali LK, Kim D, Villablanca JP, Salamon N, Razinia T, Ovbiagele B. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39:355–360. doi: 10.1161/STROKEAHA.107.490128. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet. 1997;349:79–83. doi: 10.1016/S0140-6736(96)04453-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bodhankar S, Murphy SJ, Vandenbark AA, Alkayed NJ, Offner H. Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metab Brain Dis. 2012;27:487–493. doi: 10.1007/s11011-012-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999;30:2631–2636. doi: 10.1161/01.str.30.12.2631. [DOI] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem. 2009;110:12–22. doi: 10.1111/j.1471-4159.2009.06098.x. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe K, Suzuki H. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators EAST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001:1428–1434. doi: 10.1212/wnl.57.8.1428. 2001/10/24 Edition. [DOI] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lindsberg PJ, Carpen O, Paetau A, Karjalainen-Lindsberg ML, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Offner H, Hurn PD. A Novel Hypothesis: Regulatory B Lymphocytes Shape Outcome from Experimental Stroke. Transl Stroke Res. 2012;3:324–330. doi: 10.1007/s12975-012-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006a;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006b;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, Arakawa S, Sugimori H, Kamouchi M, Kitazono T, Iida M. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- Pelidou SH, Kostulas N, Matusevicius D, Kivisakk P, Kostulas V, Link H. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–442. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- Prestigiacomo CJ, Kim SC, Connolly ES, Jr, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke. 2011a;42:2578–2583. doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011b;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011c;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Stubbe T, Ebner F, Richter D, Randolf Engel O, Klehmet J, Royl G, Meisel A, Nitsch R, Meisel C, Brandt C. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33:37–47. doi: 10.1038/jcbfm.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–347. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, Tarkowski A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Investigational anti-inflammatory agents for the treatment of ischaemic brain injury. Expert Opin Investig Drugs. 2005;14:393–409. doi: 10.1517/13543784.14.4.393. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang RL, Lu M, Krams M, Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]