Abstract
Purpose
To characterize the effect of a prostate-rectum spacer on dose to rectum during external beam radiotherapy for prostate cancer, and to assess for factors correlated with rectal dose reduction.
Materials and methods
Fifty-two patients at 4 institutions were enrolled onto a prospective pilot clinical trial. Patients underwent baseline scans, then were injected with perirectal spacing hydrogel and re-scanned. IMRT plans were created on both scans for comparison. Objectives were to establish rates of creation of ≥7.5mm of prostate-rectal separation, and decrease in rectal V70 of ≥25%. Multiple regression analysis was performed to evaluate associations between pre- vs. post-injection changes in rectal V70 and changes in plan conformity, rectal volume, bladder volume, bladder V70, PTV volume, as well as post-injection mid-gland separation, gel volume, gel thickness, length of PTV/gel contact, or gel left-to-right symmetry.
Results
Hydrogel resulted in ≥ 7.5mm prostate-rectal separation in 95.8% of patients; 95.7% had decreased rectal V70 of ≥ 25%, with mean reduction of 8.0 Gy. There were no significant differences in pre- and post-injection prostate, PTV, rectal, and bladder volumes. Plan conformities were significantly different pre- vs. post-injection (P = 0.02); plans with worse conformity indexes post-injection compared to pre-injection (n=13) still had improvements in rectal V70. In multiple regression analysis, greater post-injection reduction in V70 was associated with decreased relative post-injection plan conformity (P=0.01). Reductions in V70 did not significantly vary by institution, despite significant inter-institutional variations in plan conformity. There were no significant relationships between reduction in V70 and the other characteristics analyzed.
Conclusions
Injection of hydrogel into prostate-rectal interface resulted in dose reductions to rectum for > 90% of patients treated. Rectal sparing was statistically significant across a range of 10–75 Gy, and was demonstrated within the presence of significant inter-institutional variability in plan conformity, target definitions, and injection results.
INTRODUCTION
Adenocarcinoma of the prostate is one of the most common cancers in the world, and external beam radiation a highly effective treatment method for patients with localized disease. Despite advances in treatment planning and image guidance, rectal toxicity following treatment remains a considerable limitation and risk due to the close proximity of the rectum to posterior prostate. With dose-escalated (e.g., ≥78 Gy) IMRT treatment, rates of acute grade ≥2 rectal toxicity range from 3–20%; similarly, rates of chronic grade ≥2 rectal toxicity range from 5–21%(1)(2). The risk of late rectal toxicity has been well correlated to the volume of rectum/rectal wall receiving higher doses of radiation, in particular the volume receiving 70 Gy or more, i.e. V70(3)(4). Therefore, a method of reducing the amount of rectum treated to higher dose levels would be expected to result in reduction of the rate of rectal toxicity.
One means of affecting such reduction would be to temporarily increase the distance between the prostate and rectum. DuraSeal Dural Sealant (Covidien, Mansfield, MA) and Mynx Vascular Closure Device (AccessClosure, Mountain View, CA) are both absorbable polyethylene glycol (PEG) based hydrogels widely used in neurosurgery and interventional procedures, respectively. The safety profile of those products in these settings as well as in thoracic surgery, in conjunction with the safety profile of PEGylated drugs(5), suggests that PEG hydrogels may be ideal for use as prostatic – rectal spacers. Appendix e1 lists current FDA-approved products and relevant clinical trials. In contrast to pre-existing formulations, the formulation used in this study was tailored to polymerize over a longer period of time (12 seconds) in order to allow adequate time for injection via needle, while balancing the needs for dimensional stability ≥3 months and for hydrolysis/absorption within 6 months.
Prior pre-clinical work with PEG hydrogel demonstrated its ability to reduce rectal dose in cadaveric models(6). Herein we validate and characterize the spatial and dosimetric effects of PEG hydrogel injection in patients undergoing external beam radiotherapy for prostate cancer within the auspices of a multi-institutional trial.
METHODS AND MATERIALS
Fifty-two patients at 4 institutions (xxx) with biopsy-confirmed diagnosis of prostate cancer were enrolled onto a prospective, Ethics Committee-approved, open-label pilot study over a 16-month period. Enrolled patients met eligibility criteria defined as clinical stage T1–T2, Gleason Score ≤ 7 (primary pattern ≤ 3), PSA ≤ 20 ng/mL, and prostate volume < 80 cc. Other criteria included hematocrit > 30%, platelets > 100,000/mm3, INR within laboratory reference range, and ECOG performance status ≤ 2. Subjects with metastatic disease, prior prostate surgery or radiotherapy, chronic prostatitis, rectal or gastrointestinal surgery, or a history of inflammatory bowel disease were excluded from the study. Informed consent was obtained from all subjects.
Prior to hydrogel injection, patients were imaged with CT and/or MRI (see Table 1) in order to measure baseline prostate – rectum spacing. Under aseptic conditions and using either local or general anesthesia, an 18G needle was placed into the anterior perirectal space (potential space immediately posterior to Denonvilliers’ fascia) via a transperineal approach under transrectal ultrasound guidance. Sterile normal saline or lidocaine was first injected to expand the space, followed by 10–30cc of hydrogel precursors (SpaceOAR System, Augmenix, MA), which subsequently solidify into gel form. The hydrogel volume was limited to 10cc in the final 29 study patients, as additional volume was not required for acceptable space creation.
Table 1.
Target volumes and planning margins, by institution (limited to patients with successful injections and valid pre- and post-injection comparison plans)
| # patients | Center 1 | Center 2 | Center 3 | Center 4 |
|---|---|---|---|---|
| 5 | 19 | 17 | 4 | |
| CTV | Initial: Prostate + seminal vesicles Cone-down: Prostate | Prostate + caudal 2/3 of seminal vesicles | Prostate +/− caudal 2/3 of seminal vesicle* | Prostate (1 patient); Prostate + seminal vesicles (2 patients) |
| PTV | Initial: CTV + 7mm (4mm posteriorly) to 7200 cGy Cone-down: Prostate + 4mm to 7800 cGy | CTV + 5mm† | CTV + 7mm (4mm posteriorly) | CTV + 5mm |
7 patients with caudal 2/3 of seminal vesicles included in CTV on both pre- and post-injection plans
1 patient with 5mm margins on pre-injection and 3mm margins on post-injection plans
Approximately one week following injection, patients were again imaged (CT +/− MRI) to measure the space created. IMRT-based treatment plans for actual treatment were created using the post-implantation scan(s), with treatment plans using similarly-defined target volumes and margins created on the pre-injection scan(s) for comparison purposes. Clinical target volumes (CTVs) included prostate, +/− seminal vesicles (or a portion thereof) at the treating physician’s discretion. No patient received pelvic nodal radiation. Planning target volume (PTV) margins were institutionally determined, within protocol-defined limits of 4–10 mm (Table 1). Rectum was contoured as a solid structure from the anus (generally at the level of the ischial tuberosities) superiorly to the rectosigmoid junction. Normal tissue constraint objectives were for rectal V70 < 20% and bladder V70 <40%, with additional constraints up to institutional discretion as long as there was consistency between pre- and post-injection plans for any given patient.
Plans were reviewed by a non-treating physician and physicist (xx) and confirmed for consistency between pre- and post-injection studies. All plans were intensity-modulated, with similar beam arrangements utilized for pre- and post-treatment plans within individual patients. Dose prescribed to prostate and PTV was 78 Gy in 1.8–2.0 Gy fractions. At least 95% of the PTV was to receive 99% of the prescribed dose, with maximum dose ≤ 107% of prescription. To determine the extent and timing of hydrogel absorption, patients underwent repeat imaging at completion of treatment as well as at 3 and 6 months following radiotherapy. Patients were treated with image guidance using either cone-beam CT (2 institutions), mega-voltage CT (1 institution), or transabdominal ultrasound (1 institution).
The primary study objectives were to demonstrate functional success in creating ≥7.5mm space between rectum and prostate (at mid-gland) and clinical success in reducing the rectal V70 (volume receiving ≥ 70 Gy) by 25% compared to pre-injection treatment plan. Definition of functional success was based upon prior cadaveric data showing that 5mm thickness of hydrogel resulted in a mean relative reduction in V70 of 28.3% when prescribing 78 Gy(6) Using an expected 90% success rate for each endpoint, the expected probability of achieving both endpoints would be 81%. Given an original sample size of 52, the lower bound of the 95% confidence interval (CI) is 67%, i.e. the probability of confirming achievement of both endpoints was ≥67%.
Secondary objectives were to assess for factors associated with greater reductions in rectal V70. Several patient, treatment plan, and gel injection characteristics with potential effect on V70 reduction and gel effect were analyzed. Mid-gland prostate-rectal separation was measured on the axial image slice closest to halfway between apex and base, from posterior edge of prostate to inner rectal wall. Measurements were also taken 3–5mm caudal to the prostatic base, and 3–5mm cranial to apex. Gel thicknesses were also measured at these locations. Measurements were taken in an anterior-posterior direction at the midline (left-to-right) of the prostate-rectal interface. Gel symmetry was evaluated based on axial mid-gland images, and categorized in binary fashion based on whether the thickest portion of gel was in the midline (left-right) or skewed to one side of the prostate-rectal interface. Percent of PTV with gel contact was calculated as total PTV length (in cranial-caudal direction) divided by length of PTV having adjacent gel.
Dosimetric studies
In order to determine whether the conformity of plans was associated with the level of rectal dose reduction following injection, we assessed the relationship between absolute V70 reduction and change in plan conformity (pre- vs. post-injection). A more conformal post-injection plan could contribute to greater rectal dose reduction; conversely, a less conformal post-injection plan might result in a reduced effect of the hydrogel on rectal dose due to anterior rectal wall still falling within higher isodose lines. Since the main target coverage objective was for ≥ 99% of PTV to receive 95% of prescribed dose (=7,410 cGy), plan conformity was defined as volume of tissue receiving 7410 cGy ÷ PTV volume (for center 1, the composite dose plan and cone-down PTV were utilized).
Statistical analysis
Descriptive statistics was used to characterize the variables of interest. Each of the variables was checked for normality of distribution using Shapiro-Wilk test. Comparisons between pre- and post-injection plan characteristics and doses were performed using non-parametric Wilcoxon signed rank test. Spearman’s rank correlation coefficients were calculated to quantify the relationship between the variables of interest. Multiple linear regression analysis was further performed to evaluate the associations between pre- vs. post-injection changes in rectal V70 and the following in the presence of all other variables to control for confounding: change in conformity, change in rectal volume, change in bladder volume, change in bladder V70, change in PTV volume, injected gel volume, gel thickness at mid-gland, mean gel thickness (base-midgland-apex), gel symmetry, or % length of PTV with gel contact. Variables were selected using the backward elimination procedure with P<0.05 for retention in the model, with the independent significance of a variable in the model tested using the Wald statistic. All statistical tests were two-sided and considered statistically significant at P<0.05. Analyses were performed using IBM SPSS Statistics (v19.0.0) and SAS (version 9.2, Cary, NC).
Results
Of 52 patients enrolled, 48 had injection of hydrogel into the prostate-rectal interface. Figure 1 shows imaging of an example patient with successful injection. Four patients had unsuccessful injections, prior to the realization that utilizing brachytherapy technique was most effective due to better visualization and needle control. Details regarding unsuccessful injections, as well as safety and clinical efficacy of gel injection, are in a separate manuscript in press(7). Four physicians performed injections (one per institution). Injecting physicians reviewed post-injection images, but dosimetric results were not specifically used as feedback to alter application technique, although 1 physician was also involved in radiation treatment planning. Informally, it was observed that physicians would typically take 4–5 procedures before becoming most competent with the procedure, except those with brachytherapy experience where there seemed to be shorter time to proficiency.
Fig. 1.
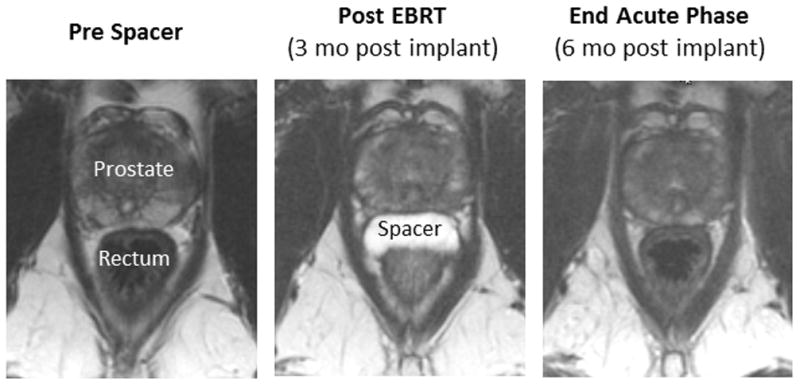
Axial T2 MRI images of a patient prior to hydrogel injection (left), post radiotherapy (middle), and 6 months after injection (right).
Measurements of separation taken pre- and post-injection are shown in Table 2. Excluding the 4 patients who were not injected into the prostate-rectal interface, functional success (≥ 7.5mm mid-gland prostate-inner rectal wall separation) was achieved in 95.8% of patients (46/48; 95% CI=85.8%–99.5%); 91.7% of patients with gel injected into prostate-rectal interface (44/48; 95% CI=80.0%–97.7%) had gel thickness at mid-gland of > 5.0mm.
Table 2.
Gel injection characteristics
| Gel thickness (n=48) | Mean (±SD), mm | Median (range), mm |
|---|---|---|
|
| ||
| Base† | 8.0 (±6.9) | 7.5 (0.0–30.3) |
| Mid-gland | 10.0 (±4.4) | 9.4 (0.0–21.5) |
| Apex†† | 6.7 (±5.6) | 7.1 (0.0–20.8) |
| Average gel thickness (base/mid/apex) | 8.1 (±4.2) | 7.1 (2.0–17.9) |
| Prostate to inner rectal wall distance (n=48) | Pre-injection, mm | Post-injection, mm | P* |
|---|---|---|---|
|
| |||
| Base† | |||
| Mean (±SD) | 9.8 (±6.6) | 18.4 (±7.2) | < .0001 |
| Median (range) | 8.2 (1.0–25.9) | 18.1 (4.7–34.7) | |
|
| |||
| Mid-gland | |||
| Mean (±SD) | 5.8 (±3.7) | 15.5 (±5.8) | < .0001 |
| Median (range) | 5.4 (1.0 – 18.6) | 14.6 (5.6 – 34.2) | |
|
| |||
| Apex†† | |||
| Mean (±SD) | 5.9 (±3.0) | 12.6 (±5.8) | < .0001 |
| Median (range) | 5.6 (1.0–14.1) | 11.7 (4.7–31.3) | |
Non-parametric Wilcoxon signed rank test
Measurement taken on axial image 3–5mm caudal to cranial-most aspect of prostate
Measurement taken on axial image 3–5mm cranial to caudal-most aspect of prostate
Dosimetric outcomes
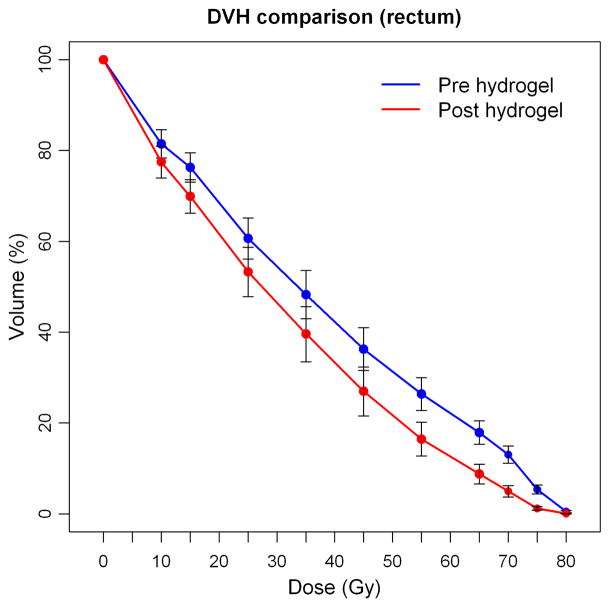
Of the 48 patients injected into the prostatic-rectal interface, 2 were excluded from the dosimetric analysis due to missing pre- or immediate post-injection treatment plans. Clinical success (≥ 25% reduction in V70) was achieved in 95.7% of these patients (44/46; 95% CI=85.2%–99.5%)). Of the two patients who were not categorized as clinical success, one patient had a 4% increase in rectal V70 on his post-injection plan; another patient had a 39% increase in rectal V70, but for this patient there was notable overlap of the prostate contoured volume into the gel on the post-injection treatment plan. This latter patient was excluded from subsequent analyses. Comparisons between pre- and post-injection plans are shown in Table 3. Significant reductions in rectal dose were seen across all levels of dose (V75 through V10 in 5–10 Gy increments, P ≤ 0.02). There were no significant differences for the cohort with regard to pre-injection vs. post-injection prostate volume, PTV volume, rectal volume, or bladder volume. The mean reduction in V70 was 8.0% (± 4.2); median 7.8% (−0.3 – 19.5). Figure 2 shows mean pre- and post-injection rectal dose volume values for the cohort.
Table 3.
Comparison of pre- and post-injection plans (n=45)
| Variable | Pre-injection | Post-injection | P* |
|---|---|---|---|
|
| |||
| Prostate volume (cc) | |||
| Mean (±SD) | 65.9 (±29.84) | 64.4 (±30.7) | |
| Median (range) | 61.0 (21.1–163.9) | 61.3 (21.2–171.0) | 0.14 |
|
| |||
| PTV volume (cc) † | |||
| Mean (±SD) | 154.6 (±48.8) | 151.2 (±46.6) | |
| Median (range) | 150.2 (44–267) | 148.1 (48–269) | 0.38 |
|
| |||
| Rectal volume (cc) | |||
| Mean (±SD) | 96.5 (±47.5) | 93.4 (±47.3) | |
| Median (range) | 82.8 (42.4 – 289.1) | 83.0 (35.6 – 233.2) | 0.19 |
|
| |||
| Bladder volume (cc) | |||
| Mean (±SD) | 228.0 (±145.0) | 299.3 (±204.6) | |
| Median (range) | 182.8 (57.3 – 776.6) | 221.0 (84.9 – 911.2) | 0.15 |
|
| |||
| Conformity index | |||
| Mean (±SD) | 1.33 (±0.30) | 1.30 (±0.29) | |
| Median (range) | 1.25 (1.00–2.98) | 1.17 (1.04–2.50) | 0.02 |
|
| |||
| Bladder V70 (%) | |||
| Mean (±SD) | 14.0 (±7.5) | 12.2 (±9.2) | |
| Median (range) | 12.4 (2.5–31.1) | 9.0 (2.7–36.9) | 0.24 |
|
| |||
| Rectal V75 (%) | |||
| Mean (±SD) | 5.5 (±3.2) | 1.2 (±1.3) | |
| Median (range) | 5.6 (0.2–11.1) | 0.9 (0.0–5.4) | <.001 |
|
| |||
| Rectal V70 (%) | |||
| Mean (±SD) | 13.0 (±6.2) | 5.1 (±4.2) | |
| Median (range) | 11.6 (3.1 – 32.7) | 4.0 (0 – 19.5) | <.001 |
|
| |||
| Rectal V65 (%) | |||
| Mean (±SD) | 17.9 (±8.4) | 8.8 (±7.0) | |
| Median (range) | 16.3 (3.7–43.4) | 6.9 (0.0–31.6) | <.001 |
|
| |||
| Rectal V55 (%) | |||
| Mean (±SD) | 26.3 (±11.8) | 16.4 (±12.1) | |
| Median (range) | 24.1 (6.5–56.6) | 13.0 (0.1–48.3) | <.001 |
|
| |||
| Rectal V45 (%) | |||
| Mean (±SD) | 36.3 (±15.6) | 27.0 (±17.8) | |
| Median (range) | 32.7 (8.8–73.5) | 21.7 (0.9–66.7) | <.001 |
|
| |||
| Rectal V35 (%) | |||
| Mean (±SD) | 48.3 (±17.5) | 39.6 (±20.0) | |
| Median (range) | 42.7 (15.1–87.4) | 35.7 (10.0–81.0) | <.001 |
|
| |||
| Rectal V25 (%) | |||
| Mean (±SD) | 60.8 (±15.0) | 53.3 (±17.9) | |
| Median (range) | 59.6 (28.8–91.9) | 54.7 (22.2–86.4) | 0.001 |
|
| |||
| Rectal V15 (%) | |||
| Mean (±SD) | 76.3 (±10.6) | 69.9 (±12.2) | |
| Median (range) | 77.2 (49.1–96.0) | 69.9 (43.6–91.6) | 0.002 |
|
| |||
| Rectal V10 (%) | |||
| Mean (±SD) | 81.5 (±10.3) | 77.4 (±11.4) | |
| Median (range) | 82.4 (53.3–98.3) | 78.7 (48.9–96.9) | 0.02 |
Non-parametric Wilcoxon signed rank test
Initial PTV volume (72 Gy) for Center 1
Fig. 2.
Dose-volume histogram showing mean rectal doses and 95% confidence intervals for all patients on pre-injection and post-injection treatment plans (confidence intervals for D10–D45 values overlap).
Rectal V70 was associated with plan conformity index for both pre-injection plans (P=0.001, correlation coefficient 0.46) and post-injection plans (P=0.008, correlation coefficient 0.39). Conformity indexes on pre-injection plans (median 1.25, range 1.00–2.98) were significantly higher (less conformal) than those from post-injection (median 1.17, range 1.04–2.50) (p=0.02). However, even patients with comparatively worse post-injection plan conformity index (n=13) still had reductions in rectal V70. In the multiple regression analysis after adjusting for all other variables, greater rectal V70 reduction was associated with decreased relative conformity index in the post-injection (vs. pre-injection) plan (regression coefficient −7.59, P = 0.013); see Figure 3. When comparisons were made between treatment centers, there were statistically significant variations in change in bladder volume (P=0.04), change in bladder V70 (P=0.02), pre-injection conformity (P<0.001), post-injection conformity (P<0.001), and change of conformity (pre- vs. post-injection, P=0.02). There were also significant differences between centers in both pre-injection and post-injection rectal V70 (P=0.002 and P=0.001, respectively), but not in reduction in rectal V70 (P=0.31). No inter-institutional differences were seen in prostate volume (pre- or post-injection), PTV volume (pre- or post-), rectal volume (pre- or post-), or pre-injection prostate-rectum midgland separation. To understand whether the associations of these variables with V70 reduction was dependent on treatment center, interactions between institution and change of conformity, change in rectal volume and change in bladder volume respectively were tested in the multiple regression and results were not statistically significant. Change in rectal volume was found to be associated with reduction in V70 with a marginal significance (P=0.05) while there were no significant associations between change in rectal V70 and change in bladder volume, injected volume of gel, gel thickness at mid-gland, mean gel thickness (base to apex), gel symmetry, % length of PTV with gel contact, or change in PTV.
Fig. 3.
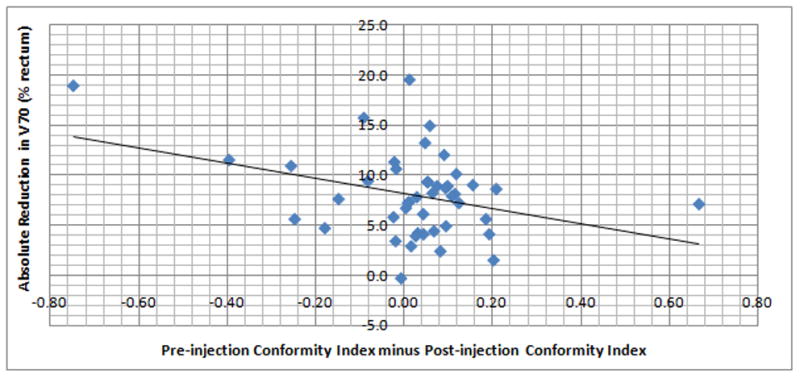
Reduction in V70 (absolute) relative to change in conformity index (pre-injection minus post-injection).
Scans taken at end of radiotherapy showed gel persistence, with no significant differences between prostate-rectal separation post-injection vs. end of treatment (p = 0.19, 0.06, 0.14 for base, mid-gland, and apex, respectively). Analysis of MRI scans taken 6 months following completion of radiotherapy found traces of gel in one patient (who had received a 10cc injection), with complete hydrogel absorption in all other patients.
Discussion
Recent pre-clinical and clinical work has been directed at evaluating the utility of injecting biomaterials as temporary spacers between the prostate and rectum(6). Clinical reports have described the use of hyaluronic acid (HA) (8)(9) as well as human collagen(10). While promising, issues related to HA radiation sensitivity (11) and the theoretical possibility of infectious agent transmission from human derived products have resulted in considerable interest in synthetic polyethylene glycol (PEG) based materials, which are currently used elsewhere in the body.
In this study, hydrogel injection was successful in creating ≥ 7.5mm of prostate-rectal separation among 95.8% of patients, with 95.7% of patients having a decrease in rectal V70 of ≥ 25%. The mean reduction in rectal V70 was 8.0 Gy. Statistically significant dose reductions were also seen at all rectal dose levels. One patient did have an unexpected increase in rectal V70 on his post-injection plan (0.3 Gy increase). This patient had 5.2mm of gel thickness at mid-gland, but baseline pre-injection V70 was relatively low (7.3%), likely due to a very large rectal volume of 289cc pre-injection, vs. 189cc post-injection. This was the third largest absolute difference in pre- vs. post-injection rectal volume within the entire cohort, and the largest decrease in post-injection rectal volume.
These results are supported by the absence of statistically significant differences in the pre- and post-injection plans overall with regard to prostate, PTV, rectal, and bladder volumes, differences which if prominent could potentially skew these results. However, conformity indexes were significantly different pre- vs post-injection. Although reductions in rectal dose among some patients may have been enhanced by more conformal post-injection plans, it is worth noting that all 13 patients whose post-injection plans had worse conformity index than pre-injection still had improvements in rectal V70. It is possible that for some subjects, there was a planning bias with more effort placed into reducing the rectal dose on the post-injection plan in order to achieve comparative dosimetric success. Another possibility is that hydrogel altered the prostate or rectal contours and therefore altered the achievable conformity. Although the prostate and rectal contours were in some cases slightly altered by the presence of hydrogel, the tendency of hydrogel to indent the prostate and thus make it more concave in shape would be likely to make dose conformity to the prostate contour more difficult rather than easier to achieve. Alternatively, it is possible that the presence of gel spacing allows for better conformity by allowing inverse planning algorithms and dosimetrists to place comparatively more weight on conformity instead of rectal avoidance.
Interestingly, there was a significant inverse correlation between treatment plan conformity and reduction in rectal V70, i.e. a greater reduction in V70 was associated with a less conformal post-injection plan compared to the pre-injection plan (Figure 3). One potential explanation is that for some patients, the planning objectives for rectal dose were readily achieved despite less conformity, leading to plans which were clinically acceptable yet not maximally optimized. In order not to bias pre- vs. post-injection comparisons, this study did not prescribe more stringent goals for post-injection plans with regard to rectal dose. Should hydrogel injection prove to be efficacious in reducing rectal complications, physicians and treatment planners may need to redefine and raise expectations for rectal dose sparing compared to current practices in order to best exploit the benefits of this technique.
With regard to the observed institutional differences in bladder volumes and bladder V70s pre- vs. post-injection, we speculate that institutional variations in practice with regard to encouraging patient bladder filling contributed to this trend, with some patients having less bladder filling on their initial simulation but remembering to fill their bladder for their second simulation.
Contrary to expectations, various measures of change in rectal separation or gel thickness did not have significant correlations to reductions in rectal V70. This may be due to other variables affecting rectal dose, such as plan conformity. It is also possible that the relationship between rectal dose reduction and hydrogel is not adequately described by one-dimensional measures utilized here, since the hydrogel was observed to vary in geometric shape as well as thickness. Other metrics such as overlap volume histograms, which take into account three-dimensional geometric relationships between target volumes and organs at risk to determine achievable dose-volume histograms, may be useful in this regard(12). In a separate study using a subset of patients, we found that an overlap-volume histogram metric was more predictive of rectal sparing than volume of injected gel(13)
The effect of plan conformity on rectal V70 was demonstrated by the presence of significant correlations between the two measures on both pre- and post-injection plans. However, the rectal-sparing effects of hydrogel were demonstrated despite significant inter-institutional variability in plan conformity, target definitions, and injection results, with no statistically significant differences in achieved reductions in V70. These results suggest that the majority of patients will have improvements in rectal dosimetry regardless of institutional technique. It is also possible that greater experience with injection technique and treatment planning on gel-injected patients may improve upon these results.
To our knowledge, this is the largest study to date on the use of temporary injectable spacers for rectal dose sparing. Although the relationship between rectal dose and volume to toxicity is well established and these results demonstrate the ability to significantly reduce rectal doses both high and low, the clinical impact of this method needs to be confirmed, and observed gastrointestinal toxicity rates in this cohort will be reported separately. Finally, clinical validation of reduced toxicity in a randomized comparison of intensity-modulated radiotherapy with or without hydrogel is warranted, and is currently underway. SpaceOAR® hydrogel is approved for this indication in several geographies outside the US. Within the US, FDA approval is pending results of the Phase III study noted above; until then, use should be confined to clinical trials.
Conclusions
Injection of PEG-hydrogel into the prostate-rectal interface successfully resulted in statistically significant dose reductions to rectum across the entire dose range for > 90% of patients treated. The rectal-sparing effects of hydrogel injection were demonstrated within the presence of significant inter-institutional variability in plan conformity, target definitions, and injection results.
Summary.
Hydrogel injection into the prostate-rectal interface resulted in significant dose reductions to rectum across the entire dose range for > 90% of patients undergoing external beam radiotherapy for prostate cancer. Rectal-sparing effects were demonstrated within the presence of significant inter-institutional variability in plan conformity, target definitions, and injection results.
Footnotes
Conflict of Interest Notification
This work was supported by funding from Augmenix Inc., including provision of research materials (hydrogel), funding for data monitoring support, and research-related travel costs. Drs. Song, DeWeese, and Ford have also received consulting fees from Augmenix Inc.
References
- 1.Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009 Mar 1;73(3):685–91. doi: 10.1016/j.ijrobp.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005 Aug 1;62(5):1297–308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 4.Huang EH, Pollack A, Levy L, et al. Late rectal toxicity: Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002 Dec 1;54(5):1314–21. doi: 10.1016/s0360-3016(02)03742-2. [DOI] [PubMed] [Google Scholar]
- 5.Jain A, Jain SK. PEGylation: An approach for drug delivery. A review. Crit Rev Ther Drug Carrier Syst. 2008;25(5):403–47. doi: 10.1615/critrevtherdrugcarriersyst.v25.i5.10. [DOI] [PubMed] [Google Scholar]
- 6.Susil RC, McNutt TR, DeWeese TL, Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010 Mar 15;76(4):1251–8. doi: 10.1016/j.ijrobp.2009.07.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhl M, van Triest B, Eble MJ, et al. Low rectal toxicity after dose escalated IMRT treatment of prostate cancer using an absorbable hydrogel for increasing and maintaining space between the rectum and prostate: Results of a multiinstitutional phase II trial. Radiother Oncol. doi: 10.1016/j.radonc.2012.11.009. (in press) [DOI] [PubMed] [Google Scholar]
- 8.Prada PJ, Fernandez J, Martinez AA, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007 Sep 1;69(1):95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Wilder RB, Barme GA, Gilbert RF, et al. Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010 Jul 1;77(3):824–30. doi: 10.1016/j.ijrobp.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 10.Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012 Apr 1;82(5):1918–22. doi: 10.1016/j.ijrobp.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Daar E, King L, Nisbet A, et al. Viscosity changes in hyaluronic acid: Irradiation and rheological studies. Appl Radiat Isot. 2010 Apr-May;68(4–5):746–50. doi: 10.1016/j.apradiso.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Wu B, Ricchetti F, Sanguineti G, et al. Data-driven approach to generating achievable dose-volume histogram objectives in intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011 Mar 15;79(4):1241–7. doi: 10.1016/j.ijrobp.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Ford EC, Wu B, et al. An overlap-volume-histogram based method for rectal dose prediction and automated treatment planning in the external beam prostate radiotherapy following hydrogel injection. Med Phys. doi: 10.1118/1.4769424. (in press) [DOI] [PubMed] [Google Scholar]