Abstract
Several models have proposed that different regions of the medial temporal lobes contribute to different aspects of episodic memory. For instance, according to one view, the perirhinal cortex represents specific items, parahippocampal cortex represents information regarding the context in which these items were encountered, and the hippocampus represents item-context bindings. Here, we used event-related functional magnetic resonance imaging (fMRI) to test a specific prediction of this model – namely, that successful retrieval of items from context cues will elicit perirhinal recruitment and that successful retrieval of contexts from item cues will elicit parahippocampal cortex recruitment. Retrieval of the bound representation in either case was expected to elicit hippocampal engagement. To test these predictions, we had participants study several item-context pairs (i.e., pictures of objects and scenes, respectively), and then had them attempt to recall items from associated context cues and contexts from associated item cues during a scanned retrieval session. Results based on both univariate and multivariate analyses confirmed a role for hippocampus in content-general relational memory retrieval, and a role for parahippocampal cortex in successful retrieval of contexts from item cues. However, we also found that activity differences in perirhinal cortex were correlated with successful cued recall for both items and contexts. These findings provide partial support for the above predictions and are discussed with respect to several models of medial temporal lobe function.
Keywords: Episodic Memory, Retrieval, Medial Temporal Lobe, Perirhinal Cortex, Parahippocampal Cortex, Hippocampus
1. INTRODUCTION
It is not an uncommon experience to come across an item that triggers memory for related contextual information or a context that calls to mind a particular item. For example, while rummaging through a junk drawer, you might encounter a shell amongst the rubble and immediately recall the beach where you enjoyed your first surfing lesson. Conversely, you might happen upon that beach sometime later, and be reminded of the shell you kept from your surfing experience. While both of these examples illustrate the act of retrieving additional information from a particular cue (i.e., either context from item or item from context), they may differentially engage brain regions known to play a critical role in successful encoding and subsequent retrieval of episodic memories.
There is broad consensus that the medial temporal lobes (MTL) are critical for long-term memory, and several models have proposed that the hippocampus and adjacent MTL cortical structures (e.g., the perirhinal and parahippocampal cortices) contribute in different ways (e.g., Brown & Aggleton, 2001; Cohen & Eichenbaum, 1993; Davachi, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Graham, Barense, & Lee, 2010). According to one influential model, perirhinal cortex supports the process of familiarity-based recognition, and the hippocampus supports successful recollection (Brown & Aggleton, 2001). Competing models have stressed differences in the representational characteristics of MTL structures, emphasizing a role for the hippocampus in relational memory (e.g., memory for relationships among items and the contexts in which they were initially encountered; Cohen & Eichenbaum, 1993) and roles for the perirhinal and parahippocampal cortices in representation of information about items and contexts, respectively (e.g., Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; Eacott & Gaffan, 2005; Eichenbaum et al., 2007; Montaldi & Mayes, 2010).
As emphasized in one of these models – the Binding of Items and Context (or BIC) model (Diana et al., 2007; Eichenbaum et al., 2007) – process-based and representational views are not necessarily incompatible because hippocampus-mediated relational memory representations may support the experience of recollection (i.e., item recognition accompanied by successful retrieval of additional details about the encoding context) and item-specific perirhinal representations may support a subjective sense of familiarity (i.e., item recognition abwasent any associated information about the encoding experience). Importantly, however, the BIC model does not rule out possible contributions of perirhinal cortex to recollection, which is consistent with results of recent functional magnetic resonance imaging (fMRI) investigations showing that perirhinal cortex contributes to successful source recollection when source (in this example, a particular color) has been encoded as an item detail (e.g., a red elephant; Staresina & Davachi, 2008). The BIC model also predicts that activity differences in the parahippocampal cortex will be associated with successful recollection to the extent that contextual representations have been recovered.
The fMRI experiment described here was designed to test a specific prediction of the BIC model – namely that cued recall of items from contexts will elicit perirhinal recruitment and that cued recall of contexts from items will elicit parahippocampal recruitment. To test this prediction, we had participants encode trial unique item-context pairs where items were pictures of common objects and contexts were pictures of indoor and outdoor scenes. During a scanned retrieval phase, participants attempted to recall contexts (i.e., studied scenes) from associated item cues and to recall items (i.e., studied objects) from associated context cues. Univariate and multivariate (i.e., pattern similarity) approaches were used to identify BOLD signal changes correlated with successful cued retrieval of items and contexts. These effects were evaluated in contrasts that compared studied cues for which the associate was successfully recalled to studied cues that were merely endorsed as familiar. In addition to predicted effects for the perirhinal and the parahippocampal cortices, we expected that successful cued recall in either condition, both of which required recovery of item-context relationships, would be supported by BOLD signal changes in the hippocampus.
2. METHODS
2.1 Participants
Twenty nine individuals (20 female) from the UC Davis community participated in this experiment and were compensated at a rate of 20 dollars per hour for their time. Data from eleven of these individuals were excluded because the number of trials (i.e., at least 8 per bin) associated with conditions of interest was insufficient for fMRI analyses or because of technical difficulties; therefore, the reported results reflect data from 18 participants (12 female). Informed consent was obtained from each individual in a manner approved by the Institutional Review Board at the University of California, Davis.
2.2 Materials
Materials included 228 items (pictures of objects - e.g., cardboard box, bandana, boomerang) and 228 contexts (full-color scenes - e.g., beach, auditorium). Because past work has shown that items with strong pre-experimental links to particular contexts (e.g., a filing cabinet) may automatically elicit retrieval of those contexts (e.g., Bar & Aminoff, 2003), we made every effort to select items for which this would not be the case. In addition, we were careful to select distinctive scene contexts from a variety of categories (e.g., there was just one bedroom scene, and contexts also included a pool hall, a cave, and a warehouse). Based on these methodological choices, it is unlikely that pre-experimental congruence between items and contexts would influence the reported outcomes.
From the above set of materials, 12 items and 12 contexts were used during a practice phase that was administered prior to the experiment. Items were sized to 150×150 pixels including a 10 pixel gray border and contexts were sized to 400×300 pixels including a 10 pixel white border; total screen resolution was set to 800×600 pixels.
2.3 Procedure and Design
After informed consent was obtained from each participant, the experimenter provided instructions in step with a 3-phase practice session. The practice session was an abbreviated version of the experiment proper and consisted of an encoding phase, a retrieval phase, and a post-test. When the experimenter was satisfied that the participant understood all of the instructions, and any remaining questions had been answered, the experiment commenced.
During the encoding phase, which took place outside of the scanner, participants were asked to commit 180 trial-unique item-context pairs to memory. Each pair remained in view for 3500ms, and was followed by a screen prompting participants to indicate whether or not they had successfully generated a story about how the item might be used in the associated context; this response requirement was meant to encourage active processing of each item-context pair. The prompt remained on the screen until a button press was made, and was then replaced with a centrally-located fixation cross that was visible for 1000ms before the next trial was initiated (see Figure 1a). All of the studied pairs were presented in a single block of trials.
Figure 1.
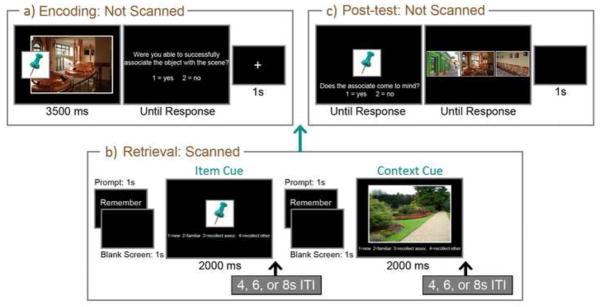
Illustration of materials and methods. A representative item-context pair (a) is presented along with associated trials from the scanned retrieval phase (b) and the unscanned post-test (c). In this example the thumbtack is a studied item cue and the garden path is a novel scene cue. Studied scene cues and novel item cues were also presented during the scanned retrieval phase, but are not illustrated here.
A scanned retrieval phase, consisting of six runs, took place shortly after encoding. During retrieval, individual pictures of items and contexts were presented for 2000ms in random order and participants were instructed to use these pictures as cues in an attempt to recall studied associates. There were 48 trials per run – 36 of these were retrieval trials and the remainders were active baseline trials that are not considered further in this report. A prompt, the word “Remember”, preceded each picture by 2000ms and distinguished retrieval trials from baseline trials; the mean inter-trial interval was 6000ms (range = 4000–8000ms). Pictures used in two-thirds of the retrieval trials were from studied pairs (12 studied items and 12 studied contexts per run; 72 of each total) and the remainders were novel (6 novel items, 6 novel contexts; 36 of each total). Importantly, if one element from a studied pair was presented as a retrieval cue, its associate was not.
Upon presentation of each picture, participants were instructed to make new responses if they felt the picture had not been seen during the corresponding study trials, familiar responses if they felt the picture was studied, but could not recall any diagnostic information to confirm this intuition, recollect-associate responses if they could call to mind the item or context with which the picture had been paired during study, and recollect-other responses if they could remember other details about the study experience, but not the associate (see also Vilberg & Rugg, 2007). Critically, and as described in more detail below (see Section 2.5), this set of response options permitted us to examine activity differences correlated with successful recall of items and contexts from studied cues and to minimize any potential contamination of fMRI data due to non-criterial recollection (i.e., by excluding trials endorsed as recollect-other from the reported contrasts). Participants were instructed to make their responses as quickly as possible without sacrificing accuracy, and were told that effortful retrieval attempts should be avoided if the associate did not immediately come to mind. When recall was successful, it was emphasized that participants should attempt to form a vivid mental image of the associate. Text was presented below each picture to remind participants of the above response mappings (see Figure 1b).
Because objective measures of associative retrieval success were not obtained during scanning, a post-test (3-alternative forced-choice recognition) was administered to confirm that participants could identify associates of studied items and contexts that had been used as retrieval cues. Across 144 trials, participants were presented with all of the studied item cues and all of the studied context cues. Following the presentation of a cue, participants were asked to indicate via button press whether or not they could recall the associate. When this response had been made, affirmative or not, the cue was replaced with three alternatives (3 contexts following an item cue; 3 items following a context cue) from which participants were instructed to identify the associate, guessing if necessary (see Figure 1c). All three of these alternatives were last seen during the study block (i.e., none were presented during the scanned retrieval phase), and each picture was used as a response option in two separate post-test trials. Performance on the recognition test was particularly important for trials classified by participants as recollect-associate during scanning as these were only included in the reported fMRI contrasts if participants successfully identified the associate when the post-test was administered. Based on this criterion, 11 percent of the trials that had been endorsed as recollect-associate were eliminated from reported fMRI contrasts.
For counterbalancing purposes each item (from the set of 216 excluding pictures used for practice) was randomly assigned to one of six lists (i.e., list 1, list 2 … list 6). Contexts were also assigned to one of six lists (i.e., list A, list B … list F) with the additional constraint that each list contained an equal number of indoor and outdoor scenes. A given list of items (e.g., list 1, which contained 36 objects) was then paired with a given list of contexts (e.g., list A, which contained 36 scenes) and individual items and contexts from corresponding lists (i.e., 1 and A) were presented as pairs during the study phase. These pairs were created systematically so that when a particular list of items was associated with the same list of contexts for more than one participant, the individual item-context pairs were always novel. Counterbalancing also ensured that paired lists rotated across fMRI scanning runs and that, within each list, individual items and contexts were presented equally often in every test condition (i.e., studied item, novel item, studied context, and novel context) across participants.
2.4 Image Acquisition and Preprocessing
MRI data were acquired with a 3T Siemens Trio scanner (Erlangen, Germany) located at the UC Davis Imaging Research Center. Each participant was provided with ear plugs to help attenuate scanner noise and padding was used to reduce head movement. Stimuli were back-projected onto a screen positioned at the foot of the scanner bed and viewed through a mirror attached to the 32-channel head coil.
Functional data were obtained with a gradient echoplanar imaging (EPI) sequence (repetition time, 2000ms; echo time, 25ms; field of view, 220; 64 × 64 matrix); each volume consisted of 34 axial slices, each with a slice thickness of 3.4mm, resulting in a voxel size of 3.4375 × 3.4375 × 3.4mm. Coplanar and high-resolution T1-weighted anatomical images were also acquired from each participant.
Preprocessing was performed using Statistical Parametric Mapping (SPM5) software. EPI data were slice-timing corrected using sinc interpolation to account for timing differences in acquisition of adjacent slices, realigned using a six-parameter, rigid-body transformation, spatially aligned to the Montreal Neurological Institute (MNI) EPI template, resliced into 3mm isotropic voxels, and spatially smoothed with an isotropic 8mm full-width at half-maximum Gaussian filter.
2.5 Univariate fMRI Data Analysis
To examine cued retrieval effects, event-related blood oxygen level-dependent (BOLD) responses associated with covariates of interest were deconvolved using linear regression (cf. Zarahn, Aguirre & D’Esposito, 1997). Covariates of interest were generated by convolving vectors of neural activity for each trial with an empirically-derived hemodynamic response function (see Hannula & Ranganath, 2008 for details). Individual trials were binned as a function of experimental condition (i.e., item cue, context cue), behavioral response (i.e., new, familiar, recollect-associate, and recollect-other), and accuracy, a classification scheme that produced 13 covariates of interest (i.e., novel item/context cue – correct rejection or false alarm; studied item/context cue – familiar, recollect-associate, recollect-other, miss; baseline trials). Additional covariates of no interest modeled spikes in the time series, global signal changes that could not be attributed to variables in the design matrix (Desjardins, Kiehl & Liddle, 2001), scan-specific baseline shifts, and an intercept. Regression analyses were performed on single-subject data using the general linear model with filters applied to remove frequencies above .25 Hz and below .005 Hz and yielded a set of parameter estimates for each participant. The magnitude of these parameter estimates can be interpreted as an estimate of the BOLD response amplitude associated with the covariates of interest described above.
The goal of this investigation was to determine whether or not MTL subregions make qualitatively different contributions to successful cued recall of items (from visible context cues) and contexts (from visible item cues). Therefore, we limit reported fMRI analyses to contrasts that examine activity differences between trials for which associative retrieval was successful (recollect-associate trials) and those for which it was not (familiar trials); data from individual trials were also separated by cue type (i.e., studied item cue, studied context cue). This approach was meant to minimize activity differences associated with visual presentation and recognition of the cue itself, and to reveal activity differences correlated with successful recollection of associates. To test hypotheses about the effects of item and context retrieval on MTL activation, we used anatomical landmarks to define specific regions of interest (ROIs) in the MTL. The MNI coordinates marking the anterior/posterior, medial/lateral and superior/inferior bounds of the hippocampus were y = −12/−39, × = ±18/±36, and z = 6/−24. The same coordinates were y = −3/−45, x = ±21/±36, and z = −3/−33, respectively, for the parahippocampal gyrus. The parahippocampal gyrus was subdivided into three sections of equal length – an anterior segment corresponding to perirhinal cortex, a posterior segment corresponding to parahippocampal cortex, and a middle segment corresponding to the transition zone between these regions (for a similar approach see Litman, Awipi, & Davachi, 2009; Staresina, Duncan, & Davachi, 2011). The same approach was used to examine activity differences in the hippocampus. The decision to subdivide the hippocampus was motivated in part by recent work that has documented preferential functional connectivity of anterior and posterior hippocampus with the perirhinal and parahippocampal cortices, respectively (Libby, Eckstrom, Ragland, & Ranganath, 2012), and by results of a multivariate pattern classification analysis (MVPA) that documented differences in the types of representational content supported by anterior (i.e., content general) and posterior (i.e., scene-specific) regions of the hippocampus (Liang, Wagner, & Preston, 2012). Activity differences in brain regions outside of the MTL are reported in Supplementary Table 1, but are not discussed here, as our predictions were specific to the MTL.
2.6 Multivariate fMRI Data Analysis: Pattern Similarity
In addition to the standard univariate approach, described above, we also performed a multivariate analysis. The logic here was that if cued retrieval involves reactivation of the corresponding associate (either item or context), then evidence for the associate should be available in brain regions that code for that type of stimulus information (e.g., Averbeck et al., 2006; Liang et al., 2012). To investigate this possibility, a multivoxel pattern similarity approach (Kriegeskorte et al., 2008a; Kriegeskorte et al., 2008b) was applied to the EPI timeseries data (preprocessed and filtered as above, but without spatial smoothing). Our goal was to examine whether or not there were activity patterns across voxels in MTL structures that were selectively sensitive to a particular type of retrieved content (i.e., recalled item content, recalled contextual content) or to successful reinstatement of bound item-context relationships (i.e., indexed by successful recall of both items and contexts from their respective cues). Activity patterns associated with studied cues endorsed as familiar were expected to contain only cue-specific information (i.e., information about the item or context in view), whereas activity patterns associated with studied cues that elicited successful recollection were expected to contain information about the bound representation of cue and associate. Therefore, multivariate analysis focused on comparing activation patterns between item and context cue trials that received either familiar or recollect-associate responses.
For each participant, a set of general linear models was constructed to estimate the BOLD response associated with individual test trials according to methods developed by Mumford et al. (2012). Covariates of no interest included a single vector modeling the convolved onsets of every other trial, motion vectors, and spikes in the time series. Single-trial parameter estimate images corresponding to the evoked response of each trial of interest were then computed using ordinary least squares regression. Each of these images indicated the magnitude of the BOLD response within each voxel associated with a single trial.
As with the univariate analysis, in order to determine the contributions of MTL subregions to cued retrieval of items and contexts, pattern analysis was restricted to anterior, middle, and posterior hippocampal and parahippocampal gyrus ROIs. For each participant, patterns of activation across voxels in each MTL ROI were extracted from single-trial parameter estimate images and vectorized. Activation pattern correlations (Pearson’s correlational coefficients) were then computed for all possible pairs of trials, resulting in twelve trial-by-trial pattern similarity matrices, one for each ROI (6 each in the left and right hemispheres).
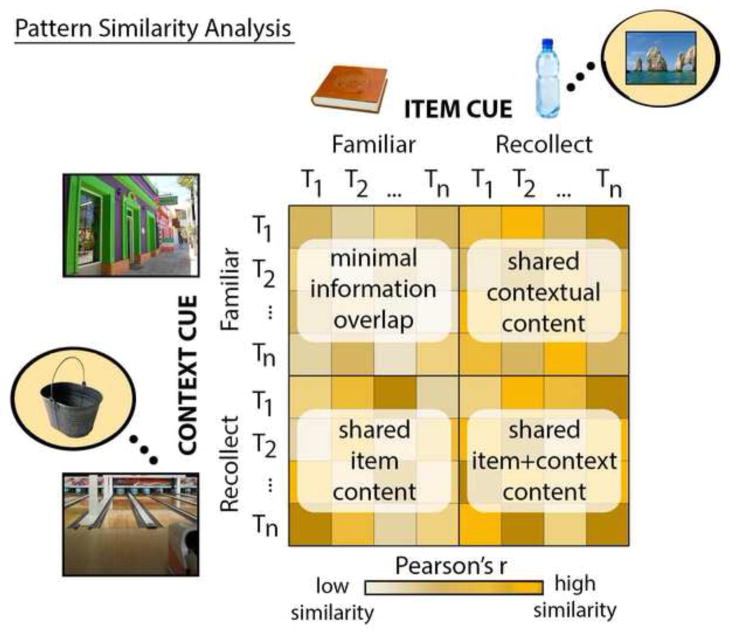
To investigate reinstatement effects associated with successful cued recall, four sets of correlations between item and context cue trials were evaluated in subsequent contrasts: 1) correlations between context and item cue trials endorsed as familiar (CF-IF), which were expected to have minimal content overlap; 2) correlations between familiar context cue trials and recollect-associate item cue trials (CF -IR), which were expected to have scene content in common; 3) correlations between recollect-associate context cue trials and familiar item cue trials (CR- IF), which were expected to have object content in common; and 4) correlations between context and item cue trials for which the associates were recollected (CR- IR), which were expected to share both scene and object information, regardless of cue type. In sum, all of the above correlations indexed pattern similarity between studied item cue trials and studied context cue trials, but the nature of shared information varied across correlations (see Figure 2). This meant that differences in the strength of pattern similarity effects could be used to examine sensitivity of MTL structures to particular types of recovered content. It is important to note that in this experiment pattern similarity measures were sensitive to shared category-level content (generic scene content, generic object content), but not to shared exemplar-level or item-specific content; this was because there was no exemplar-level (e.g., two different pencils) or item-specific (e.g., same pencil seen as a cue on one trial and recovered as an associate on another) overlap across trials. In a final step, average correlation coefficients were calculated for each participant, z-transformed, and entered into a repeated measures ANOVA (Type III sums of squares) to test our predictions.
Figure 2.
Illustration of the pattern similarity analysis approach. Correlations were calculated between item cue trials and context cue trials that were either endorsed as familiar or were associated with successful recovery of paired associates. These correlations were sensitive to shared information across trial types (e.g., item content, contextual content, or object and context content).
3. RESULTS
3.1 Behavioral Performance
Results based on behavioral performance indicated that participants could successfully distinguish old from new pictures during the scanned retrieval phase, and that performance was marginally better for item cues (d′=2.35) than for context cues (d′=1.99; t(17)=2.02, p=.06). Importantly, there were no significant differences in the proportion of studied pictures endorsed with familiar responses or with recollect-associate responses as a function of cue type, though participants did make their responses more quickly to item cues than to context cues (see Table 1). Consistent with expectations, post-test results revealed that participants successfully recognized associates of studied pictures that had been endorsed as recollect-associate more often than they recognized associates of studied pictures endorsed as familiar (F(1,17)=36.71, p<.001); there were no performance differences on the recognition test based on cue type (F(1,17)=1.84, p>.05), and the cue type by memory interaction was not significant (F(1,17), 3.06, p>.05; see Table 2).
Table 1.
Proportion of Studied Pictures Endorsed as Familiar or Recollect-Associate and Associated Response Times.
| Familiar (F) | Recollect-Associate (R-A) | |||
|---|---|---|---|---|
| Endorsement Rate | RT (ms) | Endorsement Rate | RT (ms) | |
| Item Cue | .33 (.12) | 1825.56 (437.44) | .45 (.14) | 1648.73 (361.82) |
| Context Cue | .34 (.13) | 2037.01 (526.77) | .40 (.14) | 1776.16 (324.24) |
| t(17)=.31, p>.05 | t(17)=5.69, p<.001 | t(17)=1.66, p>.05 | t(17)=4.44, p<.001 | |
Table 2.
Post-test Recognition Accuracy Sorted as a Function of Subjective Responses from the Scanned Retrieval Phase.
| Familiar | Recollect-Associate | |
|---|---|---|
| Item Cue | .82 (.10) | .94 (.05) |
| Context Cue | .86 (.12) | .94 (.08) |
| t(17)=1.83, p>.05 | t(17)=.49, p>.05 |
3.2 Univariate fMRI Results
To determine whether or not there were differences in MTL recruitment correlated with successful cued recall of items and contexts, parameter estimates associated with four conditions of interest (i.e., studied item cues endorsed as familiar, studied item cues for which the associated context was successfully recollected, studied context cues endorsed as familiar, and studied context cues for which the associated item was successfully recollected) were extracted from the anterior, middle, and posterior regions of the hippocampus and the parahippocampal gyrus for each participant. Differences in parameter estimates as a function of cue type (item cue, context cue), memory (familiar, recollect-associate), ROI (anterior, middle, posterior), and laterality (left hemisphere, right hemisphere) were then evaluated using two separate repeated measures ANOVAs, one calculated for the hippocampus and another calculated for the parahippocampal gyrus. A final set of contrasts examined whether or not there were activity differences that distinguished the hippocampus from the parahippocampal gyrus. Corrections were made using the Greenhouse-Geisser adjustment to the degrees of freedom (df) for all F tests with more than one df in the numerator; both the corrected p-value and the Greenhouse-Geisser epsilon value (έ) are reported for these tests.
3.2.1 Activity Differences in the Hippocampus Support Relational Memory Retrieval
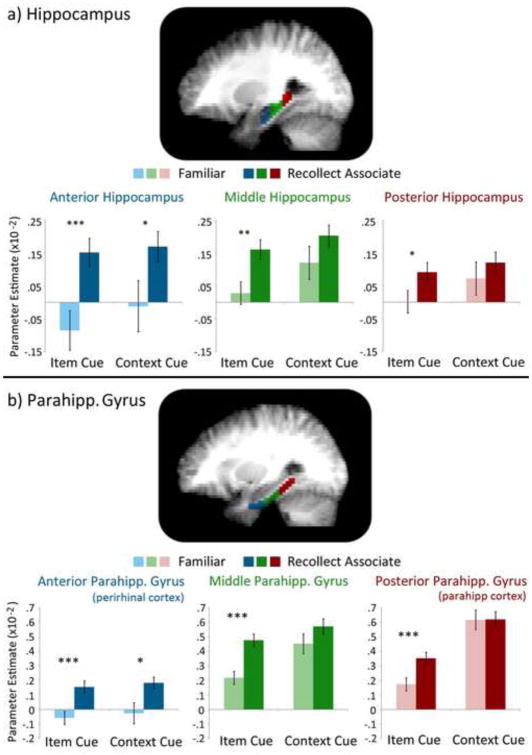
As predicted, activity differences in the hippocampus were sensitive to successful relational memory retrieval (F(1,17)=10.04, p<.01), and these differences did not interact with cue type (Memory x Cue Type interaction: F(1,17)=1.49, p>.05; Memory and Cue Type with ROI and/or Laterality: all p’s>.05). Consistent with previous experiments (e.g., Giovanello, Schnyer, & Verfaellie, 2009), this effect – greater activity for recollect-associate than for familiar trials – was most robust in the anterior hippocampus (Memory x ROI interaction: F(2,34)=15.74, p<.001, έ =.63), especially in the left hemisphere (Memory x ROI x Laterality interaction: F(2,34)=9.16, p<.005, έ =.68). In addition to these memory-based effects, activity differences were generally greater for context cues than for item cues (F(1,17)=6.03, p<.05); interactions of cue type with ROI and/or laterality were not statistically significant (all p’s >.05).
To further evaluate activity differences along the longitudinal axis of the hippocampus, individual ANOVAs were calculated separately for the left and the right anterior, posterior, and middle ROIs. Results showed that activity differences were greater for recollect-associate than familiar trials in five of six regions (F’s(1,17)≥5.07, p’s<.05); this difference was marginal for the left posterior ROI (F(1,17)=3.95, p=.06). In addition, activity differences were greater for context cues than for item cues in the posterior and middle ROIs (F’s(1,17)≥6.60, p’s<.05) – an effect of visually presented materials – though again, this difference was marginal for left posterior hippocampus (F(1,17)=3.54, p=.08). Cue-based differences were not evident in left anterior hippocampus (F(1,17)=1.05, p>.05), but there was a marginal effect in right anterior hippocampus greater for context cues than for item cues (F(1,17)=3.72, p=.07). As in the 3-way ANOVA, memory by cue type interactions were not significant in any of the hippocampal ROIs (F’s(1,17)≤1.41, p’s>.05; see Figure 3a).
Figure 3.
Activity differences to item cues and context cues endorsed with “familiar” responses and with “recollect-associate” responses for the hippocampus and the parahippocampal gyrus. (a) The locations of anterior (shown in blue), middle (shown in green) and posterior (shown in red) hippocampal ROIs are displayed on an averaged T1-weighted image (top). Parameter estimates associated with activity differences for the four conditions of interest are plotted separately for each hippocampal ROI (bottom). (b) The anatomical mask differentiating anterior (perirhinal cortex), middle (transition zone), and posterior (parahippocampal cortex) ROIs of the parahippocampal gyrus is superimposed on an averaged T1-weighted image with color coding as described above (top). Parameter estimates associated with activity differences for the four conditions of interest are plotted separately for each parahippocampal gyrus ROI (bottom). (* = p<.01, ** = p<.005, *** = p<.001)
In sum, activity differences along the length of the hippocampus were sensitive to successful relational memory retrieval whether items were recalled from context cues or contexts were recalled from item cues (i.e., main effect of memory); these content-general relational memory effects were especially robust in the anterior ROIs. Results also indicated that activity differences nearer the posterior extent of the hippocampus were greater for context cues than for item cues regardless of memory outcome (i.e., main effect of cue type), a finding that reflects preferential sensitivity of these regions to visible scene (as compared to object) content.
3.2.2. Differential Recruitment of Anterior and Posterior Parahippocampal Gyrus Associated with Successful Cued Retrieval of Items and Contexts
Having confirmed a role for the hippocampus in relational memory retrieval, our next aim was to examine activity differences in the parahippocampal gyrus. We had predicted that there would be recruitment differences along the longitudinal axis of the parahippocampal gyrus, reflecting differential sensitivity of the perirhinal and parahippocampal cortices to successful cued retrieval of items and contexts, respectively. Consistent with these expectations, and in contrast to results reported for the hippocampus, there was a significant 3-way interaction of cue type, memory, and ROI (F(2,34)=7.26, p<.01, έ =.68). Because the 4-way interaction including laterality was not significant (F(2,34)=2.09, p>.05, έ =.86), additional contrasts reported below that examine activity differences separately for the anterior, posterior and middle ROIs were collapsed across the left and right hemispheres (for a similar analysis approach see Staresina & Davachi, 2011).
Results showed that activity differences in the anterior ROI, which corresponds to perirhinal cortex, were greater for recollect-associate than for familiar trials (F(1,17)=12.37, p<.005), but there were no activity differences sensitive to cue type (F(1,17)=.60, p>.05) and the cue type by memory interaction was not significant (F(1,17)=.97, p>.05). Contrary to expectations, greater activity was evident in the anterior ROI not only when items were successfully recalled from context cues (t(17)=2.75, p=.01), but also when contexts were successfully recalled from item cues (t(17)=3.78, p=.001). In other words, activity differences were generally greater for recollect-associate than for familiar trials.
As in the anterior region, there was a significant main effect of memory in the posterior parahippocampal gyrus (F(1,17)=6.29, p<.05), which corresponds to parahippocampal cortex. However, and in contrast to results reported above, there was also a significant memory by cue type interaction (F(1,17)=13.83, p<.005). As predicted, this finding reflects the fact that activity differences in the posterior parahippocampal gyrus ROI were greater for item cues that elicited successful recall of associated scene contexts than for item cues that were merely endorsed as familiar (t(17)=4.29, p=.001); there was not a statistically significant difference to context cues based on associative retrieval success (t(17)=.05, p>.05). Notably, BOLD signal in the posterior ROI was also generally greater when context cues were presented than when item cues were presented (F(1,17)=82.38, p<.001). This result is consistent with a number of findings showing that parahippocampal cortex shows increased activation during viewing of scenes relative to viewing of objects.
Finally, evaluation of activity differences in the middle ROI revealed a pattern of results similar to those reported for the posterior ROI. Activity differences were greater for context cues than for item cues (F(1,17)=27.37, p<.001) and for trials with cues that elicited successful recollection of associates as compared to those endorsed as familiar (F(1,17)=15.23, p=.001). However, as was the case for posterior parahippocampal gyrus, these main effects were qualified by a statistically reliable interaction (F(1,17)=7.71, p=.01). Consistent with results for the posterior ROI, planned comparisons showed that activity differences were sensitive to successful retrieval of contexts from item cues (t(17)=5.57, p<.001), but here, activity differences were also marginally greater when context cues elicited successful retrieval of associated items (t(17)=1.93, p=.07; see Figure 3b).
3.2.3 Activity Differences Distinguish Middle and Posterior Parahippocampal Gyrus ROIs from the Hippocampus
A final set of contrasts were performed to determine whether or not the hippocampus and the parahippocampal gyrus contribute differently to successful cued retrieval of items and contexts. Parameter estimates for conditions of interest were entered into a repeated measures ANOVA with the factors brain area (hippocampus, parahippocampal gyrus), ROI (anterior, middle, posterior), cue type (item cue, context cue), memory (familiar, recollect-associate), and laterality (left, right). A significant 4-way interaction of area, ROI, cue type and memory (F(2,34)=15.16, p<.001, έ =.63) confirmed that there were differences in recruitment between the hippocampus and the parahippocampal gyrus associated with successful cued retrieval of items and contexts. Because the 5-way interaction including laterality was not significant (F(2,34)=.97, p>.05, έ=.89), additional contrasts that examined activity differences between the hippocampus and the parahippocampal gyrus were collapsed across the left and right hemispheres.
Because we were especially interested in whether or not activity differences along the length of the hippocampus could be distinguished from those in perirhinal cortex, parahippocampal cortex, and the transition zone between these regions, activity differences in all three subregions of the hippocampus were compared with activity differences in all three subregions of the parahippocampal gyrus. In other words, BOLD signal changes in perirhinal cortex (i.e., the anterior parahippocampal gyrus ROI) were compared with those in anterior, middle and posterior hippocampus, respectively; the same approach was used in comparisons with parahippocampal cortex (i.e., posterior parahippocampal gyrus ROI) and the middle parahippocampal gyrus ROI. Altogether, nine 2×2×2 repeated measures ANOVAs, each with the factors area (hippocampus, parahippocampal gyrus), material (item cue, context cue) and memory (familiar, recollect) were calculated to determine what exactly was driving the above interaction.
As might be expected based on the results described above in sections 3.2.1 and 3.2.2, activity differences were well-matched between the hippocampus (anterior, middle and posterior ROIs, respectively) and the most anterior parahippocampal gyrus ROI (i.e., perirhinal cortex). Consistent with findings reported separately for these regions, there were significant main effects of memory in all three of these ANOVAs (F’s(1,17)≥10.44, p≤.005) reflecting the fact that all three regions of the hippocampus and anterior parahippocampal gyrus were more active for recollect-associate trials than for familiar trials whether successful recollection involved recovery of items or contexts. This content-general relational memory effect was more robust for anterior parahippocampal gyrus than for the middle and posterior hippocampal ROIs (Area x Memory interaction: F’s(1,17)≥8.07, p≤.01), but was well-matched with anterior hippocampus (non-significant Area x Memory interaction: F(1,17)=.002, p>.05).
In contrast to results reported for the anterior parahippocampal gyrus, there were significant activity differences that distinguished all three hippocampal ROIs (anterior, middle and posterior) from the posterior and middle parahippocampal gyrus ROIs. Most critically, there were significant 3-way interactions of area, material, and memory for all of six of these ANOVAs (F’s(1,17)≥7.07, p’s≤.05). This pattern of results reflects the fact that the hippocampus was sensitive to successful recollection whether items were retrieved from context cues or contexts were retrieved from item cues, whereas middle and posterior regions of the parahippocampal gyrus were disproportionately sensitive to successful recollection of contexts from item cues.
3.3 Multivariate fMRI Results
Multivariate contrasts examined whether or not MTL subregions were disproportionately sensitive to reinstatement of specific content (i.e., items or contexts) or to successful recovery of bound item-context relationships. As indicated above, the reported contrasts were limited to correlations between 1) item and context cue trials endorsed as familiar (CF-IF), which were expected to have minimal information overlap; 2) familiar item cue trials and context cue trials for which associates were successfully recalled (CR- IF), expected to share object content; 3) familiar context cue trials and item cue trials for which associates were successfully recalled (CF -IR), expected to share scene content; and 4) item and context cue trials for which associates were successfully recalled (CR- IR), expected to share both object and scene information. Differences in the magnitude of pattern similarity estimates based on the above correlations were used to determine whether or not particular MTL regions were disproportionately sensitive to reinstatement of specific content (i.e., objects and/or scenes). As indicated in section 2.6, these contrasts were sensitive to category-level similarity effects, but could not address questions about MTL contributions to reinstatement of specific exemplars.
To determine whether or not such differences were present, a repeated measures ANOVA with the factors pattern similarity estimate (CF-IF, CF -IR, CR- IF, CR- IR), ROI (anterior, middle, or posterior), brain area (hippocampus, parahippocampal gyrus), and laterality (left or right) was calculated. A significant 3-way interaction of pattern similarity estimate, ROI and brain area confirmed that voxel patterns in MTL subregions were differentially sensitive to particular types of recovered content (F(6, 17)=7.97, p<.001, έ =.55). The 4-way interaction was not significant (F(6,17)=.94, p>.05, έ =.59) and laterality was not considered further. Subsequent analyses examined whether there was reliable evidence for context reinstatement (CF -IR > CF-IF), item reinstatement (CR- IF > CF-IF), and/or content general recovery of bound item-context relationships (CR- IR > [CF -IR and CR- IF]) in anterior, middle and posterior regions of the hippocampus and the parahippocampal gyrus, respectively. Importantly, all of the pattern similarity effects reported below (sections 3.3.1 and 3.3.2) were retained even after univariate activation magnitude was covaried out of the reported contrasts, suggesting that pattern similarity analysis provided additional information beyond what was obtained with the traditional univariate approach.
3.3.1 Pattern Similarity in the Hippocampus is Sensitive to Relational Memory
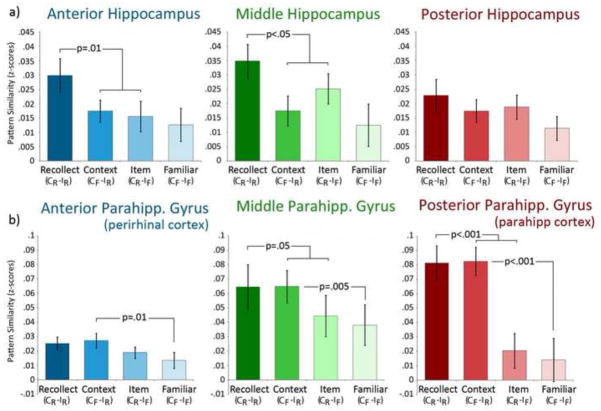
Results from subregions of the hippocampus were generally consistent with univariate outcomes described in section 3.2.1. The magnitude of pattern similarity effects between item and context cue trials was greatest in anterior and middle regions of the hippocampus when the corresponding associates were successfully recovered from memory (CR- IR > [CF -IR and CR- IF]; F’s(1,17)≥5.93, p≤.05). These regions were not sensitive to a specific type of recovered content, as there were no reliable differences in the magnitude of pattern similarity effects between item and context cue trials that shared a specific type of category-level content (i.e., CF -IR, CR- IF) versus those that did not (i.e., CF-IF; F’s(1,17)=2.33 and .32, p’s>.05 for item and context reinstatement, respectively). Results also indicated that differences in the strength of pattern similarity estimates were not statistically reliable in posterior hippocampus (all F’s≤1.19, p’s>.05; see Figure 4a). One point of departure then with respect to the univariate findings was that none of the hippocampal subregions were disproportionately sensitive to scene contexts.
Figure 4.
Pattern similarity associated with successful content-general recovery of relationships (i.e., Recollect), successful recovery of specific content (i.e., Context, Item) and successful recognition in the absence of recollection (i.e., Familiar). Results are shown separately for anterior, middle and posterior subregions of the hippocampus (a) and for anterior, middle and posterior subregions of the parahippocampal gyrus (b).
3.3.2 Pattern Similarity in the Parahippocampal Gyrus is Sensitive to Scene (Contextual) Content
Results from subregions of the parahippocampal gyrus were also generally in line with univariate outcomes (see section 3.2.2). In contrast to the hippocampus, pattern similarity in parahippocampal subregions was disproportionately sensitive to reinstatement of scenes contexts (F’s(1,17)≥6.52, p’s≤.01); in other words anterior, medial, and posterior subregions of the parahippocampal gyrus were sensitive to the type of content that was successfully recovered during cued retrieval. Pattern similarity sensitive to recovery of bound item-context relationships was also statistically reliable in the middle and posterior parahippocampal gyrus ROIs (F’s(1,17)≥4.00, p≤.05), however, evaluation of Figure 4b suggests that this effect was likely driven by sensitivity to scene reinstatement.
In contrast to univariate results, pattern similarity was not sensitive to item reinstatement in any of the ROIs, including the most anterior ROI, which corresponds to perirhinal cortex. Because this result was surprising, we examined whether or not pattern similarity was evident among trials in which novel item cues (seen for the first time at retrieval, and hence absent an associate) were presented. Evaluation of these results revealed that between-trial correlations for novel item cues were not significantly greater than zero (F(1,17)=1.68, p=.21).
4. DISCUSSION
The present study investigated the sensitivity of areas along the longitudinal extent of the hippocampus and parahippocampal gyrus to recollection of items and contexts. Consistent with expectations, hippocampal activation magnitude and pattern similarity measures were sensitive to successful content-general relational memory retrieval; notable differences in recruitment along the length of the hippocampus were evident as well, and are discussed in more detail below. Findings from the parahippocampal cortex were also as expected, revealing disproportionate sensitivity to successful cued recall of scene contexts. In contrast to these outcomes, activity differences in perirhinal cortex were not as predicted. We had expected disproportionate peririhinal recruitment when items were successfully recovered from context cues, but results showed that this region was sensitive to successful recall of both items and contexts. Below, we discuss these findings in the context of the broader literature.
4.1 Activity Differences in the Hippocampus Support Relational Memory Retrieval
As indicated above, activity differences along the length of the hippocampus were sensitive to successful relational memory retrieval whether items were successfully recalled from context cues or contexts were successfully recalled from item cues; complimentary pattern similarity measures reiterated these findings for the anterior and middle hippocampal ROIs. This content-general pattern of recruitment is consistent with results of neuropsychological investigations that have documented impaired performance in hippocampal amnesia whether memory tests required representation of spatial, non-spatial, or temporal relationships (e.g., Hannula, Tranel & Cohen, 2006; Konkel, Warren, Duff et al. 2008) and converges with additional neuropsychological and neuroimaging evidence that has implicated the hippocampus in relational memory binding (cf. Hannula & Greene, 2012; Konkel & Cohen, 2009; Olsen, Moses, Riggs & Ryan, 2012). Notably, the reported relational memory effect was most robust in anterior hippocampus, which is consistent with some findings implicating anterior hippocampus in retrieval tasks that require flexible access to learned relational memory representations (e.g., Giovanello et al., 2009; Preston, Shrager, Dudoukovic, et al., 2002). For instance, Giovanello and colleagues (2009) examined MTL activation during retrieval of word pairs that were presented in reversed order at test (e.g., arrow-surgeon), relative to the corresponding study experience (e.g., surgeon-arrow). Results showed that activity differences in left anterior hippocampus were greater for these intact pairs than for recombined pairs and that the observed activity differences were correlated with relational memory accuracy. Similarly, Preston and colleagues (2002) found that relatively anterior regions of the hippocampus showed increased activity when participants inferred relationships between studied items (i.e., faces) that were not seen together during encoding but shared a common associate (i.e., houses in this experiment; e.g., study: Michael-Brownstone, Gabby-Brownstone; test: Michael-Gabby). We would argue that task demands in the current investigation also required flexible access to learned relationships because participants were required to access associates of studied pictures via free recall and could do this successfully whether they were cued with items or contexts. Consequently, the reported results seem to support a role for the hippocampus, and perhaps especially anterior hippocampus, in flexible content-general relational memory retrieval.
4.2 Posterior Hippocampus and Parahippocampal Cortex are Disproportionately Engaged by Context (Scene) Cues
In addition to the reported sensitivity to successful content-general relational memory retrieval described above, interrogation of hippocampal ROIs revealed univariate activity differences in posterior regions that were sensitive to cue type. These regions were more active when participants were viewing context (i.e., scene) cues than when they were viewing item (i.e., object) cues, a pattern of results that was also evident in posterior regions of the parahippocampal gyrus. Because contexts were pictures of indoor and outdoor scenes, these findings converge with claims that the hippocampus and the parahippocampal cortex are critically involved in scene and/or space-based representation (see Bird & Burgess, 2008; Graham et al., 2010; Lee, Yeung & Barense, 2012 for reviews). They also complement recent results showing that the posterior hippocampus (CA1 and subiculum) exhibits strong functional connectivity with the parahippocampal cortex (Kahn, Andrews-Hanna, Vincent et al., 2008; Libby, et al., 2012).
Our findings join others that have shown parahippocampal and/or posterior hippocampal recruitment during scene processing (e.g., Awipi & Davachi, 2008; Barense, Henson, Lee & Graham, 2009; Duarte, Henson & Graham, 2011; Epstein & Kanwisher, 1998; Lee, Scahill, & Graham, 2008) and complement recent MVPA results that show above chance classification accuracy for scenes, but not for faces, words, or sounds in the posterior hippocampus (Liang et al., 2012). Importantly, findings of common recruitment may be indicative of the privileged connectivity between posterior hippocampus and parahippocampal cortex (e.g., Libby et al., 2012), but need not imply that the nature of the underlying spatial representations supported by these regions is the same. It has been proposed, for example, that the parahippocampal cortex may support inflexible view-point dependent spatial representations, while the hippocampus may support flexible spatial representations that retain more specific information about relationships among scene elements (cf. Hartley, Bird, Chan et al., 2007).
With respect to the hippocampus itself, and to the findings reported here, future studies might evaluate the possibility that anterior and posterior regions of the hippocampus are disproportionately engaged by content-general relational memory representations and spatial relational representations, respectively (cf. Liang et al., 2012). Such findings may help bridge the gap between proposals pointing to a role for the hippocampus in all manner of relational memory (cf. Konkel & Cohen, 2009) and those that link the hippocampus specifically with spatial and/or scene-based processing (e.g., Bird & Burgess, 2008; Graham et al., 2010; Lee, Yeung & Barense, 2012).
4.3 Hippocampus and Parahippocampal Cortex Contribute Differently to Successful Recollection
According to the BIC model, contributions of the hippocampus and the parahippocampal cortex to successful recollection are complementary, but qualitatively distinct. Successful retrieval of item-context bindings is proposed to depend upon the hippocampus whether items are recollected from associated context cues or contexts are recollected from associated item cues. In contrast, parahippocampal cortex is proposed to contribute in a more selective way, supporting representation of contextual information upon successful retrieval of hippocampus-supported bindings. Reported results were consistent with these predictions as direct comparison of these regions revealed a memory-based dissociation. Whereas parahippocampal cortex recruitment was specifically sensitive to successful recollection of contexts from item cues, the hippocampus exhibited content-general relational recruitment. Furthermore, the hippocampus showed robust pattern similarity contingent on successful recovery of item-context relationships, whereas the parahippocampal cortex exhibited disproportionate pattern similarity contingent on recovered content (i.e., scene contexts).
The observed dissociation between the hippocampus and the parahippocampal cortex is notable because these areas are often co-active in fMRI contrasts that tap successful recollection (see Diana et al., 2007 for review). However, most studies involve presentation of a studied word or object and recollection is operationalized in terms of successful retrieval of context (e.g., source information). According to the BIC model, such an approach would be expected to engage both the hippocampus and parahippocampal cortex.
As noted in a recent study from our group (Diana et al., 2010), efforts to document qualitative differences in recollection-based recruitment across these regions, as was done here, can be challenging. In that investigation, activity differences in both the hippocampus and the parahippocampal cortex were correlated with successful source recollection whether source (i.e., color) had been encoded as an item detail (e.g., a red elephant) or as a context with which the item was to be bound (e.g., an elephant at a red stop sign). It was proposed that parahippocampal cortex recruitment in the item detail condition may have been due to recovery of stories that participants had generated in an effort to link items with source during encoding (e.g., that the elephant was red because it had a sunburn). In other words, the stories themselves served as context. Assuming that stories linking items and contexts in the current study were retrieved contemporaneously with recollected content, it may seem surprising that there were no activity differences in parahippocampal cortex that distinguished context cues endorsed as familiar from those that elicited successful recollection of the associated item. One potential explanation for this pattern of results is that our retrieval instructions, which encouraged participants to form a vivid mental image of items or contexts successfully recovered from cues, made it less likely that participants attempted to retrieve associated stories. Alternatively, it may be the case that different types of contextual information are represented in at least partially segregated regions of the parahippocampal gyrus. This possibility is suggested by results reported by Aminoff, Gronau & Bar (2006) who showed that spatial and non-spatial contexts are processed in more posterior and more anterior regions of the parahippocampal cortex, respectively. In our work, activity differences in the posterior parahippocampal ROI may reflect sensitivity to successful recovery scene (or spatial) context and activity differences in the middle ROI, which were associated with successful recollection of both items and contexts, may reflect sensitivity to successful recovery of story-based (non-spatial) context. This proposal is admittedly speculative, as we have cannot determine whether and when stories linking items and contexts were recovered, but could be tested more directly in future experiments.
4.4 Anterior and Posterior Regions of the Parahippocampal Gyrus Contribute Differently to Successful Recollection of Items and Contexts
A primary prediction of the reported work was that there would be qualitative differences in recollection-based recruitment across anterior and posterior regions of the parahippocampal gyrus based on the type of representational content that was recovered during retrieval. Successful recollection of contexts from visible item cues was expected to elicit parahippocampal cortex (i.e., posterior ROI) recruitment and successful recollection of items from context cues was expected to elicit perirhinal cortex (i.e., anterior ROI) recruitment. Had results confirmed these expectations, they would have joined a growing number of studies that have documented dissociations in BOLD sensitivity to items and contexts (especially scenes) in the perirhinal and parahippocampal cortices during active processing of visually presented materials (e.g., Diana, Yonelinas & Ranganath, 2012; Duarte et al., 2011; Lee et al., 2008; Litman et al., 2009; but see Liang et al., 2012) and during encoding (Awipi & Davachi, 2008; Staresina et al., 2011). However, in the current study, there was only partial support for these predictions.
As indicated in section 4.2, and as predicted, the parahippocampal cortex was disproportionately engaged when contexts were recollected from visible item cues; selective content sensitivity was also evident in the multivariate findings. These results are consistent with the proposed role of parahippocampal cortex in context representation (e.g., Diana et al., 2007; Eichenbaum et al., 2007; Davachi, 2006), and complement recent work reported by Staresina and colleagues (2011) who documented selective parahippocampal cortex recruitment during encoding when subsequent recollection of scene-based source information was successful. Complementary to these results, recent findings have shown that parahippocampal cortex recruitment extends beyond scene contexts to non-spatial contextual representations (Diana et al., 2012).
The observed activity differences in parahippocampal cortex are also similar to findings reported from a pair of studies designed to examine influences of context shift on item recognition (Hayes, Nadel & Ryan, 2007; Hayes, Baena, Truong & Cabeza, 2010). Results from both experiments demonstrated that parahippocampal cortex activity was greater for recognized items that had been paired with scene contexts during an incidental encoding phase than for those that had not. Because items were presented on neutral white backgrounds in both of these conditions, the results suggest that activity differences in parahippocampal cortex were indexing automatic retrieval of associated scene contexts. Importantly, results reported here showed that parahippocampal activity is related to successful recollection of scene context even when intentional encoding and retrieval instructions have been administered, and that this enhancement is seen when participants indicate that the scene context had been subjectively recollected.
While results from parahippocampal cortex confirmed our predictions, evaluation of findings from the perirhinal cortex did not. In contrast to expectations, univariate activity differences in perirhinal cortex distinguished recollect-associate trials from familiar trials whether participants were retrieving items or contexts. These results seem similar to recent findings reported by Watson, Wilding, and Graham (2012) who found that activity differences in the perirhinal cortex during encoding predicted successful subsequent recollection of contexts that had been studied with particular items. However, contexts used by Watson and colleagues were characterized by processing that seems to have required evaluation of item-specific details as participants were required to indicate at test whether they had evaluated individual items with respect to whether they had more edges or curves (context 1) or with respect to whether they would be considered common or uncommon (context 2). This conceptualization of context is different from our own and may be more closely aligned with work described in the introduction that has linked activity differences in perirhinal cortex to successful source recollection when recollection involves recovery of item-specific details (e.g., Staresina & Davachi, 2008). If this interpretation is correct, then the results reported by Watson and colleagues may be more easily accommodated by the BIC model than those reported here.
Although the present results are not easy to reconcile with the view that the perirhinal cortex contributes selectively to the process of familiarity-based recognition (Brown & Aggleton, 2001), they are also problematic for views that predict domain-specific representational contributions for this region (e.g., Davachi, 2006; Diana et al., 2007; Graham et al., 2010). In addition, the absence of statistically significant pattern sensitivity to item content in perirhinal cortex as assessed via multivariate analyses was surprising. Several fMRI studies have documented preferential item-based responding in this region (e.g., Awipi & Davachi, 2008; Barense et al., 2010; Diana et al., 2010; Diana et al., in press; Duarte et al., 2011; Lee et al., 2008; Litman et al., 2009; Pihlajamaki, Tanila, Kononen et al., 2004; Staresina et al., 2011; Staresina & Davachi, 2008, 2010), but it is worth mentioning that these investigations reported relative differences in response magnitude between objects and scenes (cf. Litman et al., 2009), and preferential responses to items as compared to other materials have not always been documented in the perirhinal cortex (e.g., Liang et al., 2012; Liang et al., 2012).
In contrast to past work, the current findings imply that items and scene contexts enjoy similar status in the perirhinal cortex, but there may be alternative explanations for the reported outcomes. As can be seen in Figure 1, scenic pictures used in our work were often defined by a prominent item (or items; e.g., the copper kettles in the brewery scene). Consequently, it may have been the case that participants classified a subset of the item cue trials as recollect-associate based on successful retrieval of a particularly notable item embedded in the associated scene context. In other words, this item may have been dominant in the mental image that participants formed when retrieval of contexts from item cues was successful. To address this possibility, a new set of univariate analyses were performed in which we only classified trials as recollect-associate in the item cue condition when the to-be-retrieved scene did not have obvious item content. In a first pass, we narrowed the set of viable scenes from 224 to 96. As a consequence of this reduction, data from 3 participants were dropped because they no longer had a sufficient number of “recollect-associate” trials in the item cue condition. Results were identical to those reported with the full complement of scene stimuli1. An even more conservative attempt (i.e., with just 52 of 224 scenes) yielded the same outcome. These results confirm those reported above, and suggest that perirhinal recruitment elicited by successful retrieval of scene contexts in the item cue condition is not attributable to retrieval of objects embedded in scenes.
Alternatively, it may be the case that the lack of selectivity in perirhinal cortex reflects 1) the tendency for scene contexts to be treated as objects, or 2) the existence of stronger projections from parahippocampal cortex to perirhinal cortex than vice-versa (Suzuki & Amaral, 1994). Regarding the first possibility, it seems that if both scene contexts and items were conceptualized as “objects”, pattern similarity analyses would not have been disproportionately sensitive to scene content, but rather, should have been sensitive to both types of information; this was not the case. Regarding the second possibility, successful cued recall of contexts via parahippocampal cortex may have led to reinstatement of those representations in perirhinal cortex by way of parahippocampal-perirhinal interactions. This possibility cannot be examined in the current investigation, but seems ripe for evaluation because, to our knowledge, the functional role of this asymmetry has not been investigated.
4.5 Concluding Remarks
The reported investigation documented several differences in patterns of MTL recruitment associated with successful cued recall of items from contexts and contexts from items. There were also notable differences in the hippocampus and the parahippocampal gyrus based on cue type. Findings converge with models of MTL function that link hippocampal function with relational memory binding and representation (Cohen & Eichenbaum, 1993) and with more recent models that link parahippocampal cortex function to representation of contextual information (e.g., Davachi, 2006; Diana et al., 2007; Eichenbaum et al., 2007). The findings are less clear with respect to the proposed role for perirhinal cortex in representation of items. Nonetheless, and as has been emphasized in the above discussion, the reported results raise a number of interesting questions and suggest several different avenues for future work.
Supplementary Material
Highlights.
A prediction of the Binding of Items and Contexts (or BIC) model was tested.
Contributions of MTL structures to cued recall of items and contexts were examined.
Parahippocampal cortex supports successful retrieval of contexts from item cues.
Perirhinal cortex supports item and context retrieval from associated cues.
Hippocampus supports flexible content-general relational memory retrieval.
Footnotes
As reported in section 3.2.2, activity differences in the anterior ROI were sensitive to successful relational memory retrieval (F(1,14)=8.32, p=.01), but there was not a significant cue type by memory interaction (F(1,14)=.116, p>.05).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex. 2006;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nature Reviews Neuroscience. 2006;7:358–366. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. Journal Experimental Psychology: Learning, Memory, and Cognition. 2008;34:769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Henson RN, Lee AC, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2010;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Science. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of Cognitive Neuroscience. 2010;22:1808–18. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Adapatation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.07.035. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Research. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan EA. The roles of the perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Quarterly Journal of Experimental Psychology B. 2005;58:202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Reviews of Neuroscience. 2007;30:23–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Greene AP. The hippocampus reevaluated in unconscious learning and memory: at a tipping point? Frontiers in Human Neuroscience. 2012;6:80. doi: 10.3389/fnhum.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. The Journal of Neuroscience. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. The Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effects of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Baena E, Truong TK, Cabeza R. Neural mechanisms of context effects on face recognition: automatic binding and context shift decrements. Journal of Cognitive Neuroscience. 2010;22:2541–2554. doi: 10.1162/jocn.2009.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:129–39. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Memory and the hippocampus: representations and methods. Frontiers in Human Neuroscience. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis – connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience. 2008a;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Ruff DA, Kiani R, Bodurka J, Esteky H, Tanaka K, Bandettini PA. Matching categorical object representations in the inferior temporal cortex of man and monkey. Neuron. 2008b;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgments for faces and scenes. Cerebral Cortex. 2008;18:683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Lee AC, Yeung LK, Barense MD. The hippocampus and visual perception. Frontiers in Human Neuroscience. 2012;6:91. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JC, Conser CJ, Wattenberger AM, Preston AR. Cortical reinstatement during episodic retrieval: Content-specific contributions of the medial temporal lobe. Program No. 194.20, Society for Neuroscience Meeting; 2012. Online. [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Cerebral Cortex. 2012. Content representation in the human medial temporal lobe. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Eckstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. The Journal of Neuroscience. 2012;32:6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19:308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;39:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Frontiers in Human Neuroscience. 2012;6:146. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. European Journal of Neuroscience. 2004;19:1939–49. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudoukovic NM, Gabrielli JD. Hippocampal contribution to novel use of relational information in declarative memory. Hippocampus. 2002;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–89. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. The Journal of Neuroscience. 2010;30:9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. The Journal of Neuroscience. 2011;31:8739–47. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociaiton of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HC, Wilding EL, Graham KS. A role for perirhinal cortex in memory for novel object-context associations. The Journal of Neuroscience. 2012;32:4473–81. doi: 10.1523/JNEUROSCI.5751-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.