Abstract
Background
JC virus (JCV) seropositivity is a risk factor for progressive multifocal leukoencephalopathy (PML) in patients on natalizumab. Accordingly, the JCV serological antibody test is of paramount importance in determining disease risk.
Methods
We tested the accuracy of the JCV serum antibody test by comparing the results of JCV serology to JC viruria and viremia in 67 patients enrolled in a single-center, retrospective cohort study. Bodily fluids (urine and blood) were assessed for JCV DNA by real time quantitative polymerase chain reaction 6 to 47 months earlier (mean 26.1 months) before JCV antibody testing. In 10 individuals, blood and urine samples were obtained on two separate occasions at 6 month intervals.
Results
Forty (59.7%) of the 67 patients were JCV seropositive. Of 27 JCV seronegative patients, 10 (37%) had JC viruria. Urine JCV DNA copy numbers were significantly higher in the seropositive group (mean log copy number: 5.93; range 1.85 – 9.21) than the seronegative group (mean log copy number: 2.41; range 1.85 – 5.43) (p=0.0026). Considering all body fluid test results, 50 (74.6%) of the 67 patients were previously infected with JCV.
Conclusions
The false negative rate of the JCV serology in this study was 37%; therefore, JCV serostatus does not appear to identify all patients infected with JCV. Thus, a negative JCV antibody result should not be conflated with absence of JCV infection. This discordance may be important in understanding JCV biology, risk for PML and PML pathogenesis.
Keywords: JC virus, JC virus antibody, multiple sclerosis, natalizumab, progressive multifocal leukoencephalopathy
Introduction
Progressive multifocal leukoencephalopathy (PML) remains a significant risk in individuals receiving natalizumab. The initial risk estimate based on the seminal three cases1–3 was that approximately 1 in 1000 persons would develop PML after a mean of 17.9 months.4 With greater experience, that risk estimate has been refined. As of January 2, 2013, there have 323 confirmed cases of natalizumab-associated PML among more than 108,000 patients exposed. The risk appears to peak after 24 months and can be stratified not only on the basis of duration of natalizumab therapy, but also with prior exposure to immunosuppressive therapies and whether an individual is JC virus (JCV) antibody positive or negative.5 In persons who are JCV seronegative, the estimated risk of PML is <0.09/1000, whereas, in JCV seropositive patients with no prior immunosuppression, the risk is approximately 0.56/1000 at 1 to 24 months of therapy and 4.6/1000 after >25 months of therapy.5 The risk is substantially higher in JCV seropositive patients with prior immunosuppression and is estimated to be 1.6/1000 at 1 to 24 months of therapy and 11.1/1000 with longer durations of treatment.5
JCV, the etiological agent of PML, is ubiquitous6 and is frequently isolated from the urine of otherwise healthy individuals.7–9 The mechanism of contagion remains uncertain, but the evidence points to PML resulting from the recrudescence of a latent or persistent JCV infection rather than the consequence of newly acquired infection.10 Early serological studies for JCV infection employing hemagglutination inhibition assays11 indicated that approximately 10% of children to age 5 were seropositive and 40–60% adults.12–14 More refined serological studies using immunoassays for JCV show rates varying between 35%15 and 91%16 among adults. In as much as the seminal step for the development of PML is acquisition of JCV infection, a reliable serological test is of paramount importance in determining disease risk.
Methods
As of October 26, 2011, 120 patients had enrolled in the STRATIFY II study of JCV antibody in patients with multiple sclerosis (MS) at the University of Kentucky College of Medicine. The Stratify II study was designed to assess the presence of JCV antibody in the blood and risk of PML while under treatment with natalizumab. The study was supported by BiogenIdec. Sixty seven of these patients had been previously enrolled in a study that examined the effects of MS disease modifying therapies (DMTs) on JCV expression and viral copy numbers in blood and urine.17 This study was supported by EMD Serono. Blood and urine samples had been obtained from patients 6 to 47 months (mean 26.1 months) before their enrollment in the Stratify II study. Blood and/or urine specimens for JCV serology were obtained at a second visit six month later from ten patients. Both studies were approved by the University of Kentucky Institutional Review Board.
The JCV antibody test was performed as part of the STRATIFY II study. Blood was shipped to Covance Laboratories, Indianapolis, Indiana. Tests were performed on serum using a 2-step assay consisting of an enzyme-linked immunosorbent assay and supplemental confirmation test that used soluble JCV virus like particles to pre-absorb antibodies against JCV prior to evaluation.18 The false negative rate has been reported to be 2.5%.18
Quantification of JCV DNA in blood and urine was performed by real time quantitative PCR (qPCR). Total DNA was purified from enriched buffy coat, derived from the equivalent of 1.5 ml whole blood, using the QIAGEN Blood kit and eluted in 200 μl buffer AE. Total DNA was purified from 1 ml urine using the QIAamp Viral RNA Minikit and eluted in 70 μl water. Ten μl of purified DNA was subjected to qPCR using primers and probes specific for JCV detection, i.e., the large tumor (T) antigen as described by MacKenzie et al.19 The JCV probe was labeled at the 3′ end with the quencher fluorochrome, 6-carboxytetramethyl-rhodamine (TAMRA) (PE Applied Biosystems). The 5′ end of the probe was labeled with the reporter fluorochrome, 6-carboxy-fluorescein (6-FAM). Real time-PCR was performed on an ABI Prism 7700 Sequence Detection system (PE Applied Biosystems). Cycling parameters were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Each PCR run contained negative controls, including reaction mixtures without DNA template and several specimens that were known to contain no JCV DNA. Positive controls consisted of a 10-fold dilution series (1 X 10° to 1 X 106 genome equivalents per reaction) of cloned JCV (MAD1 plasmid) sequences kindly provided by E.O. Major (NINDS). Each specimen was analyzed in duplicate. Results were scored positive if either reaction yielded a threshold cycle value (Ct) above the limit of detection for the standards. In the few cases in which amplification was detected in only one of the replicate reactions, the qPCR assay profiles were manually inspected to ensure true logarithmic amplification curves. No such amplification curves have been obtained in hundreds of negative control assays (data not shown). PCR product was reliably detected in the control standards containing 10 copies/μl of purified JCV DNA template allowing for high confidence quantification of samples at or above this concentration (i.e., limit of quantitation was 700 copies/ml of urine). At lower template concentrations (i.e., 1 copy/μl, 10 copies/assay) detection of qPCR product was variable (hit or miss), indicating that the limit of detection (LOD) was 70 copies/ml of urine. Quality control of these assays was performed in conjunction with the help of E. O. Major (NINDS) as previously reported.17 Patients were classified as JCV positive if at any time test results showed positive antibody or viremia.
Results
Sixty seven patients with MS participated; 45 (67%) were women and 22 (33%) were men with ages ranging from 22 to 68 years (mean 43.2 and 48.5, respectively). Blood was available from all subjects at all visits; 10 provided blood at two visits. Sixty two patients provided urine samples; 9 provided urine at two visits. Serology was performed 6 to 47 months (mean 26.1 months) after the first urine and blood samples were obtained. Forty patients were antibody positive and 27 were antibody negative (Figure 1). JCV serostatus was indeterminate on the first step of the antibody assay in 12 subjects; 3 of these were seronegative on the second step of the assay and 9 were seropositive. JCV DNA was detected in the urine from ten (37%) of 27 patients who were antibody negative and in the urine from 23 (66%) of the 35 patients who were antibody positive and provided urine. No gender differences were detected. Viral copy numbers in urine ranged from 70 copies/ml to 1.62 × 109 copies/ml. Only one blood sample was positive for JCV DNA (at the limit of detection and less than the level of quantitation) Of those sera which were “indeterminate” on the first step; one of the 3 JCV seronegatives had detectable JCV in the urine as did 4 of the seropositives.
Figure 1.
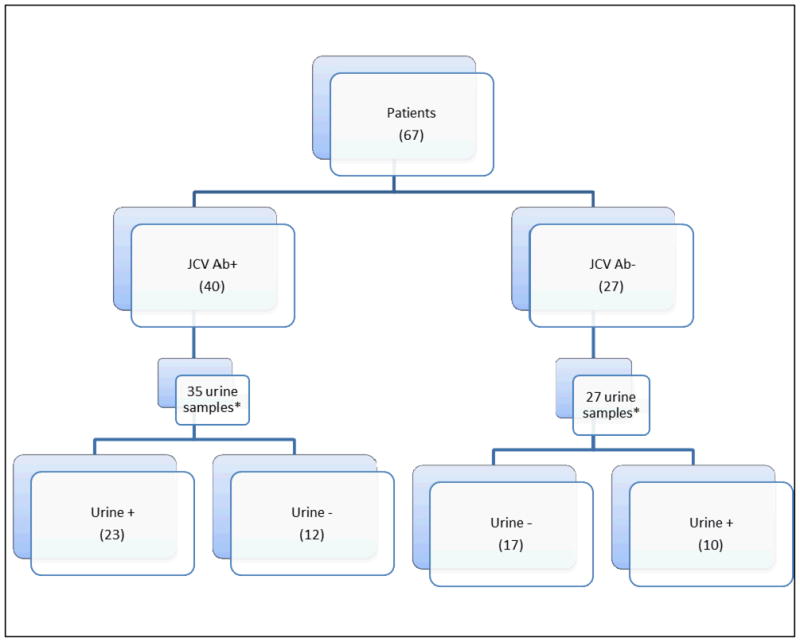
Comparison of JC virus antibody status in serum versus JC virus DNA status in urine.
* - Not all patients provided urine at study visit.
Thirty three (53.2%) of the 62 patients who provided urine were JCV viruric on at least one occasion. An additional patient, who did not provide urine at either visit, was JC viremic 39 months before JCV seropositivity was demonstrated. The analysis of these 67 patients is shown in Figure 1.
Among the 10 JC virus seronegative patients who were JC viruric, viral copy numbers were significantly lower than in those who were antibody positive. In these 10 patients, antibody testing was performed 17 to 38 months (mean 29.7) after the first urine and blood samples were obtained. When detected, JCV DNA copy numbers in this group ranged from 70 to 2.71×105 copies/ml (mean 2.00 ×104; median 70, i.e. LOD). Seven (70%) of 10 patients with JCV viruria had less than 700 copies/ml at one or both visits. One patient had 1,150 copies/ml urine at the first visit, but no JCV DNA was detected at the second. Only two patients who were JCV seronegative had urine JCV copy numbers exceeding 2000 copies/ml (7,600 and 271,000). In two JCV seronegative patients, urine specimens were positive at one visit, but not the other. JCV DNA was detected in the urine of 23 (65.7%) of the 35 seropositive patients who provided urine and was detected in the blood of one of the five patients who did not provide urine. JCV antibody testing was performed 7 to 43 months (mean 26.6 months) after detection of JCV viruria. In the JCV antibody positive group with JCV viruria, viral copies per ml ranged between 70 (LOD) and 1.62 ×109 (mean 1.44 ×108; median 2.36 ×106). Six (26%) of 23 seropositive patients had <700 copies/ml in urine at one or both visits. In one of these patients, JCV DNA was not detected at the first visit but 1.9 X 107 copies/ml were detected at the second.
JCV DNA was not detected in the urine of twelve (34.3%) of the 35 seropositive patients who provided urine. The interval between collection of blood and urine and the performance of the JCV antibody test was 7 to 47 months (mean 27.4 months). Seventeen (27.4%) of the 62 patients who provided urine were JCV seronegative and JCV DNA urine negative. The time from specimen collection to the performance of the JCV antibody test was 6 to 33 months (mean18.8 months). Therefore, only 27.4% of the study population lacked evidence, either by JCV antibody or PCR, of prior JCV infection. The mean log10 value of viral copy numbers/patient/visit in urine in the JCV antibody negative group was 2.66 and the median was 2.24 (Figure 2). In contrast, the mean log10 value of viral copy numbers in urine of the JCV antibody positive group was 5.968 and the median 6.99 (p=0.0026 by Wilcoxon rank sum statistic).
Figure 2.
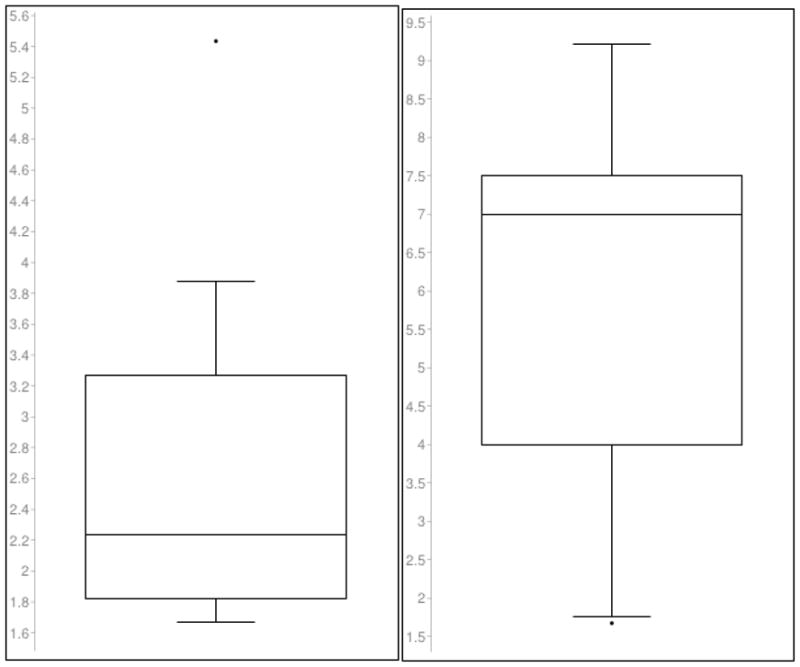
Log10 values of urine virus in the JC virus antibody negative and positive groups
| JCV antibody negative population (n=10) | JCV antibody positive population (n=23) |
| Mean log10 value = 2.657 | Mean log10 value = 5.968 |
| Median log10 value = 2.2385(line) | Median log10 value = 6.991 (line) |
Discussion
The frequency of JCV exposure in the population determined by antibody studies has been reported between 33% and 91%.18 These differences have been largely ascribed to differences in assay methods, although other factors may be important. We tested the sensitivity of a recently described JCV antibody test18 against JCV DNA PCR results from urine and blood obtained months earlier. Our findings indicate that 74% of the studied patients had evidence of prior JCV infection by serostatus or viral detection in blood or urine. The sensitivity of the JCV antibody assay in this study was 63%. Urine copy numbers of JCV in the falsely seronegative group were significantly lower than in the seropositive group. Seventy percent had <700 viral copies/ml in comparison to 22% of the JCV seropositive group. The lower viral copy numbers are consistent with a lesser antigenic stimulus which may account for the failure to detect antibody in these patients. The sensitivity of our PCR assay is similar to that of others18, 20 and the specificity of the assay has been previously demonstrated with controls from Dr. E.O. Major’s laboratory.21, 22
Humoral responses to JCV during treatment with natalizumab appear to remain stable.23 JCV antibody is virtually always detected in association with PML24, 25 Intrathecal synthesis of antibody to JCV has been suggested as a diagnostic measure for PML24, 26 and rising intrathecal antibody titers during the course of PML have been described.27 Similarly, rising serum JCV antibody titers have been observed in individuals developing PML in association with human immunodeficiency virus (HIV) infection; only 58% of HIV-associated PML were antibody positive 1–1.5 years before the diagnosis, but 96% were seropositive in the 6 months following diagnosis.28
Our study found a significantly higher frequency of false negatives for the JCV antibody test than the previously reported 2.5% with an upper confidence limit of 4.4%;18 however, a second generation JCV antibody test appears to improve detection of low titers of the JCV antibody.29 The detection of JCV antibodies by this second generation antibody assay in 29.1% of a French cohort who had been seronegative one year earlier using the first generation assay30 used in our study suggests that it is substantially more sensitive and is concordant with our observation. Although the numbers are small, 25% (2 of 8 subjects) with detectable JCV in their urine were JCV antibody negative in a study designed to address the effects of corticosteroids on JCV-T cell response.31 We also found a relationship between the false negative serologies and viral copy numbers in the urine, whereas, the previous study did not find a correlation between antibody titers as measured by optical density and urine JCV copy numbers.18 JCV presence in urine is dynamic and a negative urine PCR for JCV does not preclude infection. As an example of this, only 28.6% of study participants (2/7) were persistent JCV urine shedders on longitudinally collected urine specimens.
Despite the number of false negative in this study, the available evidence suggests that the serum antibody test is a useful method of assessing PML risk. At the time of the initial description of the methodology,18 17 samples collected before the development of natalizumab associated PML were antibody positive. Similarly, all 9 patients with natalizumab-associated PML in a study of JCV antibody in a German MS cohort were JCV seropositive in blood specimens that had been collected 2.0 to 37.6 months earlier.32 Using different methodology to detect JCV antibodies, Ryschkewitsch and colleagues found that 3 of 25 PML patients had absent or borderline titers;33 however, all were being plasma exchanged at the time of blood collection perhaps lowering antibody titers and results of the 2-step ELISA on each was positive.34
As of August 1, 2012, 85 individuals were tested for JC virus antibody by the 2-step ELISA at least 6 months before the time of natalizumab-associated PML diagnosis.5 Eighty three (98%) of the 85 were JCV seropositive. One patient was JCV seronegative at 9 months and seropositive at 6.5 months prior to PML diagnosis.5 Another patient was JCV seronegative 9 months prior to PML diagnosis and not retested.5 A third patient, not included among the 85, was seronegative 15 months before diagnosis, but positive when retested 2 months before diagnosis.5, 35 There are at least three possible explanations for this event, namely, that the initial test was insufficiently sensitive and the antibody was simply not detected, antibodies developed over time during the administration of natalizumab, or the patient was infected by JCV proximate to the development of PML. While the latter is possible, it is not particularly likely given the hurdles that must be overcome for the disease to occur.36 That the shortest interval yet reported from time of natalizumab initiation to the development of PML is 8 months and the highest incidence for PML is not reached until beyond 24 months of treatment duration5 suggests that the development of PML with natalizumab is a dynamic process involving latent or persistent infection.
As in our study, Major and colleagues have detected both JC viremia and cell mediated immune responses to JCV in seronegative patients, but with a different antibody assay (E.O. Major, personal communication, April 24, 2012). The negative antibody studies in the face of JCV detection in the urine 6 to 38 months before the performance of the antibody test is revealing about JCV biology. This observation and the apparent strikingly reliable predictive nature of the JCV antibody test for PML suggest that in some, if not all individuals, JCV is actively replicating and disseminating from sites of latency or low grade persistent infection prior to the development of PML. In some individuals, antibody titers prior to this event are below the limits of detection; however, with active viral replication and dissemination, humoral immunity is amplified as evidenced by an increase in antibody titers.
In the seminal study of the 2-step ELISA for JCV antibodies, approximately 3% of seropositive patients became seronegative over 5 years 18 and in the German study 4.7%.32 This variance was attributed to fluctuations of the antibody at the threshold of detection.32 Discordance between antibody positivity and viral detection is not unique to JCV37–39 and largely reflects the sensitivity of the test, although other factors, such as, timing of infection, are important.
Our study has several limitations including small sample size, missing samples, the possibility of seroreversion or seroconversion from recently acquired infection in the months between JCV PCR and antibody determination, and the episodic nature of JCV urine expression. Also, our assays were performed using urine and buffy coats, thus by not assessing sera or plasma, the incidence of JCV viremia may have been underestimated as these sources may be JCV PCR positive with undetectable JCV in the buffy coat.40 Nevertheless, the results of our study strongly support the need for repeat testing of MS and other patients on natalizumab whose JCV antibody is initially negative. Performing the test at 6 month intervals in such persons as has recently been recommended seems prudent; however, clinical studies may prove that shorter time intervals are needed. Importantly, while JCV antibody positivity appears to be an excellent test for PML risk stratification, it is not an efficient means of determining prior JCV infection. This observation suggests that in falsely negative JCV seronegative person, viral replication during the weeks or months leading up to PML provides a sufficient stimulus for antibody production to surpass the threshold of antibody detection. The observation of moderate to high or rising JCV antibody titers in 22 of 25 patients with PML33 is supportive of this hypothesis.
Acknowledgments
Study Support: Supported by grants to Dr. Berger from EMD Serono and BiogenIdec and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000117).
Footnotes
Financial Disclosure:
Dr. Berger serves on PML Adjudication Committees for Millennium Pharmaceuticals, Inc and Amgen; has received speaker honoraria from Bayer Schering Pharma and BiogenIdec; serves on the editorial boards of the Journal of Neurovirology, ISRN Education, Neuroscience, World Journal of Rheumatology, and MS and Other Related Disorders; has served as a consultant to Bayer Schering Pharma, BiogenIdec, Eisai, Genentech, Millennium Pharmaceuticals, Inc., Novartis, Pfizer, and Sanofi; and receives research support from the PML Consortium.
Dr. Houff has no disclosures.
Dr. Gurwell has no disclosures.
Ms. Vega has no disclosures.
Dr. Miller is on the editorial board of Oral Surgery Oral Medicine Oral Pathology and Oral Radiology. He has received grants from the National Institutes of Health and BeechTree Labs.
Dr. Danaher has no disclosures.
References
- 1.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 2.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 3.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–8. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 4.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–33. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed August 20, 2012];TYSABRI Safety Update. 2012 at https://medinfo.biogenidec.com/medinfo/secure/pmlresource.do?resource=TYSABRIPMLSafetyUpdate.
- 6.Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol. 2000;66:238–45. doi: 10.1128/aem.66.1.238-245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JR, Miller CS, Mootoor Y, Avdiushko SA, Kryscio RJ, Zhu H. JC virus detection in bodily fluids: Clues to transmission. Clin Infect Dis. 2006 doi: 10.1086/504947. [DOI] [PubMed] [Google Scholar]
- 8.Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–60. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 9.Whiley DM, Arden KE, Mackay IM, Syrmis MW, Sloots TP. Simultaneous detection and differentiation of human polyomaviruses JC and BK by a rapid and sensitive PCR-ELAHA assay and a survey of the JCV subtypes within an Australian population. J Med Virol. 2004;72:467–72. doi: 10.1002/jmv.20005. [DOI] [PubMed] [Google Scholar]
- 10.Berger JR, Khalili K. The pathogenesis of progressive multifocal leukoencephalopathy. Discov Med. 2012;12:495–503. [PubMed] [Google Scholar]
- 11.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973;127:467–70. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26:1057–64. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker D, Padgett B. The epidemiology of human polyomaviruses. In: Sever J, Madden D, editors. Polyomaviruses and human neurological disease. New York: Alan R. Liss, Inc; 1983. pp. 99–106. [Google Scholar]
- 14.Walker D, Padgett B. Progressive multifocal leukoencephalopathy. In: Fraenkel-Conrat H, Wagner R, editors. Comprehensive Virology. New York: Plenum; 1983. pp. 161–93. [Google Scholar]
- 15.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 16.Matos A, Duque V, Beato S, da Silva JP, Major E, Melico-Silvestre A. Characterization of JC human polyomavirus infection in a Portuguese population. J Med Virol. 2010;82:494–504. doi: 10.1002/jmv.21710. [DOI] [PubMed] [Google Scholar]
- 17.Miller CS, Houff SA, Hopper J, et al. Disease modifying drugs for multiple sclerosis and JC virus expression. J Neurovirol. 2012;18:411–5. doi: 10.1007/s13365-012-0107-0. [DOI] [PubMed] [Google Scholar]
- 18.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie J, Wilson KS, Perry J, Gallagher A, Jarrett RF. Association between simian virus 40 DNA and lymphoma in the United kingdom. J Natl Cancer Inst. 2003;95:1001–3. doi: 10.1093/jnci/95.13.1001. [DOI] [PubMed] [Google Scholar]
- 20.Bozic C, Richman S, Plavina T, et al. Anti-John Cunningham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol. 2011;70:742–50. doi: 10.1002/ana.22606. [DOI] [PubMed] [Google Scholar]
- 21.Arthur RR, Dagostin S, Shah KV. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989;27:1174–9. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–21. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Jilek S, Jaquiery E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol. 2010;9:264–72. doi: 10.1016/S1474-4422(10)70006-5. [DOI] [PubMed] [Google Scholar]
- 24.Knowles WA, Luxton RW, Hand JF, Gardner SD, Brown DW. The JC virus antibody response in serum and cerebrospinal fluid in progressive multifocal leucoencephalopathy. Clin Diagn Virol. 1995;4:183–94. doi: 10.1016/0928-0197(95)00012-w. [DOI] [PubMed] [Google Scholar]
- 25.Padgett BL, Walker DL. Virologic and serologic studies of progressive multifocal leukoencephalopathy. Prog Clin Biol Res. 1983;105:107–17. [PubMed] [Google Scholar]
- 26.Weber T, Weber F, Petry H, Luke W. Immune response in progressive multifocal leukoencephalopathy: an overview. J Neurovirol. 2001;7:311–7. doi: 10.1080/13550280152537166. [DOI] [PubMed] [Google Scholar]
- 27.Guillaume B, Sindic CJ, Weber T. Progressive multifocal leukoencephalopathy: simultaneous detection of JCV DNA and anti-JCV antibodies in the cerebrospinal fluid. Eur J Neurol. 2000;7:101–6. doi: 10.1046/j.1468-1331.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 28.Viscidi RP, Khanna N, Tan CS, et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin Infect Dis. 2011;53:711–5. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P, Plavina T, Castro A, et al. An enhanced ELISA for detection of anti-JC virus antibodies in human serum and plasma to support porgressive multifocal leukoencephalopathy risk stratification. 22nd Annual Meeting of the European Neurological Society; Prague, Czech Republic. 2012. p. P467. [Google Scholar]
- 30.Etxeberria A, Outteryck O, Ongagna JC, et al. Annual rate of JCV seroconversion in a French cohort of MS patients under natalizumab. European Committee for Treatment and Research in Multiple Sclerosis; Lyon, France. 2012. p. P996. [Google Scholar]
- 31.Antoniol C, Jilek S, Schluep M, et al. Impairment of JCV-specific T-cell response by corticotherapy: effect on PML-IRIS management? Neurology. 2012;79:2258–64. doi: 10.1212/WNL.0b013e3182768983. [DOI] [PubMed] [Google Scholar]
- 32.Trampe AK, Hemmelmann C, Stroet A, et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology. 2012;78:1736–42. doi: 10.1212/WNL.0b013e3182583022. [DOI] [PubMed] [Google Scholar]
- 33.Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2010;68:384–91. doi: 10.1002/ana.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goelz SE, Gorelik L, Subramanyam M. Assay design and sample collection can affect anti-John Cunningham virus antibody detection. Ann Neurol. 2011;69:429–30. doi: 10.1002/ana.22304. author reply 30–1. [DOI] [PubMed] [Google Scholar]
- 35.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Eng J Med. 2012;366:1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 36.Berger JR, Khalili K. The pathogenesis of progressive multifocal leukoencephalopathy. Discov Med. 2011;12:495–503. [PubMed] [Google Scholar]
- 37.Hasan KN, Rumi MA, Hasanat MA, et al. Chronic carriers of hepatitis B virus in Bangladesh: a comparative analysis of HBV-DNA, HBeAg/anti-HBe, and liver function tests. Southeast Asian J Trop Med Public Health. 2002;33:110–7. [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Miller LG, Daar ES. Diagnostic discordance for hepatitis C virus infection in hemodialysis patients. Am J Kidney Dis. 2005;46:290–300. doi: 10.1053/j.ajkd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–7. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbeeck J, Van Assche G, Ryding J, et al. JC viral loads in patients with Crohn’s disease treated with immunosuppression: can we screen for elevated risk of progressive multifocal leukoencephalopathy? Gut. 2008;57:1393–7. doi: 10.1136/gut.2007.145698. [DOI] [PubMed] [Google Scholar]


