Abstract
Fanconi anemia patients suffer from progressive bone marrow failure. An overactive p53 response to DNA damage contributes to the progressive elimination of Fanconi anemia hematopoietic stem and progenitor cells (HSPC), and hence presents a potential target for therapeutic intervention. To investigate whether the cell cycle regulatory protein p21 is the primary mediator of the p53-dependent stem cell loss, p21/Fancd2 double-knockout mice were generated. Surprisingly double mutant mice displayed even more severe loss of HSPCs than Fancd2−/− single mutants. p21 deletion did not rescue the abnormal cell cycle profile and had no impact on the long-term repopulating potential of Fancd2−/− bone marrow cells. Collectively, our data indicate that p21 has an indispensable role in maintaining a normal HSPC pool and suggest that other p53-targeted factors, not p21, mediate the progressive elimination of HSPC in Fanconi anemia.
Keywords: Bone Marrow Failure, Fanconi Anemia, Hematopoietic Stem Cell, p21
Introduction
Fanconi anemia (FA) is a bone marrow failure disorder caused by the disruption of FA-BRCA network, which consists of at least 15 FA genes (including FANCD2 and FANCC) and FA-associated genes (Bagby and Alter, 2006; Kim and D’Andrea, 2012).
Bone marrow failure is the primary cause of early mortality for FA patients (Kutler et al., 2003). It has been reported that a subset of FA patients with lower levels of CHK1 and p53 expression and abrogation of the G2 cell cycle checkpoint display much milder bone marrow deficiency (Ceccaldi et al., 2011). Further mechanistic studies established that an overactive p53 response to cellular stress and DNA damage drives the progressive elimination of hematopoietic stem and progenitor cells (HSPC) in FA patients, suggesting p53 down-regulation as a potential target for FA drug design (Ceccaldi et al., 2012).
It is also known that loss of p53 promotes carcinogenesis in both FA mouse models and human FA patients (Ceccaldi et al., 2011; Houghtaling et al., 2005; Rajagopalan et al., 2009). Given the detrimental tumorigenic effects of p53 loss, it is desirable to shut down only those p53-target genes specifically involved in HSPC elimination in FA. Further understanding the molecular targets of p53-mediated HSPC elimination could guide the future design of therapeutic regimens exploiting this approach. p21, encoded by the Cdkn1a gene, is a key p53-target gene and the main factor responsible for p53-mediated cell cycle arrest and apoptosis (Abbas and Dutta, 2009). p21 could even be the sole mediator of the overactive p53 response to DNA damage in FA HSPC, pointing to p21 inhibition as a way to prevent the progressive HSPC loss in FA (Murray et al., 2010; Sax et al., 2002). Interestingly, p21 deletion is already known to rescue stem cell self-renewal from mice suffering from DNA damage provoked by dysfunctional telomerase (Choudhury et al., 2007). On the other hand, it has also been reported that p21 has an important role in the regulation of FA-BRCA pathway activation (Rego et al., 2012). Therefore, the precise outcome of p21 deletion in FA patients warrants further investigation.
Fancd2−/− mice on the 129S4 genetic background recapitulate major FA patient phenotypes, displaying tumor susceptibility and hematopoietic defects (Houghtaling et al., 2003; Zhang et al., 2010). Here we characterized p21 and Fancd2 double-knockout mice to understand whether p21 is involved in the FA pathway and whether the rescue effects of p53 deletion on HSC maintenance are p21-related.
Methods
Mice
Fancd2 and ROSA26 transgenic mice were maintained on the 129S4 background. Cdkn1a deficient mice were originally ordered from Jackson Laboratories (Bar Harbor, Maine) and backcrossed to the 129S4 background for more than 10 generations (Brugarolas et al., 1995). Heterozygotes were crossed inter se to generate mutant mice and littermate controls. All mice used were 4 months old. All animals were treated in accordance with the guidelines of the Institutional Animals Care and Use Committee.
Complete blood count (CBC)
Comprehensive CBC tests were measured by IDEXX Laboratories to monitor hematological parameters.
Histopathology
Testis sections were fixed in 10% phosphate-buffered formalin and stained with hematoxylin and eosin (H&E) as reported previously (Zhang et al., 2008).
Flow cytometry
Bone marrow cells were isolated from Fancd2−/−/Cdkn1a−/− mice and littermate controls, immunostained, and analyzed by flow cytometry, as previously described (Zhang et al., 2010).
In vivo competitive repopulation assay
Competitive repopulation assays were done as previously described (Zhang et al., 2010). Briefly, donor bone marrow cells were isolated from Fancd2−/−/Cdkn1a−/− mice (or littermate controls) and ROSA26Tg/O mice. Test donor cells were mixed with ROSA26Tg/O bone marrow cells at a 1:1 ratio and then transplanted into lethally irradiated Fancc−/− recipient mice. Quantitative real-time PCR (qPCR) analysis of the peripheral blood was performed 6 months after transplantation.
Statistical analysis
Two-tailed, unpaired student’s t-tests were performed to calculate P values using Prism 4.0 software (GraphPad Software Inc). A P value less than 0.05 was considered significant.
Results and Discussion
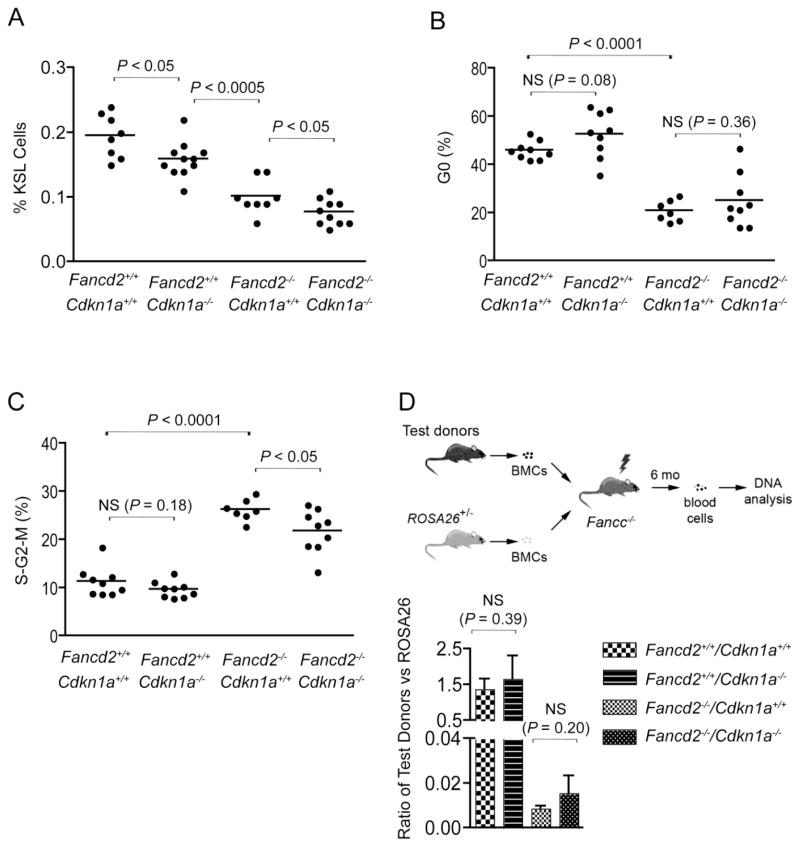
We reported previously that Fancd2−/− mice have readily detectable hematopoietic defects, including fewer cKit+Sca1+Lin− (KSL) hematopoietic stem and progenitor cells, compromised HSC repopulating capacity, and abnormal cell cycle status of KSL cells (loss of quiescence and increased cycling) (Zhang et al., 2010). To determine whether lack of p21 has an impact on any of these parameters in Fancd2−/− mice, we characterized hematopoietic phenotypes of Fancd2−/−/Cdkn1a−/− mice first by measuring the size of their KSL pool. As compared to wild-type controls, both Fancd2−/− mice and Cdkn1a−/− mice suffered a significant reduction in the size of the KSL HSPC pool (Figure 1A). Fancd2−/−/Cdkn1a−/− mice had the smallest HSPC population among the different genotypes. This observation indicates that p21 and Fancd2 deletion independently cause loss of hematopoietic stem and progenitor cells, suggesting that both Fancd2 and p21 are important for maintaining the normal size of HSPC pool.
Figure 1. p21 deletion reduced KSL pool but did not change stem cell function.
(A) Quantification of KSL hematopoietic stem and progenitor cell frequencies in the bone marrow of Fancd2−/−/Cdkn1a−/− mice and controls. The percentage of the KSL gate refers to the proportion of KSL cells in the whole nucleated bone marrow. n=8 for Fancd2+/+/Cdkn1+/+ mice, n=11 for Fancd2+/+/Cdkn1a−/− mice, n=8 for Fancd2−/− /Cdkn1+/+ mice, and n=10 for Fancd2−/−/Cdkn1a−/− mice. (B, C) Cell cycle profiles of bone marrow KSL cells. n=9 for Fancd2+/+/Cdkn1+/+ mice, n=9 for Fancd2+/+/Cdkn1a−/− mice, n=7 for Fancd2−/−/Cdkn1+/+ mice, and n=9 for Fancd2−/−/Cdkn1a−/− mice. NS denotes not significant. (D) Upper panel: Strategy used in the competitive repopulation experiment. BMCs denotes bone marrow cells. Lower panel: In vivo competitive repopulation of Fancd2−/−/Cdkn1a−/− (or control) donor and ROSA26Tg/O bone marrow cells. qPCR analyses were performed to evaluate donor contribution to the peripheral blood cells from each donor. Three independent qPCR analyses were performed for each sample and results from 5 animals were pooled together for each experimental group. Data are presented as mean ± SD. NS denotes not significant.
The ability to maintain quiescence is critical for HSC function (Orford and Scadden, 2008). Considering that p21 is an important cell cycle regulator, we next analyzed the cell cycle profiles of Fancd2−/−/Cdkn1a−/− KSL cells. As shown in Figure 1B, 1C & Figure 2, p21 deficiency did not significantly correct the abnormal cell cycle status of Fancd2−/− KSL cells.
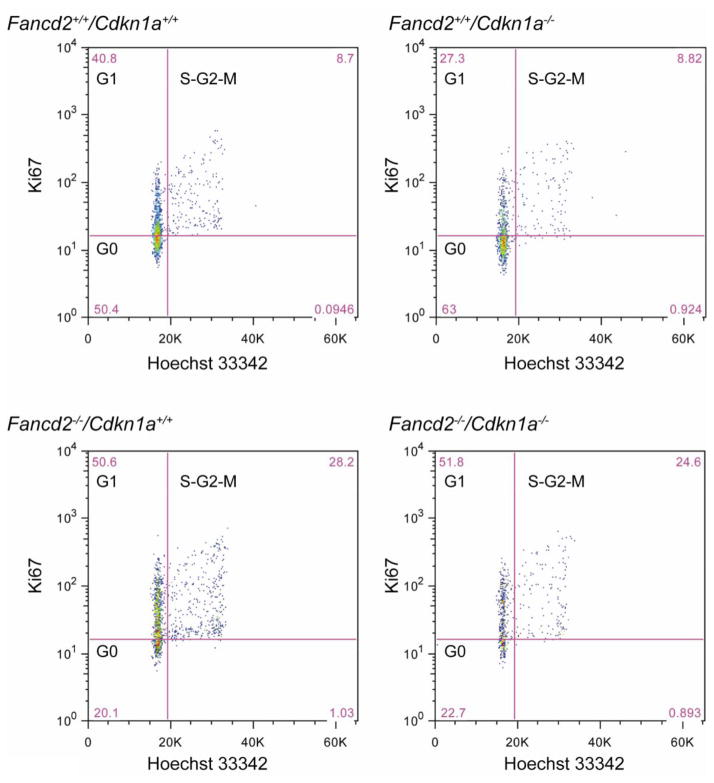
Figure 2. Representative flow cytometry profile of cell cycle analysis.
p21 deletion did not change the abnormal cell cycle status of Fancd2−/− mice. Hoechst 33342 and FITC-conjugated anti-mouse Ki67 (BD Sciences) were used in combination to distinguish cells in G0, G1, and S-G2-M phases of the cell cycle.
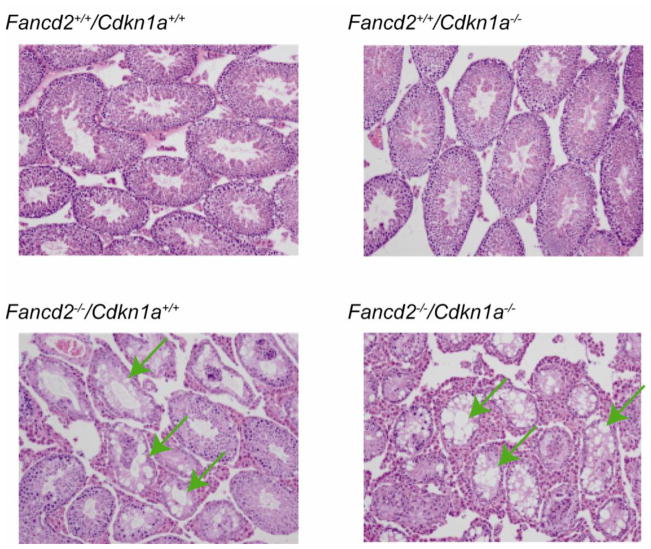
To further assess how Fancd2−/−/Cdkn1a−/− HSCs perform functionally in a stressful environment, we next performed in vivo competitive repopulation assays, in which test donor bone marrow cells competed with wild-type bone marrow bearing the ROSA26Tg/O marker. We transplanted equal numbers of nucleated whole bone marrow cells into each lethally irradiated recipient and analyzed the recipient mice 6 months after transplantation. Fancd2−/−/Cdkn1a−/− bone marrow cells performed similarly to their single mutant Fancd2−/− counterparts (Figure 1D). This is in stark contrast to the effect of p53 deletion, which significantly enhanced hematopoietic stem cell activity in Fancd2−/− mice (Ceccaldi et al., 2012). Since Fancd2−/− mice show germ cell loss (Houghtaling et al., 2003), we further examined whether p21 deletion would have any impact on this phenotype. As shown in Figure 3, testes from both Fancd2−/− mice and Fancd2−/−/Cdkn1a−/− mice displayed tubules with vacuolated Sertoli cell cytoplasm and decreased numbers of spermatocytes and spermatids, once again demonstrating a lack of substantial rescue by p21 loss.
Figure 3. Representative pictures after H&E staining of testis sections.
p21 deletion did not correct the germ-cell-loss phenotype of Fancd2−/− mice. The mice used were 4 months old. The arrows indicate tubules with vacuolated Sertoli cell cytoplasm. Original magnification x100.
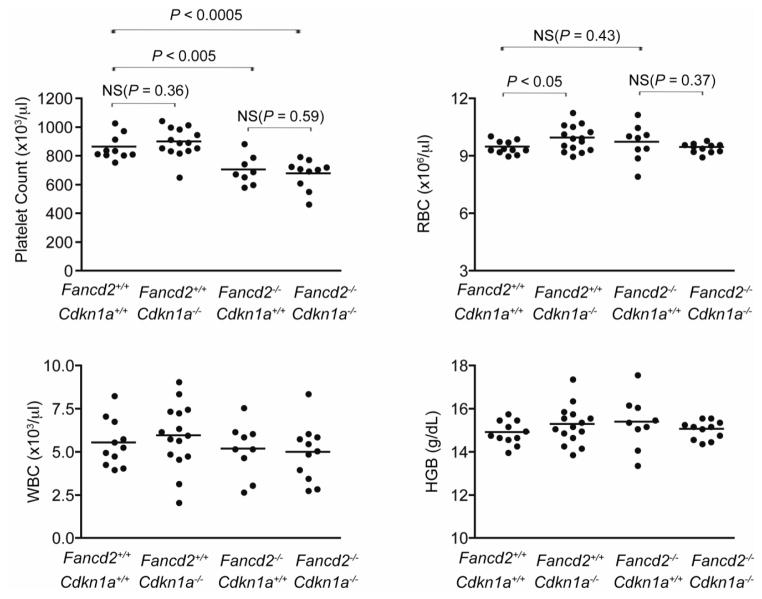
We also performed complete blood counts from 4-month old Fancd2−/−/Cdkn1a−/− mice and controls. Hematologic parameters of Cdkn1a−/− mice were slightly higher than those of wild-type mice (Figure 4). However, the changes were insignificant. Overall Fancd2−/−/Cdkn1a−/− double mutant mice showed the worst hematopoietic parameters such as lowest platelet counts among all four genotype groups tested.
Figure 4. p21 deletion did not affect hematologic parameters of peripheral blood.
Comprehensive CBC tests of peripheral blood from Fancd2−/−/Cdkn1a−/− mice and controls. n=11 for Fancd2+/+/Cdkn1+/+ mice, n=15 for Fancd2+/+/Cdkn1a−/− mice, n=9 for Fancd2−/−/Cdkn1+/+ mice, and n=11 for Fancd2−/−/Cdkn1a−/− mice. WBC denotes white blood cells; RBC, red blood cells; HGB, hemoglobin; and NS, not significant. Unless specified otherwise on the figure, P values were not significant.
In summary, we observed that p21 deficiency did not rescue any of the major hematopoietic phenotypes of Fancd2−/− mice, such as abnormal cell cycle status of HSPC and long-term repopulating potential. To the contrary, p21 deletion caused a further reduction in the HSPC pool. These results are in clear contrast to the obvious rescue effects from p53 deletion in mice on the same genetic background (Ceccaldi et al., 2012) and argue against the possibility that p21 functions as a primary mediator of p53-driven HSPC elimination in FA. Clearly other factors regulated by p53 play a more important role. It is noteworthy that p21 depletion in FA patient HSPCs is able to rescue their clonogenic growth defects (Ceccaldi et al., 2012). This discrepancy to our in vivo results may be partly due to the differences in the p53-mediated responses between human and mice. It is also possible that an acute knockdown of p21 may provide temporary benefits to hematopoiesis but that this is not sustained over time.
It has been under debate whether p21 is involved in normal HSC function (Tesio and Trumpp, 2011). In this study, we also uncovered an indispensable role of p21 in maintaining the normal size of the HSPC pool in 129S4 mice. This novel role was not seen in previous studies using p21 murine models on other genetic backgrounds (Cheng et al., 2000; van Os et al., 2007). Further investigation is needed to reconcile these discrepancies.
Highlights.
Hyperactive p53 activity in Fanconi anemia stem cells is not mediated by p21.
p21 is important for maintaining the normal size of HSPC pool.
Fancd2 and p21 work independently in maintain normal hematopoiesis.
Acknowledgments
This work was supported by NIH grant 2 P01 HL048546-16A1.
Abbreviations
- FA
Fanconi anemia
- HSPC
hematopoietic stem and progenitor cells
- H&E
hematoxylin and eosin
- CBC
complete blood count
- KSL
cKit+Sca1+Lin−
- FITC
fluorescein isothiocyanate
- qPCR
quantitative real-time PCR
Footnotes
Authorship
Contributions: Q.S.Z. designed the study, performed research, analyzed and interpreted data, and wrote the manuscript; K.W.S., K.S, L.M.L., A.M., E.B., A.E.N., and E.J. performed research; A.M.S. and S.O. interpreted data and wrote the manuscript; and M.G. designed the study, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43:147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Ceccaldi R, Briot D, Larghero J, Vasquez N, Dubois d’Enghien C, Chamousset D, Noguera ME, Waisfisz Q, Hermine O, Pondarre C, et al. Spontaneous abrogation of the G(2)DNA damage checkpoint has clinical benefits but promotes leukemogenesis in Fanconi anemia patients. J Clin Invest. 2011;121:184–194. doi: 10.1172/JCI43836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Parmar K, Mouly E, Delord M, Kim JM, Regairaz M, Pla M, Vasquez N, Zhang QS, Pondarre C, et al. Bone Marrow Failure in Fanconi Anemia Is Triggered by an Exacerbated p53/p21 DNA Damage Response that Impairs Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- Houghtaling S, Granville L, Akkari Y, Torimaru Y, Olson S, Finegold M, Grompe M. Heterozygosity for p53 (Trp53+/−) accelerates epithelial tumor formation in fanconi anemia complementation group D2 (Fancd2) knockout mice. Cancer Res. 2005;65:85–91. [PubMed] [Google Scholar]
- Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–2035. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Pierce JG, Kagan VE, et al. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In vivo (Athens, Greece) 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- Rego MA, Harney JA, Mauro M, Shen M, Howlett NG. Regulation of the activation of the Fanconi anemia pathway by the p21 cyclin-dependent kinase inhibitor. Oncogene. 2012;31:366–375. doi: 10.1038/onc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax JK, Dash BC, Hong R, Dicker DT, El-Deiry WS. The cyclin-dependent kinase inhibitor butyrolactone is a potent inhibitor of p21 (WAF1/CIP1 expression) Cell cycle (Georgetown, Tex) 2002;1:90–96. [PubMed] [Google Scholar]
- Tesio M, Trumpp A. Breaking the cell cycle of HSCs by p57 and friends. Cell Stem Cell. 2011;9:187–192. doi: 10.1016/j.stem.2011.08.005. [DOI] [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, Van Waes C, Grompe M. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–1608. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Marquez-Loza L, Eaton L, Duncan AW, Goldman DC, Anur P, Watanabe-Smith K, Rathbun RK, Fleming WH, Bagby GC, et al. Fancd2−/− mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 2010;116:5140–5148. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]