Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide, with limited treatment options. AKT/mTOR and Ras/MAPK pathways are frequently deregulated in human hepatocarcinogenesis. Recently, we generated an animal model characterized by the co-expression of activated forms of AKT and Ras in the mouse liver. We found that concomitant activation of AKT/mTOR and Ras/MAPK cascades leads to rapid liver tumor development in AKT/Ras mice, mainly through mTORC1 induction. To further define the role of mTORC1 cascade in AKT/Ras induced HCC development, the mTORC1 inhibitor Rapamycin was administered to AKT/Ras mice at the time when small tumors started to emerge in the liver. Of note, Rapamycin treatment significantly delayed hepatocarcinogenesis in AKT/Ras mice. However, some microscopic lesions persisted in the livers of AKT/Ras mice despite the treatment and rapidly gave rise to HCC following Rapamycin withdrawal. Mechanistically, Rapamycin inhibited mTORC1 and mTORC2 pathways, lipogenesis and glycolysis, resulting in inhibition of proliferation in the treated livers. However, activated ERK and its downstream effectors, Mnk1 and eIF4E, were strongly upregulated in the residual lesions. Concomitant suppression of AKT/mTOR and Ras/MAPK pathways was highly detrimental for the growth of AKT/Ras cells in vitro. The study indicates the existence of a complex interplay between AKT/mTOR and Ras/MAPK pathways during hepatocarcinogenesis, with important implications for the understanding of HCC pathogenesis as well as for its prevention and treatment.
Keywords: AKT, Rapamycin, Ras, liver cancer, mTOR, mouse models
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent solid tumors worldwide, with limited treatment options and a poor prognosis.1,2 Thus, there is a strong need to expand the basic and translational research on HCC in order to improve the patients’ prognosis. Furthermore, the establishment of mouse models recapitulating the major molecular alterations that occur along human hepatocarcinogenesis would be highly beneficial for preclinical drug testing.
Activation of v-akt murine thymoma viral oncogene homolog (AKT)/mammalian target of Rapamycin (mTOR) and ras viral oncogene homolog (Ras)/mitogen-activated protein kinase (MAPK) cascades is frequently observed and associated with aggressive tumor phenotype and poor prognosis in human HCC.3-7 To dissect the functional interaction between these two pathways in liver cancer, we generated a model characterized by the co-expression of activated forms of AKT and Ras in the mouse liver. In this model, activation of AKT/mTOR and Ras/MAPK pathways promotes rapid liver tumor development via mTOR-dependent and -independent mechanisms.8 Here, we summarize the data from the latter study and present new evidence showing that Rapamycin, an inhibitor of mTOR complex 1 (mTORC1), restrains AKT/Ras-driven hepatocarcinogenesis when administrated during the early stages of tumor development. Nevertheless, we found that microscopic lesions persist in Rapamycin-treated livers. Mechanistically, Rapamycin inhibited mTORC1 and mTORC2 pathways, lipogenesis and glycolysis, resulting in inhibition of proliferation and induction of apoptosis in the treated livers. On the other hand, activated extracellular-related kinase (ERK) and its downstream effectors were strongly upregulated in the microscopic, residual lesions. Subsequent experiments in vitro, using a cell line derived from an AKT/Ras HCC showed that concomitant suppression of AKT/mTOR and Ras/MAPK pathways is highly detrimental for AKT/Ras-induced growth. Altogether, our studies indicate the existence of a functional crosstalk between AKT/mTOR and Ras/MAPK pathways along hepatocarcinogenesis, whose inhibition might be highly beneficial for the treatment of HCC patients.
AKT/mTOR Signaling Pathway in HCC Development
The phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway is a central regulator of multiple cellular processes, including metabolism, proliferation and survival.9,10 Once induced, PI3Ks in turn activate AKT, resulting in activation of mTOR kinases.9,10 mTOR kinases are assembled into two distinct complexes: mTORC1 and mTORC2.9,10 mTORC1 phosphorylates S6 kinases and 4E binding protein 1 (4EBP1) downstream targets, thus regulating protein synthesis, cell growth and metabolism.9,10 mTORC2 regulates the AGC kinase subfamily, which includes AKT, and plays a key role in cell proliferation and cytoskeleton organization.9,10
In HCC, deregulation of the PI3K/AKT/mTOR pathway is the result of multiple molecular mechanisms, including activated mutations of PI3K p110 (PIK3CA) catalytic subunit, loss of expression of its negative regulator, phosphatase and tensin homolog (Pten) or aberrant activation of receptor tyrosine kinases.11,12 The importance of the PI3K/AKT/mTOR pathway in hepatocarcinogenesis is underscored by the finding that mTOR inhibition suppresses HCC growth in vitro and xenograft models.6 In addition, either specific ablation of Pten or overexpression of myristoylated/activated form of AKT leads to HCC development in the mouse.3,13 Furthermore, clinical studies with mTOR inhibitors, such as RAD001, are currently in progress, with some promising, yet limited, preliminary benefits for HCC treatment.14
Rapamycin and Rapamycin analogs (Rapalogs) are allosteric partial inhibitors of mTORC1 that have been extensively tested clinically as anticancer agents.15,16 However, most studies suggest that these drugs possess only mild anticancer activity.15,16 Several mechanisms contribute to the weak in vivo antitumor potency of these drugs.17,18 On the one hand, Rapamycin only partially inhibits mTORC1 by efficiently suppressing phosphorylation of ribosomal protein S6 (RPS6), but not 4EBP1.18,19 4EBP1/eukaryotic translation initiation factor 4E (eIF4E)-mediated translation control has been shown to be the key signal downstream of mTORC1 in many cancer types.20 On the other hand, mTORC1 inhibition may trigger the feedback activation of either the PI3K/AKT or the MAPK cascades.21-24
Ras/MAPK Signaling Pathway
Ras proteins are small guanosine triphosphatases regulating cellular response to many stimuli.25 Growth factors bind to cell surface receptors, which then recruit and activate guanine nucleotide exchange factors. The latter activation, in turn, stimulates the formation of Ras-GTP, which binds and activates effector proteins, such as members of the MAPK cascade, to regulate various cellular functions, including proliferation, survival and differentiation.25
In human HCC, previous evidence indicates ubiquitous activation of the Ras/MAPK pathway, supporting the critical role(s) of this signaling cascade during liver tumor initiation and progression.7 Interestingly, Ras family members are rarely mutated in human HCC.7 Mechanisms alternative to somatic mutations leading to the activation of Ras/MAPK pathways in HCC have been discovered and include the overexpression and activation of receptor tyrosine kinases as well as the loss of expression of Ras pathway inhibitors.7,26-28 In mouse models, no tumor formation is observed when an activated mutant form of Ras alone is expressed in hepatocytes, indicating that the sole activation of Ras/MAPK pathway is not sufficient to induce HCC formation in vivo.6,26 A possible scenario in Ras-overexpressing livers is the occurrence of senescence and the clearance of senescent hepatocytes by the immune system.29 Thus, a second oncogenic event is required to promote hepatocarcinogenesis when Ras is activated.
AKT and N-Ras Co-activation in the Mouse Liver Promotes Rapid Carcinogenesis via mTORC1, FOXM1/SKP2 and c-Myc Pathways
To investigate the functional crosstalk between the AKT/mTOR and Ras/MAPK pathway in hepatocarcinogenesis, we recently generated a mouse model characterized by the co-expression of activated forms of AKT and N-Ras protooncogenes in the liver (referred to as AKT/Ras mouse in this paper). Specifically, we co-expressed myristoylated (myr)-AKT1 and N-RasV12 via hydrodynamic gene delivery.8 While overexpression of N-RasV12 alone did not induce histological abnormalities in the mouse liver, overexpression of myr-AKT1 alone (which will be referred to as AKT mouse) induced lipogenesis and hepatocyte proliferation that resulted in HCC development 24 wk post-injection.3,8 In contrast, co-expression of myr-AKT1 and N-RasV12 in the mouse liver significantly accelerated tumorigenesis, leading to abdomen enlargement and lethality by 4 and 6 wk post-injection, respectively.8 At the cellular level, AKT/Ras co-activation resulted in increased proliferation and angiogenesis when compared with AKT mice, leading to rapid malignant transformation and tumor progression.8 At the molecular level, higher levels of mTORC1 and its downstream effectors involved in protein translation, angiogenesis and apoptosis were mostly detected in AKT/Ras mice when compared with AKT mice.8 In contrast, a similar upregulation of mTORC2 targets was detected in AKT and AKT/Ras mice when compared with wild-type mice.8 Of note, the increased mTORC1 activation in AKT/Ras tumor cells was found to be the consequence, at least partly, of the Ras/MAPK mediated phosphorylation/inactivation at the serine 664 residue of the tuberous sclerosis 2 (TSC2) protein, an mTORC1 suppressor.8 In addition, a strong upregulation of c-Myc and the forkhead box M1 (FOXM1) transcription factor was detected almost exclusively in AKT/Ras livers.8 Interestingly, in vitro assays demonstrated that the increased expression of FOXM1 and c-Myc in AKT/Ras tumor cells was independent of mTORC1.8
Altogether, our study demonstrates that activation of AKT and Ras cascades synergizes to promote rapid hepatocarcinogenesis via both mTORC1-dependent and -independent mechanisms. In particular, mTORC1, FOXM1/SKP2 and c-Myc pathways all significantly contribute to AKT/Ras accelerated hepatocarcinogenesis.
Rapamycin Treatment Restrains AKT/Ras Hepatocarcinogenesis, but Triggers the Feedback Activation of MAPK Signaling in the Residual Tumor Cells
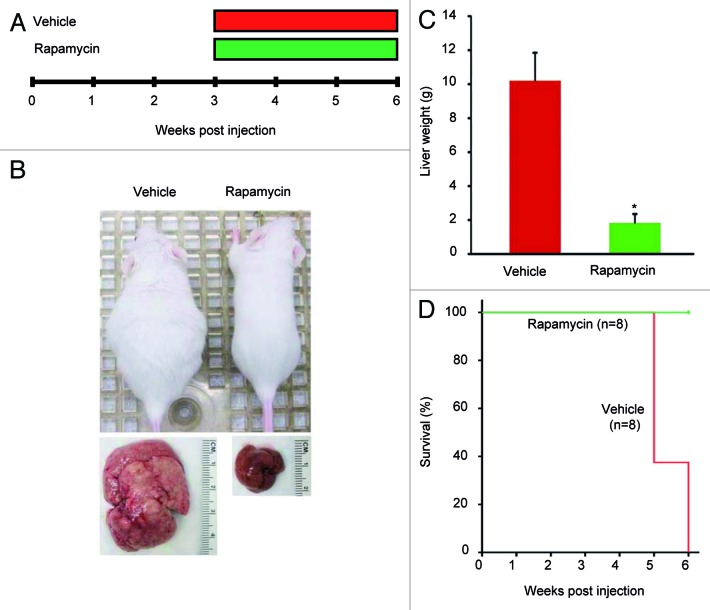
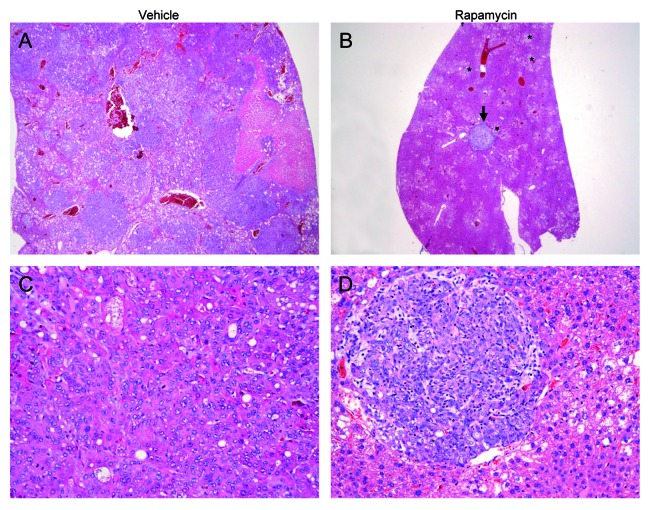
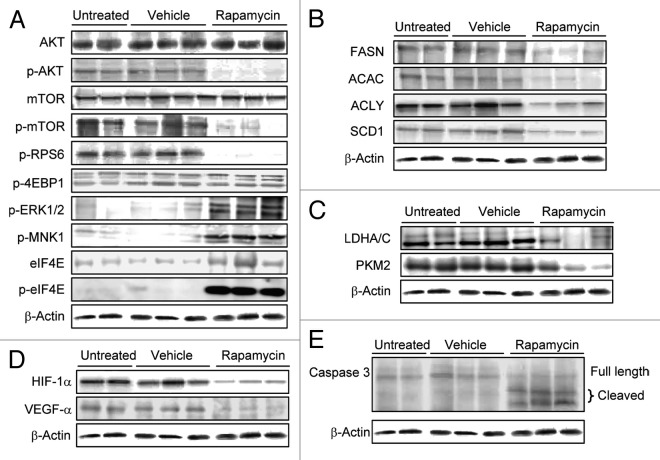
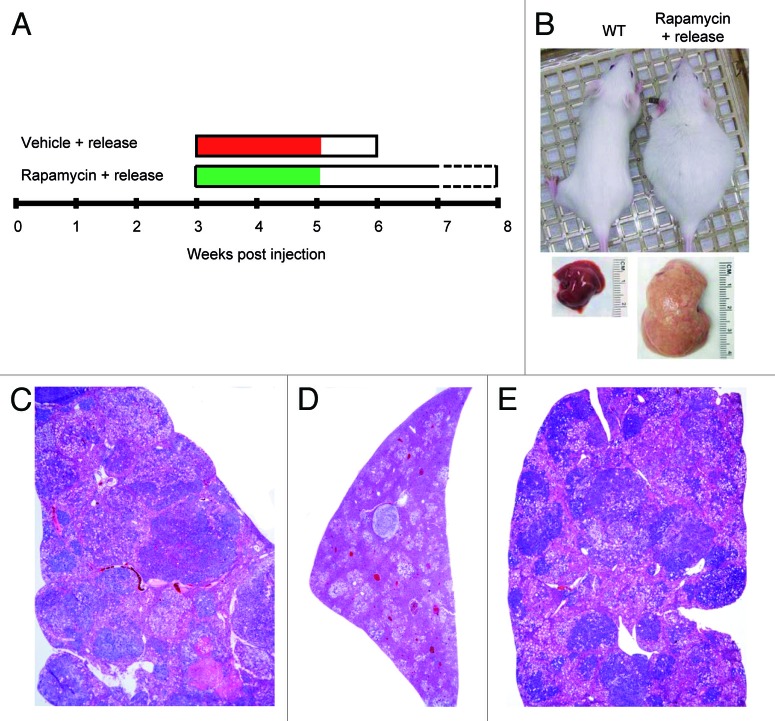
Next, we determined whether pharmacological inhibition of mTORC1 could inhibit ATK/Ras-driven hepatocarcinogenesis. For this purpose, AKT/Ras mice were subjected to either Rapamycin or vehicle administration for 3 wk (Fig. 1A), starting 3 wk post hydrodynamic injection, when small nodules become microscopically visible in AKT/Ras livers.8 Noticeably, all Rapamycin treated mice appeared to be healthy, while all the vehicle-treated mice developed large liver tumors and needed to be euthanized (Fig. 1B and D). Macroscopically, the livers of the Rapamycin-treated mice looked normal and weighed only approximately one-fifth of the livers of vehicle-treated mice (Fig. 1B and C). Histological evaluation revealed that small, microscopic lesions, consisting of foci of altered hepatocytes and small tumors, persisted in Rapamycin-treated AKT/Ras livers (Fig. 2B and D), whereas full-blown liver tumors occupied the whole liver parenchyma in vehicle-treated mice (Fig. 2A and C). The results suggest that Rapamycin treatment partially inhibits liver tumor progression initiated by AKT and Ras proto-oncogenes. The molecular mechanisms underlying Rapamycin mediated tumor inhibition activity were also investigated. As neoplastic lesions only occupied small parts of Rapamycin-treated AKT/Ras liver tissues (Fig. 2B), they were macrodissected for biochemical analysis. Importantly, immunoblotting showed that Rapamycin effectively inhibited the expression of phosphorylated/activated AKT, mTOR and RPS6 proteins (Fig. 3A), while levels of phosphorylated/inactivated 4EBP1 remained unaffected (Fig. 3A). Furthermore, proteins involved in lipid biosynthesis (fatty acid synthase or FASN, acetyl-CoA carboxylase or ACAC, ATP-citrate lyase or ACLY and stearoyl-CoA desaturase or SCD1) were downregulated in Rapamycin-treated AKT/Ras liver tissues (Fig. 3B). Rapamycin also inhibited glycolysis inducers (lactic dehydrogenase or LDHA/C, pyruvate kinase isozyme M2 or PKM2) and angiogenesis (hypoxia-inducible factor 1alpha or HIF-1α, and vascular endothelial growth factor or VEGF-α), and promoted apoptosis (as assessed by induction of the cleaved/activated form of the apoptotic mediator caspase 3) in the treated livers (Fig. 3C–E). Thus, all these factors likely contributed to the strong tumor inhibitory activity by Rapamycin.
Figure 1. Rapamycin treatment restrains hepatocarcinogenesis driven by AKT and Ras co-expression in the mouse liver. (A) Study design. AKT/Ras mice were subjected to either Rapamycin or vehicle administration for 3 wk, starting 3 wk post hydrodynamic injection. (B) Phenotype of vehicle-treated and Rapamycin-treated AKT/Ras mice and respective livers before being euthanized. (C) Liver weight of vehicle-treated and Rapamycin-treated AKT/Ras mice before being euthanized. Student’s t-test: p < 0.0001*, vs. vehicle-treated AKT/Ras mice. (D) Survival curve of vehicle-treated and Rapamycin-treated AKT/Ras mice.
Figure 2. Rapamycin treatment restrains hepatocarcinogenesis in AKT/Ras mice. (A and C) Liver from an AKT/Ras mouse, 6 wk post hydrodynamic injection and subjected to 3 wk vehicle administration, showing size enlargement and complete replacement of the liver parenchyma by highly malignant liver lesion, such as the HCC depicted in (C). (B and D) Liver from an AKT/Ras mouse treated with Rapamycin for 3 wk showing a drastic regression of both preneoplastic (asterisks) and neoplastic lesions [the arrow indicates a small tumor, detailed in (D)]. Original magnifications: 100× in (A and B); 200× in (C and D).
Figure 3. Rapamycin administration inhibits the AKT/mTOR pathway but triggers the compensatory induction of the Ras/MAPK cascade in AKT/Ras mice. Representative immunoblot analysis on the effect of Rapamycin treatment over the AKT/mTOR and Ras/MAPK pathways (A) as well as on lipogenesis (B), glycolysis (C), angiogenesis (D) and apoptosis (E) in AKT/Ras mice. Apoptosis activation is underlined by the induction of caspase 3 cleavage forms following Rapamycin administration. Untreated (n = 8), vehicle-treated (n = 8) and Rapamycin-treated (n = 8) mice were used for the analysis.
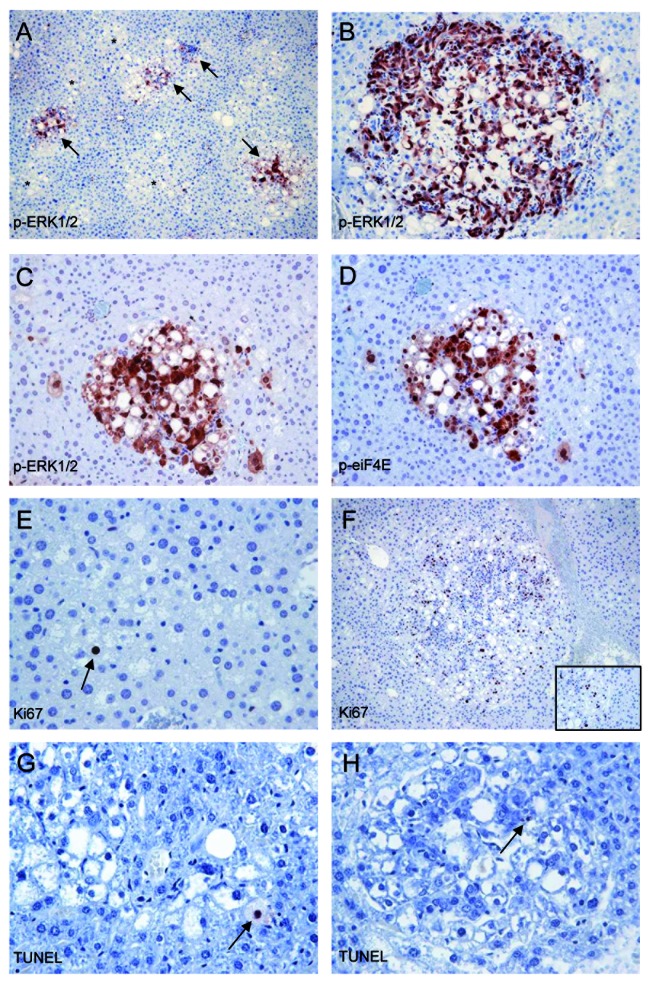
Recent studies suggest the existence of multiple feedback loops between AKT and Ras pathways during tumor development.21-24,30,31 In particular, a previous investigation has shown that mTORC1 inhibition leads to MAPK pathway activation through a RPS6-dependent feedback loop in cancer.31 Thus, we investigated whether the same occurs in AKT/Ras mice after Rapamycin treatment. Noticeably, high levels of phosphorylated/activated ERK1/2 and its downstream effectors, including MAP kinase interacting serine/threonine kinase 1/2 (MNK1/2) and eIF4E, were detected in Rapamycin-treated livers by immunoblotting (Fig. 3A). Accordingly, immunohistochemistry showed an intense staining for phosphorylated/activated ERK and eIF4E, associated with some proliferative activity and scarce apoptosis, in the residual tumor cells (Fig. 4A–H).
Figure 4. Rapamycin treatment induces the compensatory activation of the ERK/eIF4e pathway in the residual tumor cells from AKT/Ras mice. (A and B) Strong ERK activity, as assessed by positive immunolabeling for phosphorylated/activated (p-)ERK1 and 2 proteins in some (arrows) but not all (asterisks) the residual preneoplastic hepatocytes (A) and in a regressing tumor (B) following 3 wk of Rapamycin treatment. (C and D) Co-localization of phosphorylated/activated ERK1/2 and eIF4E immunoreactivity in one AKT/Ras preneoplastic lesion following Rapamycin treatment. (E and F) Proliferative activity in one preneoplastic lesion (E) and a small tumor (F), as assessed by Ki67 immunolabeling. The inset in (F) shows the different proliferative activity between the tumor (left part of the picture) and the surrounding non-tumorous liver (right). (G and H) Apoptotic bodies, indicated by arrows, in one preneoplastic lesion (G) and a small tumor (H), as assessed by TUNEL immunolabeling. Original magnifications: 100× in (A and F); 200× in (B–D); 400× in (E, G and H).
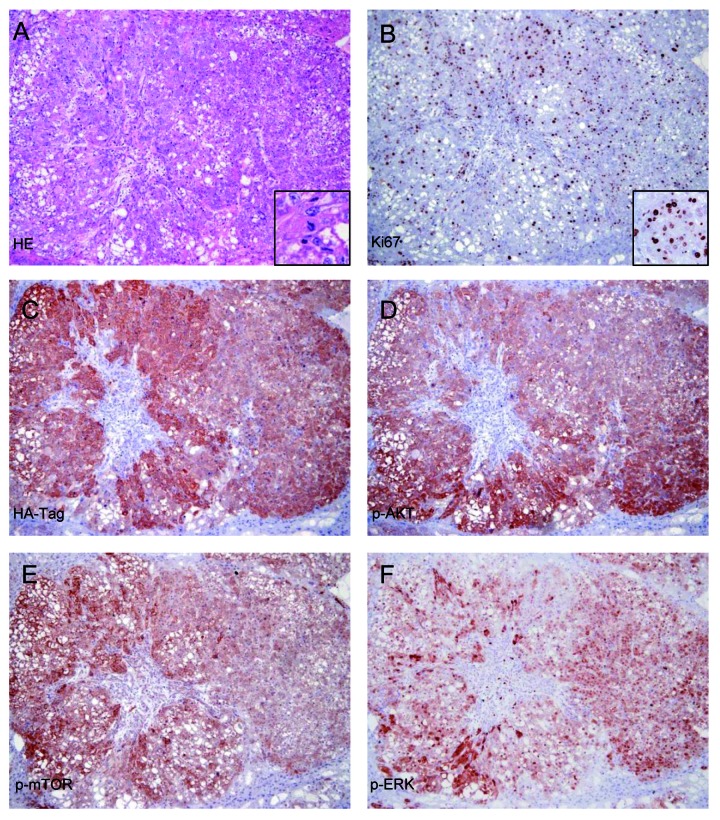
To assess whether the residual cells following Rapamycin administration were still able to form large tumors, a group of AKT/Ras mice was subjected to Rapamycin administration for 2 wk, starting 3 wk after hydrodynamic injection. Subsequently, Rapamycin treatment was suspended (Fig. 5A). Strikingly, Rapamycin withdrawal led to unrestrained proliferation of residual cells, resulting in the development of large tumors replacing the whole liver parenchyma within 2–3 wk (Fig. 5B,E). Of note, both histopathological (Fig. 5E) and immunohistochemical analysis of the main components of the AKT/mTOR and Ras/MAPK pathways (Fig. 6) showed that the tumors developed following Rapamycin withdrawal are identical to the ones from untreated AKT/Ras mice.
Figure 5. Rapamycin withdrawal triggers rapid tumor development in AKT/Ras mice. (A) Study design. A group of five AKT/Ras mice was subjected to Rapamycin administration for 2 wk, starting 3 wk post hydrodynamic injection. Next, Rapamycin administration was suspended and mice were followed for additional 2–3 wk. (B) Phenotype of wild-type mice and AKT/Ras mice subjected to Rapamycin treatment and subsequent drug withdrawal before being euthanized. (C) Liver from an untreated AKT/Ras mouse, 6 wk post hydrodynamic injection, showing that the tumor tissue has replaced almost completely the normal liver tissue. (D) Liver from an AKT/Ras mouse treated with Rapamycin for 3 wk showing the dramatic regression of preneoplastic and neoplastic lesions. (E) Withdrawal of Rapamycin results in the rapid growth of the residual lesions occupying the whole liver parenchyma 2 wk after the drug suspension. Original magnifications: 100× in (C, D and E).
Figure 6. Rapamycin withdrawal leads to the growth of neoplastic lesions exhibiting the aberrant activation of the same signaling pathways underlying hepatocarcinogenesis in AKT/Ras untreated mice. (A–E) Serial sections of the same hepatocellular tumor, 2 wk after withdrawal of Rapamycin. (A) Hematoxylin and eosin (HE) staining showing the presence of a large hepatocellular carcinoma (HCC) in active proliferation (mitosis shown in inset). (B) The elevated proliferation rate of the HCC is further confirmed by the high density of Ki67-positive tumor cell nuclei. (C–F) Strong activation of AKT/mTOR and Ras/MAPK pathways in the same HCC, as indicated by immunoreactivity for HA-Tag (implying the presence of the AKT construct in these cells), and phosphorylated/activated (p-)AKT, mTOR and ERK. Original magnification: 100×, except insets (200×).
In summary, our study indicates that Rapamycin treatment restrains, without complete inhibition, AKT/Ras driven hepatocarcinogenesis by suppressing the mTORC1/RPS6 cascade. Rapamycin treatment triggers the compensatory upregulation of the MAPK pathway in AKT/Ras mouse livers. Withdrawal of Rapamycin results in the development of aggressive liver tumors starting from the residual cells.
Co-Targeting of mTORC1 and Ras/MAPK Pathways is Detrimental for the Growth of AKT/Ras Cells In Vitro
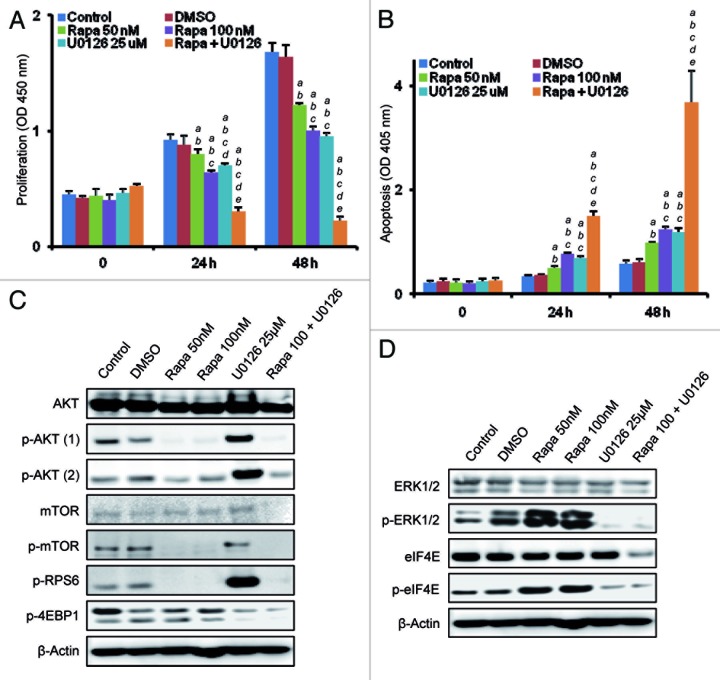
Finally, we assessed the effect of the combined inhibition of AKT/mTOR and Ras/MAPK pathways in AKT/Ras cells. For this purpose, we used a cell line derived from an AKT/Ras HCC (which will be referred to as AKT/Ras cell line).8 Treatment with either the mTORC1 inhibitor, Rapamycin or the MEK inhibitor, U0126, resulted in a significant reduction of the growth of the AKT/Ras cell line due to decreased proliferation and increased apoptosis (Fig. 7A and B). Strikingly, concomitant administration of Rapamycin and U0126 resulted in the complete suppression of cell growth in the AKT/Ras cell line due to strong reduction in cell proliferation and massive apoptosis (Fig. 7A and B). At the molecular level, treatment with Rapamycin suppressed the AKT/mTOR pathway, but had no effect on phosphorylated/inactivated 4EBP1 levels (Fig. 7C), which were instead remarkably inhibited by U0126 treatment (Fig. 7D). Furthermore, Rapamycin induced the compensatory activation of ERK1/2 and eIF4E proteins (Fig. 7D). Intriguingly, a strong upregulation of phosphorylated/activated AKT and RPS6 proteins was triggered by U0126 treatment (Fig. 7C), implying the existence of a compensatory induction of the AKT/mTOR pathway in response to MAPK suppression. The compensatory feedback loops were efficiently blunted when Rapamycin and U0126 were co-administered (Fig. 7C and D).
Figure 7. Concomitant suppression of the mTORC1 and Ras/MAPK pathways is highly detrimental for AKT/Ras-driven growth in vitro. (A and B) Inhibition of mTORC1 and MAPK via Rapamycin and U0126, respectively, induces a remarkable restraint over the growth of the AKT/Ras cell line, as determined by the decrease in cell proliferation and induction of massive apoptosis when compared with administration of either Rapamycin or U0126 alone. Each bar represents mean ± SD. Tukey-Kramer test: p < 0.001 a, vs. control (untreated cells); b, vs. vehicle (DMSO); c, vs. Rapamycin- (50 nM) treated cells; d, vs. Rapamycin- (100 nM) treated cells; e, vs. U0126-treated cells. (C and D) Representative immunoblot analysis showing that Rapamycin induces downregulation of mTORC1 and AKT activity in a concentration-independent manner but is ineffective on 4EBP1 protein and induces the compensatory activation of ERK and eIF4E proteins. On the other hand, treatment with U0126 alone suppresses phosphorylated/inactivated (p-)4EBP1 but induces the compensatory activation of phosphorylated/activated (p-)AKT and RPS6. The compensatory feed-back loops are abolished by the combination of Rapamycin and U0126 treatment. Note that combined treatment downregulates both the total and phosphorylated/activated levels of mTOR and eIF4E. The abbreviations p-AKT (1) and p-AKT (2) indicate phosphorylated/activated levels of AKT protein at threonine 308 and serine 473 residue, respectively.
Discussion
Concomitant activation of AKT/mTOR and Ras/MAPK pathways is frequently observed along human hepatocarcinogenesis.3-7 Here, we summarize our recent data obtained using a novel mouse model of liver cancer induced by activated AKT and Ras protooncogenes. Our study is significant in many ways. To the best of our knowledge, this is the first study demonstrating the functional interplay between AKT/mTOR and Ras/MAPK pathways in promoting rapid hepatocarcinogenesis in vivo. Also, we uncovered novel molecular mechanisms involved in malignant transformation and tumor progression driven by AKT/mTOR and Ras/MAPK pathways. Specifically, the study demonstrates the importance of mTORC1-dependent and -independent mechanisms, which involve FOXM1 and c-Myc activation, in AKT/Ras-induced hepatocarcinogenesis. In addition, the study provides a valuable preclinical model that can be used to characterize the chemopreventive and therapeutic potential of small molecules interfering with AKT/mTOR and/or Ras/MAPK pathways. Our mechanistic studies also support the development of drugs targeting c-Myc and FOXM1 pathways for HCC treatment.
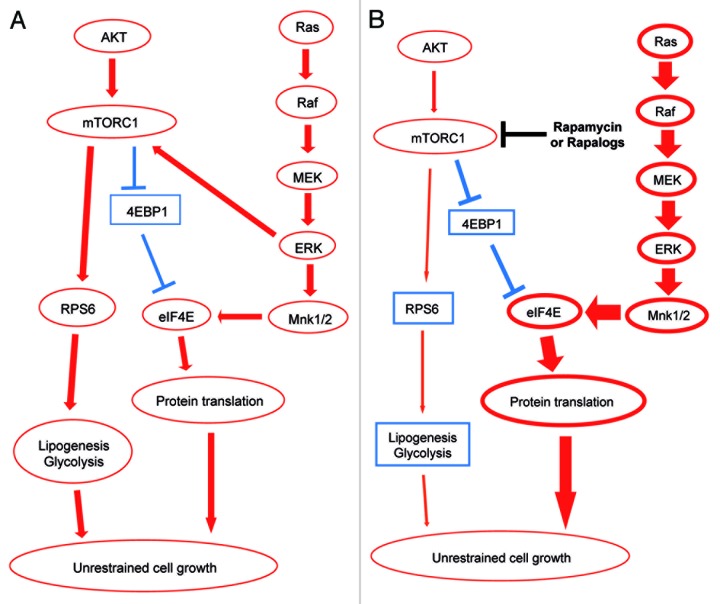
We also presented novel data showing that Rapamycin treatment significantly inhibits AKT/Ras hepatocarcinogenesis by blocking the mTORC1/RPS6 cascade (Fig. 8A). However, we showed that Rapamycin does not affect the levels of phosphorylated/inactivated 4EBP1 protein (Fig. 3A), a key downstream effector of mTORC1 in some tumor types.20 Whether the 4EBP1/eIF4E axis is required for AKT/Ras-driven hepatocarcinogenesis remains to be defined.
Figure 8. Schematic representation of the molecular mechanisms whereby AKT and Ras promote hepatocarcinogenesis in the mouse and the effect of Rapamycin treatment. (A) AKT induces activation (red arrows) of mTORC1, with consequent activation of RPS6, lipogenesis and glycolysis as well as inactivation (blunted, blue arrows) of 4EBP1 protein. Through inactivation of 4EBP1, mTORC1 releases the eIF4E protein, leading to protein translation. Ras activates instead the Raf/MEK/ERK/eIF4E cascade, which in turn activates mTORC1 and eIF4E. The crosstalk between activated AKT and Ras ultimately leads to unrestrained cell growth. (B) Rapamycin induces downregulation (thin, red arrows) of mTORC1 and AKT activity but is ineffective on 4EBP1 protein and induces the compensatory activation (thick, red arrows) of Ras/Raf/MEK/ERK and eIF4E pathways.
Furthermore, we showed that Rapamycin treatment triggers the feedback activation of the MAPK cascade, which is presumably responsible for the survival of residual tumor cells in Rapamycin-treated AKT/Ras mice (Fig. 8B). These observations have important implications. Indeed, our in vivo studies suggest that Rapamycin or Rapalogs might be beneficial for HCC patients with activated AKT and Ras pathways, and these drugs may be particularly useful to prevent recurrences after curative resection or liver transplantation. However, Rapamycin or Rapalogs only partially block mTORC1 signaling, leading to the feedback activation of Ras/MAPK cascade, which may contribute to drug resistance or tumor recurrence. Some approaches are likely to be effective in circumventing the feedback activation mechanisms. For instance, the use of dual mTOR/PI3K inhibitors, such as NVP-BEZ235, BGT226, SF1126 and PKI-587,32 should be able to block the AKT compensatory induction following mTORC1 inhibition. A second possibility is to combine Rapamycin with MEK inhibitors. In accordance with the latter hypothesis, previous studies have shown that combination therapy of Rapamycin with AZD6244, a MEK inhibitor or Sorafenib, a Raf inhibitor, effectively inhibits tumorigenesis in HCC xenografts.33,34 The important clinical implications of simultaneous targeting the AKT/mTOR and Ras/MAPK pathways have been recently demonstrated in other tumor types. For instance, it has been shown that sustained activation of AKT1 induces resistance to chemotherapy, hormonal-based drug approaches and radiation in human breast cancer cells.35 In these cells, administration of chemotherapeutic drugs and hormonal-based drugs were shown to induce the Ras/MAPK pathway. Thus, suppression of both AKT/mTOR and Ras/MAPK cascades might be helpful in treating breast cancer more effectively. Furthermore, it is noteworthy to mention that suppression of the AKT inhibitor Pten was found to increase chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors in breast cancer cells.36 The importance of dual target therapy has also been envisaged in malignant melanoma. Indeed, it was found that combined targeting of Ras/MAPK and PI3K/AKT/mTOR pathways is necessary to effectively inhibit N-Ras mutant melanoma in vitro and in vivo.37
At the cellular level, it has been demonstrated that combined suppression of the AKT/mTOR and Ras/MAPK cascades affects important features of tumor cells, not limited to proliferation and apoptosis. In a recent study, it was shown that AKT, when co-expressed with an activated form of Ki-Ras, promotes carcinogenesis in the mouse pancreas by inhibiting Ras-induced senescence.38 Similarly, it has been found that concomitant upregulation of the AKT/mTOR and Ras/MAPK pathways can contribute to drug resistance by diminishing cell senescence in response to chemotherapy in breast cancer cells.39 Thus, suppression of the two pathways might contribute to the anti-growth effect of chemotherapy also by favoring the induction of senescence in cancer cells.
In summary, we have recently developed a mouse model of liver cancer that exhibits concomitant activation of AKT/mTOR and Ras/MAPK pathways, two signaling cascades often activated in human HCC. This mouse model provides an ideal system to test the efficacy of AKT/mTOR and Ras/MAPK inhibitors on HCC development and progression. Ongoing studies using the AKT/Ras mouse model will advance the knowledge of targeted therapy for HCC and provide solid preclinical evidence for utilizing Ras/MAPK and AKT/mTOR inhibitors in human HCC treatment.
Materials and Methods
Hydrodynamic injection and mouse treatment
Hydrodynamic injection was performed as described.3,8,26,27 Briefly, 10 μg of myr-AKT1 and N-RasV12 plasmids along with sleeping beauty transposase in a ratio of 25:1 were diluted in 2 mL 0.9% NaCl, filtered through a 0.22-μm filter and injected into the lateral tail vein of 6–8-wk-old FVB/N mice in 5–7 sec. Additional groups of AKT/Ras-injected mice were subjected, 3 wk after hydrodynamic injection, to administration of either vehicle (n = 8) or Rapamycin (5 mg/kg, n = 8) by daily intraperitoneal injection for either 2 or 3 wk. Mice treated for 2 wk with Rapamycin were then left untreated for 3 wk and then sacrificed. Livers were harvested 5 h after the last dose. Mice were housed, fed and treated in accordance with protocols approved by the Committee for Animal Research at the University of California, San Francisco.
Histology and immunohistochemistry
Livers were fixed in 4% paraformaldehyde and processed for paraffin embedding. Liver lesions were assessed by two board-certified pathologists (M.E. and F.D.). Immunohistochemical staining was performed using the following antibodies: mouse monoclonal anti-HA-Tag (Cell Signaling Technology; 1:2,000), rabbit monoclonal anti-phosphorylated AKT, anti-phosphorylated ERK1/2, anti-phosphorylated eIF4E, anti-phosphorylated mTOR (Cell Signaling Technology; 1:100) and rabbit polyclonal anti-Ki67 (Bethyl Laboratories; 1:2,000), as previously described.3,8 TUNEL staining was performed using the ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Millipore), following the manufacturer’s instructions.
Immunoblotting
Frozen livers were processed as reported.3,8 Aliquots of 40 µg proteins were denatured, separated by SDS-PAGE and transferred onto nitrocellulose membranes by electroblotting. Membranes were blocked in 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h and probed with specific antibodies as previously described.3,8
In vitro treatment and assessment of proliferation and apoptosis
The primary AKT/Ras HCC cell line was isolated from a AKT/Ras mouse HCC as has been previously described.3,8 AKT/Ras cells were plated at 2.0 × 103/well in 96-well plate and grown for 12 h. After 24 h serum deprivation, Rapamycin (50 or 100 nM; Cell Signaling Technology) and or U0126 (25 μM; Cell Signaling Technology) were added to the medium and incubated for 24 and 48 h. Proliferation and apoptosis were assessed using the BrdU Cell Proliferation Reagent (Cell Signaling Technology) and Cell Death Detection Elisa Plus Kit (Roche Molecular Biochemicals), respectively, following the manufacturers’ protocol. Experiments were repeated at least three times in triplicate.
Statistical analysis
Student’s t- and Tukey-Kramer tests were used to evaluate statistical significance. Values of p < 0.05 were considered significant. Data are expressed as means ± SD.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft DFG (grant number Do622/2-1) to F.D. and (grant number Ev168/2-1) to M.E. and NIH R01CA136606 to X.C. We would like to thank UCSF Liver Center (P30DK026743) for the histology support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25099
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–83. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–5. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 5.Ladu S, Calvisi DF, Conner EA, Farina M, Factor VM, Thorgeirsson SS. E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology. 2008;135:1322–32. doi: 10.1053/j.gastro.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(83 e1-11):1972–83, e1-11. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of Rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55:833–45. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M. Molecular targeted therapy for hepatocellular carcinoma: bench to bedside. Dig Dis. 2011;29:273–7. doi: 10.1159/000327558. [DOI] [PubMed] [Google Scholar]
- 13.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci USA. 2004;101:2082–7. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–61. doi: 10.1586/14737140.9.2.247. [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 16.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–41. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, Rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–72. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 18.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–9. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–61. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, et al. Mammalian target of Rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–82. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 24.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 26.Lee SA, Ho C, Roy R, Kosinski C, Patil MA, Tward AD, et al. Integration of genomic analysis and in vivo transfection to identify sprouty 2 as a candidate tumor suppressor in liver cancer. Hepatology. 2008;47:1200–10. doi: 10.1002/hep.22169. [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Ladu S, Evert M, Dombrowski F, De Murtas V, Chen X, et al. Synergistic role of Sprouty2 inactivation and c-Met up-regulation in mouse and human hepatocarcinogenesis. Hepatology. 2010;52:506–17. doi: 10.1002/hep.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvisi DF, Ladu S, Conner EA, Seo D, Hsieh JT, Factor VM, et al. Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J Hepatol. 2011;54:311–9. doi: 10.1016/j.jhep.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 30.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Huynh H. AZD6244 (ARRY-142886) enhances the antitumor activity of Rapamycin in mouse models of human hepatocellular carcinoma. Cancer. 2010;116:1315–25. doi: 10.1002/cncr.24863. [DOI] [PubMed] [Google Scholar]
- 34.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, et al. Ras pathway activation in hepatocellular carcinoma and antitumoral effect of combined sorafenib and Rapamycin in vivo. J Hepatol. 2009;51:725–33. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steelman LS, Navolanic P, Chappell WH, Abrams SL, Wong EW, Martelli AM, et al. Involvement of Akt and mTOR in chemotherapeutic- and hormonal-based drug resistance and response to radiation in breast cancer cells. Cell Cycle. 2011;10:3003–15. doi: 10.4161/cc.10.17.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH, et al. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27:4086–95. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci USA. 2013;110:4015–20. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, et al. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Steelman LS, McCubrey JA. Cooperative effects of Akt-1 and Raf-1 on the induction of cellular senescence in doxorubicin or tamoxifen treated breast cancer cells. Oncotarget. 2011;2:610–26. doi: 10.18632/oncotarget.315. [DOI] [PMC free article] [PubMed] [Google Scholar]