Abstract
Inhibitors of EZH2 methyltransferase activity have been demonstrated to selectively suppress the growth of diffused large B cell lymphoma (DLBCL) cells with gain-of-function mutations in EZH2, while exhibiting very limited effects on the growth of DLBCL cells with wild-type EZH2. Given that EZH2 is often overexpressed but not mutated in solid tumors, it is important to investigate the determinants of sensitivity of solid tumor cells to EZH2 inhibitors. In the current study, we show that three-dimensional (3D) culture of epithelial ovarian cancer (EOC) cells that overexpress EZH2 sensitizes these cells to EZH2 methyltransferase inhibition. Treatment of EOC cells with GSK343, a specific inhibitor of EZH2 methyltransferase, decreases the level of H3K27Me3, the product of EZH2’s enzymatic activity. However, GSK343 exhibited limited effects on the growth of EOC cells in conventional two-dimensional (2D) culture. In contrast, GSK343 significantly suppressed the growth of EOC cells cultured in 3D matrigel extracellular matrix (ECM), which more closely mimics the tumor microenvironment in vivo. Notably, GSK343 induces apoptosis of EOC cells in 3D but not 2D culture. In addition, GSK343 significantly inhibited the invasion of EOC cells. In summary, we show that the 3D ECM sensitizes EOC cells to EZH2 methyltransferase inhibition, which suppresses cell growth, induces apoptosis and inhibits invasion. Our findings imply that in EZH2 wild-type solid tumors, the ECM tumor microenvironment plays an important role in determining sensitivity to EZH2 inhibition and suggest that targeting the ECM represents a novel strategy for enhancing EZH2 inhibitor efficacy.
Keywords: epithelial ovarian cancer, EZH2, EZH2 inhibitor GSK343, 3D culture, apoptosis
Introduction
Enhancer of zeste homolog 2 (EZH2) is the catalytic subunit of polycomb repressive complex 2 (PRC2), which plays an important role in epigenetic gene silencing.1,2 PRC2 functions to silence gene expression by tri-methylating the lysine 27 residue of histone H3 (H3K27) associated with target genes.1,2 EZH2 has been shown to target genes involved in a variety of biological processes, such as cell proliferation and apoptosis.3 Epithelial ovarian cancer (EOC) is currently the deadliest of all gynecological cancers,4 highlighting the urgent need to identify new targets for developing therapeutics for EOC. EOCs are classified into distinct histological types, including serous, mucinous, endometrioid and clear cell. The most common histology of EOC is serous (~60% of all cancers).5 Recently, an alternative classification has been proposed, in which EOC is broadly divided into two types.6 Type I EOC includes mucinous, low-grade serous, low-grade endometrioid and clear cell carcinomas, and type II EOC includes high-grade serous carcinomas, which is the most lethal histosubtype.6 EZH2 is often overexpressed in all histosubyptes of EOCs, and its expression promotes cell proliferation and invasion, inhibits apoptosis and enhances angiogenesis in EOCs.7,8 Therefore, inhibiting EZH2/PRC2 activity might represent an attractive strategy for developing urgently needed EOC therapeutics.9
Normal epithelial tissues exist as well-organized polarized single cell layers regulated by the surrounding microenvironment and extracellular matrix (ECM).10-12 During cancer progression this organization is disrupted as cancer cells proliferate and invade into the ECM.12,13 Significantly, this process is not well replicated in the conventional two-dimensional (2D) tissue culture environment that is often used to assay potential therapeutics. It has been shown that non-transformed epithelial cells cultured with reconstituted basement membrane form hollow, growth-arrested, polarized three-dimensional (3D) structures that have many features of epithelial cells grown in vivo.12,14 Tumorigenic cells cultured in the same way often form large, solid, proliferating and invasive structures characteristic of in vivo tumors.12 These 3D models have led to powerful insights into tumor growth, behavior and drug responses that would not be possible in conventional 2D monolayer cultures.11
GSK343 is a cofactor S-(S’-adenosyl)-L-methionine competitive EZH2 methyltransferase inhibitor.15 Notably, GSK343 is highly selective for EZH2 over a number of other methyltransferases such as SUV39H1 and G9a, with selectivity greater than 1,000-fold.15 Here we examined the effects of GSK343 on the growth and invasion of human EOC cells. Interestingly, our data indicates that EZH2 inhibition is significantly more potent in suppressing the growth of EOC cells in 3D, which more closely mimics the tumor microenvironment in vivo compared with conventional 2D monolayer culture.11 In addition, we show that this correlates with induction of apoptosis of human EOC cells in 3D but not 2D cultures. Further, we show that GSK343 suppresses the invasion of human EOC cells. These data establish that 3D ECM plays an important role in determining the sensitivity of EOC cells to EZH2 inhibitors and imply that EZH2 methyltransferase activity promotes aberrant 3D phenotypes in EOC cells.
Results
EZH2 inhibitor exhibited limited effects on the growth of human EOC cells under conventional 2D monolayer culture
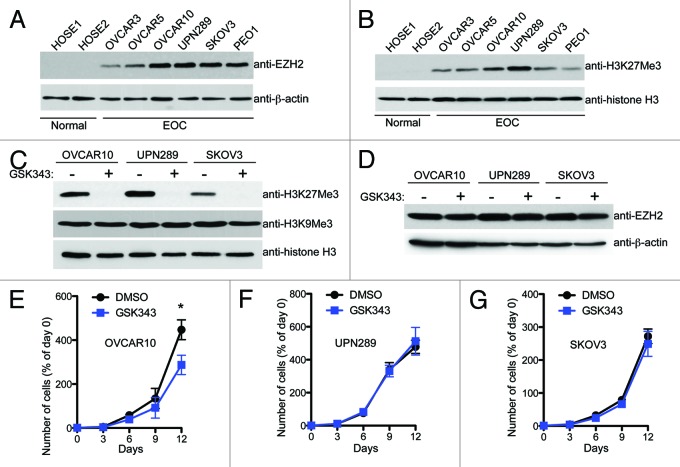
Compared with normal human ovarian surface epithelial (HOSE) cells, EZH2 is expressed at a higher level in EOC cell lines (Fig. 1A). Consistently, the levels of H3K27Me3, the product of EZH2 methyltrasferase enzymatic activity,1 are also higher in EOC cells compared with HOSE cells (Fig. 1B). We sought to determine the effects of GSK343 on the malignant phenotypes of EOC cells. Toward this goal, we titrated GSK343 concentration in two EOC cells lines that express high levels of EZH2, OVCAR10 and SKOV3 (Fig. S1A and B). We observed a dose-dependent decrease in the level of H3K27Me3 in cells treated with GSK343 and a > 90% reduction in the level of H3K27Me3 in EOC cells treated with 1 μM GSK343 for 72 h (Fig. 1C; Fig. S1A and B). In contrast, levels of H3K9Me3, which are generated by different histone methyltransferases such as SUV39H1 and SETDB116 were not affected by GSK343 (Fig. 1C). This further demonstrates the specificity of GSK343 as an EZH2 methyltransferase inhibitor. Notably, GSK343 treatment had no appreciable effect on EZH2 expression (Fig. 1D; Fig. S1A and B), suggesting that the effects observed in GSK343 treated cells are not due to loss of EZH2 expression. Together, we conclude that the EZH2 inhibitor GSK343 efficiently decreases the level of H3K27Me3 in EOC cells.
Figure 1. The EZH2 inhibitor GSK343 exhibits limited effects on the growth of human EOC cells under conventional 2D monolayer culture. (A) Expression of EZH2 and β-actin in two individual batches of normal human ovarian surface epithelial (HOSE) cells and indicated human EOC cell lines was determined by immunoblotting. (B) Same as (A) but for H3K27Me3 and histone H3 expression determined by immunoblotting. (C) Expression of H3K27Me3, H3K9Me3 and histone H3 was determined in the indicated EOC cell lines by immunoblotting after 3 d of treatment with GSK343 (1 μM) or vehicle control (0.1% DMSO). (D) Same as (C) but for EZH2 and β-actin expression determined by immunoblotting. (E–G) Cell growth curves for OVCAR10, UPN289 and SKOV3 cell lines treated with GSK343 (1 μM) or vehicle control (0.1% DMSO) over 12 d. Media was changed and cells counted every 3 d, time points represent the mean of three independent experiments with SD, *p < 0.05.
Expression of EZH2 positively correlates with markers of cellular proliferation in primary EOC specimens, and its knockdown suppresses the growth of human EOC cells.7 Thus, we sought to determine the effects of GSK343 on growth of EOC cells with high EZH2 expression, such as OVCAR10, UPN289 and SKOV3, under conventional 2D monolayer cultures. We treated these EOC cells with 1 μM GSK343 for a period of 12 d, with fresh treatment every 3 d, because we observed a > 90% reduction of H3K27Me3 levels in these cells after 3 d of GSK343 treatment (Fig. 1C; Fig. S1A and B). Interestingly, although treatment with GSK343 resulted in a minor but statistically significant decrease in growth of OVCAR10 cells over 12 d, GSK343 had minimal to no effects on the growth of UPN289 and SKOV3 cells (Fig. 1E–G). Similarly, we observed that GSK343 has no statistically significant effects on the growth of EOC cells with relatively low EZH2 expression, such as OVCAR3, OVCAR5 and PEO1 (Fig. S1C–E), despite efficient reduction of H3K27Me3 levels (Fig. S1F). This suggests that the inability of GSK343 to suppress the growth of EOC cells is not due to variations in the levels of EZH2 expression. Based on these results, we conclude that EZH2 inhibitor has limited effects on the growth of human EOC cells under conventional 2D monolayer culture.
EZH2 inhibitor significantly suppresses the growth of human EOC cells in 3D cultures
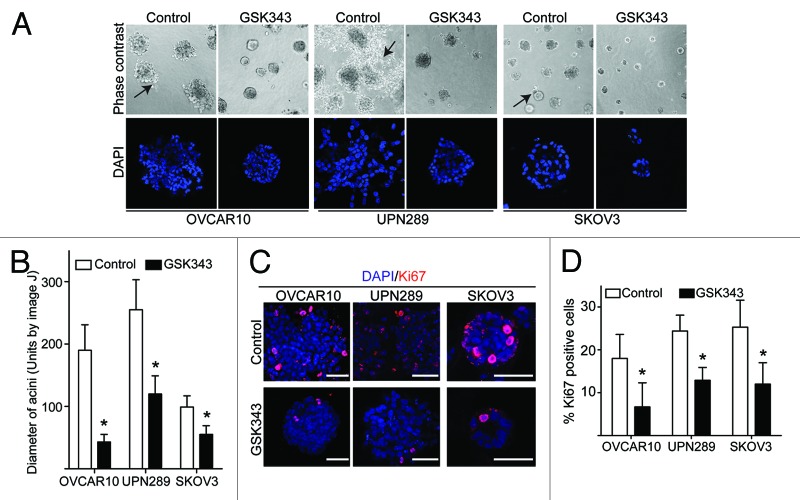
It is well established that the tumor microenvironment and ECM play an important role in regulating tumor phenotypes, in part through epigenetic mechanisms.17 Accordingly, 3D culture utilizing ECM components is often used to mimic the in vivo tumor microenvironment.10 We hypothesized that cellular interactions with the ECM might regulate the effects that EZH2 inhibition has on EOC cells. Thus, we sought to determine the effects of GSK343 on the growth of EOC cells in 3D cultures using a Matrigel basement membrane ECM. Significantly, we observed that GSK343 suppressed the size of acini formed by EOC cell lines (Fig. 2A and B). In addition, we observed that treatment of EOC cells with GSK343 resulted in a more compact acini structure that is characteristic of normal epithelial cells with decreased appearance of invasive characteristics (Fig. 2A). Furthermore, when acini were stained for Ki-67, a marker of cell proliferation, we observed a significant decrease in Ki-67-positive cells in GSK343-treated cells compared with controls (Fig. 2C and D). Indeed, cell number was also decreased by GSK343 in 3D cultures (Fig. S2). Similar observations were made using multiple EOC cell lines (Fig. 2), suggesting that the observed effects are not cell line-specific. Together, we conclude that EZH2 inhibition suppresses the growth of human EOC cells in 3D culture conditions.
Figure 2. The EZH2 inhibitor GSK343 significantly suppresses the growth of human EOC cells in 3D cultures. (A) Indicated EOC cells were cultured in 3D Matrigel and treated with GSK343 (1 μM) or vehicle control (0.1% DMSO) for 12 d. 3D acini were examined by phase contrast (top) or stained with the nuclei fluorescence dye DAPI (bottom). Note differences in acini size and shape. Arrows indicate examples of invasive structures observed in EOC 3D cultures. (B) Quantification of acini size formed by the indicated EOC cells treated with GSK343 (1 μM) or vehicle control (0.1% DMSO) after 12 d of growth in Matrigel. Mean of three independent experiments with SD, *p < 0.05. (C) Same as (A) but stained for Ki-67 expression Bar = 40 μm. (D) Quantification of (C). Ki-67 positive staining cells in the indicated EOC cells treated with GSK343 (1 μM) or vehicle control (0.1% DMSO) after 12 d of growth in Matrigel. Numbers represent mean number of cells counted in 5 acini for each condition. Mean of three independent experiments with SD, *p < 0.05.
EZH2 inhibitor suppresses the invasion of human EOC cells
Since we observed a decrease in the appearance of invasive characteristics in GSK343-treated EOC cells under 3D culture conditions (Fig. 2A), we next sought to directly determine the effects of GSK343 on the invasion of human EOC cells. Indeed, GSK343 significantly inhibited the invasion of SKOV3 EOC cells as determined by a Boyden chamber assay (Fig. 3A and B). Interestingly, while GSK343 inhibited the invasion of SKOV3 EOC cells, it does not statistically affect the migration of SKOV3 cells (Fig. 3A and B). This is consistent with the report that EZH2 knockdown suppresses the invasion of human EOC cells while having no effects on the migration of these cells.7 Notably, GSK343 has no appreciable effects on the growth of human SKOV3 cells in 2D (Fig. 1), suggesting that the observed effects are not due to a decrease in the proliferation of these cells. Inhibition of invasion was also observed in UPN289 and OVCAR10 EOC cells (Fig. S3), suggesting that these effects are not cell line-specific. Based on these results, we conclude that EZH2 inhibition suppresses the invasion of human EOC cells.
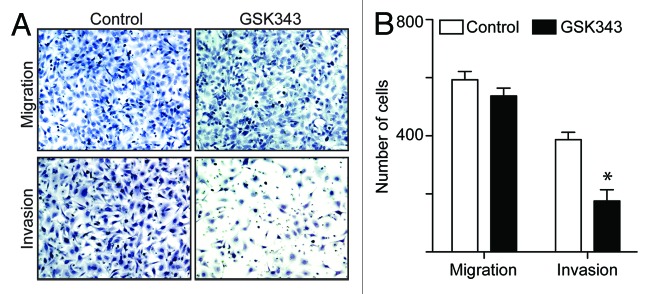
Figure 3. The EZH2 inhibitor GSK343 suppresses invasion of human EOC cells. (A) Equal number of SKOV3 cells treated with 1 μM GSK343 or vehicle control (0.1% DMSO) were assayed for migration through uncoated control membrane or invasion through Matrigel-coated membrane. The cells migrated through control membrane or invaded through Matrigel-coated membrane were stained with 1% crystal violet in PBS. (B) Quantification of (A). Number of SKOV3 cells migrated through control membrane or invaded through Matrigel-coated membrane. Numbers represent mean of three independent experiments. *p < 0.05.
EZH2 inhibitor induces apoptosis of EOC cells in 3D but not 2D culture conditions
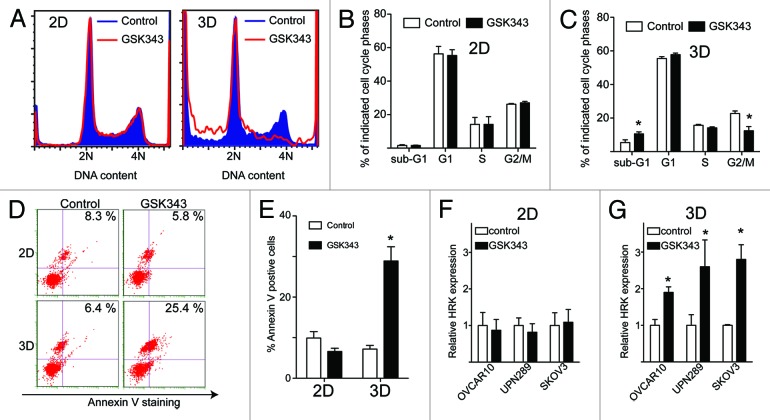
We next sought to determine the mechanism by which GSK343 inhibits the growth of human EOC cells in 3D but not 2D culture conditions. Toward this goal, we preformed cell cycle distribution analysis on cells grown in 2D vs. 3D conditions using flow cytometry. Consistent with the idea that GSK343 induces apoptosis of EOC cells in 3D culture, we observed a significant increase of sub-G1 phase population in GSK343-treated SKOV3 cells compared with controls (Fig. 4A–C). In contrast, there were no effects of GSK343 on sub-G1 population of EOC cells cultured in 2D conditions (Fig. 4A–C). Indeed, the percentage of Annexin V-positive cells was increased by GSK343 treatment compared with controls (Fig. 4D and E). This further supports the notion that GSK343 induces apoptosis of EOC cells cultured in 3D. In contrast, GSK343 failed to increase the percentage of Annexin V positivity in EOC cells cultured in 2D (Fig. 4D and E). In addition, we observed that there was a significant decrease in G2/M phase of the cell cycle in EOC cells treated with GSK343 compared with controls in 3D but not 2D culture conditions (Fig. 4A–C). This is consistent with our observation that expression of cell proliferation marker Ki-67 is decreased by GSK343 in 3D cultures (Fig. 2C and D). In contrast, a decrease in G2/M phase of the cell cycle is not observed in conventional 2D cultures treated with GSK343 (Fig. 4A–C). Similarly, we observed an increase in sub-G1 phase and a decrease in G2/M phase of the cell cycle in GSK343-treated OVCAR10 and UPN289 EOC cells cultured in 3D conditions (Fig. S3), suggesting that the observed effects are not cell line-specific. Interestingly, GSK343 significantly decreases the S phase population of the cells in OVCAR10 cells in 2D cultures (Fig. S4). This is consistent with the observed minor suppression of growth by GSK343 in OVCAR10 cells in 2D cultures (Fig. 1D). However, GSK343 has no significant effects on cell cycle distribution of UPN289 or SKOV3 cells in 2D cultures (Fig. 4; Fig. S4), which is consistent with the observation that GSK343 does not significantly affect the growth of these cells in 2D cultures (Fig. 1F and G). Taken together, we conclude that EZH2 inhibition induces apoptosis and decreases the G2/M phase population of EOC cells in 3D, but not 2D cultures. These results suggest that cell interactions with the ECM in the tumor microenvironment play an important role in determining the efficacy of EZH2 inhibitors.
Figure 4. The EZH2 inhibitor GSK343 induces apoptosis and decreases the G2/M phase population of EOC cells cultured in 3D conditions. (A) Representative cell-cycle distribution as determined by FACS analysis for SKOV3 cells treated with 1 μM GSK343 (red) or 0.1% DMSO vehicle control (blue) after 4 d of growth on tissue culture plastic (left) or in Matrigel (right). (B) Quantitation of (A). The percentage of sub-G1, G1, S and G2/M of SKOV3 cells treated with 1 μM GSK343 (black bars) or 0.1% DMSO vehicle control (white bars) after 4 d of growth in 2D monolayer culture. Mean of three independent experiments with SD (C) Same as (B) but for cells cultured in 3D in Matrigel. Mean of three independent experiments with *p < 0.05. (D) Same as (B and C) but stained for Annexin V, a marker of apoptosis. Percentage of Annexin V positive cells was indicated. (E) Quantification of (D). Mean of three independent experiments with SD, *p < 0.05. (F and G) Same as (B and C) but examined for relative HRK expression in the indicated EOC cells treated with 1 μM GSK343 (black bars) or 0.1% DMSO vehicle control (white bars) after 4 d of growth in 3D in Matrigel as determined by q-RT PCR. Numbers represent expression relative to β-2-microglobulin (B2M) expression. Mean of three independent experiments with SD, *p < 0.05.
We next sought to determine the effects of GSK343 on the expression of PRC2/H3K27Me3 target genes in human EOC cells. It has previously been established that HRK, a pro-apoptotic PRC2/H3K27Me3 target gene, plays a key role in regulating apoptosis of EOC cells induced by decreasing H3K27Me3 levels in these cells.18 Thus, we determined the effects of GSK343 on the expression of HRK in 2D vs. 3D cultures. Indeed, we observed a significant upregulation of HRK in EOC cells treated with GSK343 in 3D cultures but not in 2D cultures (Fig. 4F and G). Notably, upregulation of HRK by GSK343 was observed in multiple EOC cell lines (Fig. 4F and G), suggesting that this is not a cell line-specific effect. From these results, we conclude that EZH2 inhibition upregulates the pro-apoptotic H3K27Me3 target gene HRK in 3D cultures.
Discussion
There is no evidence of EZH2 mutation in EOCs based on the recently released The Cancer Genome Atlas (TCGA) ovarian cancer database (http://tcga-data.nci.nih.gov/).19 In addition, based on the TCGA ovarian database, EZH2 gene amplification (> 4 copy) is rare (~2%) in EOC.19 However, EZH2 is often overexpressed in EOCs.7,8,18 In this study, we found that inhibition of EZH2 methyltransferase activity by GSK343 had little effect on the growth of human EOC cells in conventional 2D cultures (Fig. 1). This is consistent with the multiple reports in diffuse large B cell lymphoma (DLBCL), where, although EZH2 inhibitors are similarly effective in decreasing H3K27Me3 levels in both EZH2 wild-type overexpressed and mutant DLBCL cells, EZH2 mutant DLBCL cells are selectively growth-inhibited by EZH2 inhibitors.20-22 Significantly, we show that growth of human EOC cells in 3D Matrigel ECM significantly sensitizes them to the effects of EZH2 inhibition (Fig. 2). In the context of solid tumors, our present study suggests that the ECM in the tumor microenvironment plays an important role in determining the sensitivity of cancer cells to EZH2 inhibitors. Interestingly, EZH2 has been shown to play an active role in ECM remodeling by repressing the expression of ECM-regulating enzymes in prostate cancer cells.23,24 The ECM is known to play a critical role in regulating cell growth, therefore it is plausible that EZH2 inhibitors restrain the growth of tumor cells in 3D by repressing their ability to remodel the ECM.25,26 In addition, integrins are cell-surface receptors that regulate adhesion of tumor cells to the ECM, and certain integrin genes, whose expression is known to suppress cell proliferation, are regulated by EZH2.27 Thus, it is also possible that EZH2 inhibition may alter the interaction between tumor cells and ECM to restrain the proliferation of tumor cells. Regardless, it is likely that EZH2 methyltransferase activity regulates the way cells interact with ECM, which is responsible for the efficacy of EZH2 inhibitors like GSK343 observed in 3D culture.
It has previously been demonstrated that GSK343 displays high clearance rate in pharmacokinetic studies,15 which prevented us from testing its effects in vivo in an animal model. Here we utilized an in vitro 3D model for tumor growth that bridges the gap between animal models and traditional monolayer cultures that lack important determinants of cell growth and treatment response such as the ECM tumor microenvironment.10 In addition, similar effects are also observed using another EZH2 inhibitor, namely GSK926 (data not shown),15 suggesting that the observed effects are not unique to GSK343. Together, these findings suggest that strategies to alter ECM in tumor microenvironment may enhance the tumor-suppressive activity of EZH2 inhibitors. Notably, previous studies have demonstrated that 2D vs. 3D culture conditions lead to different sensitivity of cancer cells to cytotoxic drugs such as PI3K inhibitor LY294002, where cancer cells are more sensitive to cytotoxic drugs in 2D compared with 3D culture conditions.28
Notably, EZH2 inhibition decreases the invasive characteristics of human EOC cells in 3D cultures (Fig. 2A) and in the classical Boyden chamber assay, while it has no significant effects on the migration of these cells (Fig. 3). Consistently, it has previously been demonstrated that EZH2 knockdown suppresses the invasion of human EOC cells, while having no effects on the migration of human EOC cells.7 Together, these data are consistent with the idea that EZH2 plays an important function in regulating how tumor cells interact with the surrounding ECM to promote the invasion of human EOC cells. Thus EZH2 inhibitors may represent a potential therapeutic tool to reverse this phenotype.
EZH2 knockdown will have global effects on a cell in many biological processes. For example, EZH2 knockdown by short hairpin RNA is known to inhibit cell growth, induce apoptosis and suppress invasion of human EOC cells under conventional 2D cultures.7,8 This correlates with a decrease in the level of H3K27Me3 in these cells.7,8 However, we found that EZH2 inhibitor exhibited very limited effects on cell growth and apoptosis of EOC cells in 2D monolayer cultures (Fig. 1; Fig. S1). This suggests that additional methyltransferase-independent function of EZH2 may account for the knockdown phenotype observed under 2D cultures. Significantly, we show that growth in a 3D Matrigel ECM model sensitizes human EOC cells to EZH2 inhibition to induce growth inhibition and apoptosis (Figs. 2 and 4). These results suggest that cancer therapeutics which show little efficacy in conventional 2D models may still have therapeutic benefit within the ECM tumor microenvironment. Together, these findings show that EZH2 methyltransferase activity is required to promote cell growth and suppress apoptosis in the context of the solid tumor microenvironment. Furthermore, they suggest that targeting the ECM in the tumor microenvironment is a novel strategy to enhance the tumor-suppressive effects of EZH2 inhibition. They also imply that EZH2 inhibitors have the potential to be developed as a novel EOC therapeutics, which are urgently needed. Toward this goal, our future studies will investigate how the ECM tumor microenvironment enhances the tumor-suppressive effects of EZH2 inhibitors in EOC. In addition, it will be important to profile the changes in gene expression and, in particular, changes in the expression of direct EZH2 target genes in EOC cells cultured in 2D vs. 3D conditions induced by EZH2 inhibitors. The expression pattern of these genes could serve as potential biomarkers for predicting the response of solid tumors, such as EOC to EZH2 inhibitors.
Materials and Methods
EZH2 inhibitor, antibodies and cell culture
EZH2 methyltransferase inhibitor GSK343 was obtained through the Structure Genomics Consortium (SGC Toronto, M5G1L7). GSK343 was dissolved in DMSO at a stock concentration of 5 mM. Human EOC cell lines OVCAR3, OVCAR5, OVCAR10, UPN 289, SKOV3 and PEO1 cells were described previously7 and were maintained in RPMI-1640 medium, supplemented with 10% fetal bovine serum, penicillin (100 units/mL) and streptomycin (100 μg/mL). The following antibodies from the indicated suppliers were used: anti-EZH2 (BD Biosciences), anti-histone H3 (Millipore), anti-H3K9Me3 (AbCam), anti-H3K27Me3 (Cell Signaling), anti-β-actin (Sigma-Aldrich), anti-Ki-67 (Dako).
Cell proliferation assay
1 x103 cells were plated in triplicate per well in a 12-well plate. Cells were treated the next day, day 0, with RPMI-1640 media supplemented with 10% FBS with either vehicle control (DMSO) or 1 μM of GSK343. Every 3 d for 12 d total, cells were trypsinized and counted using a hemocytometer. At the end of the experiments, the control cells reach ~90% of confluence.
Matrigel invasion assay
BD BioCoat™ Matrigel™ Invasion Chamber was used to measure cell invasion according to manufacturer’s instruction and described previously.7 Briefly, EOC cells were treated with either vehicle control (DMSO) or 1 μM GSK343 for 48 h. Cells were allowed to invade for 48 h toward 5% FBS and 2 ng/ml EGF (Invitrogen) and were subsequently fixed with 4% formaldehyde and stained with 0.05% crystal violet in PBS. The number of cells that migrated across control membrane or invaded through Matrigel-coated membrane was determined in four fields in rectangle across each membrane.
3D cell culture and immunofluorescence staining
3D culture of EOCs was adapted from previously published methods for breast epithelial cell lines.14 Briefly, 40 μL of growth factor reduced-Matrigel (GFR-Matrigel™; BD Biosciences) was pipetted into each well of an 8-well chamber slide (BD Biosciences). Single-cell suspensions of each cell lines (400 μL of 1 × 104 cells/mL) in RMPI-1640 supplemented with 5% FBS, 2 ng/ml of EGF (Invitrogen), 3% Matrigel and either vehicle control (DMSO) or drug was pipetted into each pre-coated well. Matrigel media with either vehicle control (DMSO) or drug was changed every 3 d and cells were grown for 12 d total. Immunofluorescence was performed on day 12 by fixing samples in 2% paraformaldehyde, permeabilizing in 2% paraformaldehyde with 0.5% Triton-X. Samples were incubated with primary antibodies for 2 h at room temperature, highly cross absorbed secondary antibodies (Invitrogen) for 1 h at room temperature and mounted with prolong anti-fade reagent (Invitrogen).
Flow cytometry
Briefly, 1 × 105 cells were either plated directly into each well of a 12-well plate and treated with either vehicle control (DMSO) or 1 μM GSK343 (for 2D), or plated into a well of a 12-well plate that was pre-coated with 120 μl of Matrigel and treated either vehicle control (DMSO), or 1 μM GSK343 (for 3D). Cells were grown in RMPI-1640 supplemented with 5% FBS, 2 ng/ml of EGF Invitrogen), cells in 3D were also supplemented with 3% Matrigel. Cells were analyzed on day 4. To release cells from 3D cultures, cells were treated with ice-cold trypsin for 10 min, and Matrigel was disrupted with a wide orifice pipette. For cell cycle analysis, cells were pelleted by centrifugation at 200 g at 4°C for 5 min, washed with ice cold PBS and fixed in 1 ml of ice-cold 70% ethanol for 60 min. The cells were then centrifuged, washed in 1 ml PBS and resuspended in 0.5 ml propidium iodide solution with RNase (Sigma) and incubated for 30 min at 37°C. Cells were then analyzed for DNA content using a BD LSR14 Flow Cytometer. For Annexin-V analysis, cells were pelleted by centrifugation at 200 g at 4°C for 5 min, washed and resuspended at 2 × 106 cells/ml in fresh media. One hundred μl of cells were then added to three wells of a 96-well plate. Cells were incubated with 100 μl of Guava Nexin Reagent for 20 min at RT and analyzed on the Guava system.
RNA isolation and qRT-PCR
RNA was isolated using Trizol (Invitrogen) according to manufacturer’s instruction. For quantitative real-time PCR (qRT-PCR), Trizol-isolated RNA was further purified using an RNeasy kit (Qiagen) following manufacture's instruction. The primers for HRK genes used for qRT-PCR are: forward: 5′-GCAACAGGTTGGTGAAAACCCT-3′ and reverse: 5′-ATTGGGGTGTCTGTTTCTGCAGC-3′. Expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression.
Supplementary Material
Aknoweldgements
We thank Structure Genomics Consortium for GSK343 and Dr Caretha Creasy at GlaxoSmithKline Pharmaceuticals for critical reading of the manuscript. R.Z. is an Ovarian Cancer Research Fund (OCRF) Liz Tilberis Scholar. This work was supported by the National Cancer Institute of the National Institutes of Health (R01CA163377 to R.Z.) and in part by a DOD ovarian cancer academy award (OC093420 to R.Z.). Support of Core Facilities used in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Glossary
Abbreviations:
- 2D
two-dimensional culture
- 3D
three-dimensional cuture
- DLBCL
diffuse large B cell lymphoma
- ECM
extracellular matrix
- EOC
epithelial ovarian cancer
- EZH2
enhancer of zeste homolog 2
- H3K27Me3
lysine 27 tri-methylated histone H3
- HOSE
human ovarian surface epithelial cells
- PRC2
polycomb repressive complex 2
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/25163
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25163
References
- 1.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 2.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–96. doi: 10.1016/S0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 3.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Atlanta: American Cancer Society Cancer Facts & Figures. 2012.
- 5.Arulkumaran S, Regan L, Farquharson DIM. Obstetrics and gynaecology. Oxford: Oxford University Press, 2011. [Google Scholar]
- 6.Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/S0002-9440(10)63708-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Cai Q, Godwin AK, Zhang R. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res. 2010;8:1610–8. doi: 10.1158/1541-7786.MCR-10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Zhang R. Role of EZH2 in Epithelial Ovarian Cancer: From Biological Insights to Therapeutic Target. Front Oncol. 2013;3:47. doi: 10.3389/fonc.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 12.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 14.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 15.Verma SK, Tian XR, LaFrance LV, Duquenne C, Suarez DP, Newlander KA, et al. Identification of Potent, Selective, Cell-Active Inhibitors of the Histone Lysine Methyltransferase EZH2. ACS Med Chem Lett. 2012;3:1091–6. doi: 10.1021/ml3003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenuwein T. The epigenetic magic of histone lysine methylation. FEBS J. 2006;273:3121–35. doi: 10.1111/j.1742-4658.2006.05343.x. [DOI] [PubMed] [Google Scholar]
- 17.Weaver VM, Gilbert P. Watch thy neighbor: cancer is a communal affair. J Cell Sci. 2004;117:1287–90. doi: 10.1242/jcs.01137. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Cai Q, Wu H, Vathipadiekal V, Dobbin ZC, Li T, et al. SUZ12 promotes human epithelial ovarian cancer by suppressing apoptosis via silencing HRK. Mol Cancer Res. 2012;10:1462–72. doi: 10.1158/1541-7786.MCR-12-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–6. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 21.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 22.Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA. 2012;109:21360–5. doi: 10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS One. 2012;7:e30393. doi: 10.1371/journal.pone.0030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–74. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraro A, Mourtzoukou D, Kosmidou V, Avlonitis S, Kontogeorgos G, Zografos G, et al. EZH2 is regulated by ERK/AKT and targets integrin alpha2 gene to control Epithelial-Mesenchymal Transition and anoikis in colon cancer cells. Int J Biochem Cell Biol. 2013;45:243–54. doi: 10.1016/j.biocel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.