Abstract
Borrelia species comprise a unique genus of bacterial pathogens. These organisms contain a segmented genome with up to two dozen plasmids ranging in size from 5kb up to about 200 kb. The plasmids have also been referred to as mini-chromosomes or essential genetic elements, as some of them carry information important for infection of vertebrates or for survival in the tick vector. Most of the plasmids are linear with covalently closed hairpin telomeres and these linear plasmids are in a constant state of genetic rearrangement. The mechanisms of plasmid replication, maintenance and partitioning remain largely obscure and are complicated by a long doubling time, the requirement for expensive media and inefficient genetic manipulation. A set of five parologous protein families (PFs) are believed to confer the ability for autonomous replication and plasmid maintenance. The number of plasmids also complicates analyses because of the possibility that PFs from one plasmid may sometimes function in trans on other plasmids. Two papers in the last year have moved the field forward and their combined data suggest that trans complementation amongst Borrelia plasmids may sometimes occur.
Keywords: plasmid replication, plasmid maintenance, Lyme disease
Introduction
Borrelia species are important human pathogens (Samuels and Radolf, 2010) causing either Lyme borreliosis (Radolf et al., 2012; Stanek et al., 2011; Steere et al., 2004) or relapsing fever (Barbour, 2006; Dworkin et al., 2008). These spirochetes display a variety of unusual features, one of which is that Borrelia species carry more plasmids than reported for any other type of bacteria. Plasmid compatibility and maintenance (Reyes-Lamothe et al., 2012) in this genus appears to be at the pinnacle of evolutionary development, although our understanding of the process is still in its early stages with most of our information coming from studies on Lyme borreliae. The type strain B. burgdorferi B31, carries 22 plasmids (Casjens et al., 2000; Fraser et al., 1997; Miller et al., 2000). The plasmids are a mix of circular and linear forms and exist in low copy number (Beaurepaire and Chaconas, 2005; Chaconas, 2010; Hinnebusch and Barbour, 1992). Most of the circular plasmids (those in the cp32 family) appear to be prophages or prophage derived (Damman et al., 2000; Eggers et al., 2001; Eggers and Samuels, 1999). The linear plasmids are terminated by covalently closed hairpin telomeres (Barbour and Garon, 1987; Casjens et al., 1997; Hinnebusch and Barbour, 1991; Tourand et al., 2009), which are generated from replicative intermediates by a specialized enzyme (Chaconas et al., 2001; Kobryn and Chaconas, 2002), the telomere resolvase, ResT (see (Chaconas and Kobryn, 2010)). Moreover, the linear plasmids vary in size and DNA content from strain to strain with many gene duplications and DNA rearrangements present near the plasmid telomeres (Casjens et al., 2000; Casjens et al., 2012). The DNA sequence scrambling on the linear plasmids is believed to occur through telomere fusions generated by ResT (Chaconas, 2005; Chaconas, 2010; Chaconas and Kobryn, 2010; Kobryn and Chaconas, 2005).
A number of genes essential for survival or required for the tick-vertebrate lifecycle are carried on Borrelia plasmids (see (Norris et al., 2010)). For this reason the plasmids have also been referred to as mini-chromosomes (Barbour and Zückert, 1997) or essential genetic elements (Stewart et al., 2005), which seem to be reasonable alternative descriptors for the term plasmids in Borrelia. The reason for the segmented genome and for the very large plasmid complement typically found in Borrelia species, as well as the molecular details of how the plasmids are replicated and partitioned, remain largely unknown (Chaconas, 2010; Chaconas and Kobryn, 2010). Genetic experiments in borreliae are slow and arduous (Brisson et al., 2012; Rosa et al., 2010) and the study of plasmid maintenance genes is complicated by the unanswered question of whether these genes can function in trans, providing complementation of defects on other plasmids. Biochemical studies through the use of replication extracts have also been hindered by the requirement of expensive media (Barbour, 1984) to grow an organism that reaches stationary phase at about 1 × 108 cells per ml. There has been little elucidation of plasmid maintenance mechanisms in B. burgdorferi in the last decade or more. However, the last year has seen two important advances (Lin et al., 2012; Tilly, Checroun et al., 2012) in studying plasmid maintenance in B. burgdorferi. These works will be summarized and the results contrasted and discussed.
Plasmid Maintenance Genes
A set of related, but not identical, plasmid maintenance genes is carried on all Borrelia plasmids (Casjens et al., 2000). Most plasmids carry an entire set of four genes, usually in a clustered array; however, a few of the smaller plasmids carry incomplete sets. These genes are present on DNA regions from several circular and linear plasmids that are capable of conferring autonomous replication (Beaurepaire and Chaconas, 2005; Byram et al., 2004; Dulebohn et al., 2011; Eggers et al., 2002; Stewart et al., 2003; Stewart et al., 2001). These genes encode five paralogous families (PFs) of proteins entitled PF32, PF49, PF50, PF57, and PF62 (Casjens et al., 2000; Fraser et al., 1997). The predicted sizes of the PF32, PF49, and PF50 proteins are relatively small (30, 23, and 23 kDa average MW, respectively), whereas the PF57 and PF62 predicted products are somewhat larger (45 and 42 kDa on average). While all Borrelia species (including Lyme disease and relapsing fever organisms) contain orthologs of these protein families, no significant homology is found in other organisms (except for the occurrence of conserved motifs such as a Walker box ATPase domain and magnesium binding region in the PF 32 family). All of these predicted proteins are basic (with isoelectric points ranging from 8.1 to 10.9), consistent with the properties of DNA-binding proteins. The proteins seem to be “plasmid-specific”, as indicated by divergence among PF members within a strain. For example, the nearest paralog to the lp28-3 associated PF32 gene product in B. burgdorferi B31 is only 69% identical; however, a close ortholog with 96% sequence identity is found in B. garinii Far04, as well as other diverse Borrelia species and strains. These results indicate that the PF family homologs have been ‘specialized’ to permit parallel but independent maintenance of coexisting plasmids, and that these specialized properties have been highly conserved over millions of years of Borrelia species divergence.
PF57/62
The distantly-related family 57 and 62 genes (Casjens et al., 2000) are each believed to encode a replication initiator protein, and every plasmid carries one of these genes. Their apparent duplicity of function coupled with their limited homology has led to their grouping together as PF57/62. Members of this dual family group confer the ability for autonomous replication (Beaurepaire and Chaconas, 2005; Eggers et al., 2002; Stewart et al., 2003; Stewart et al., 2001). The PF62 protein from lp17 has been purified, but its only activity identified thus far is the ability to physically interact with the PF32 family member from lp17 (Deneke and Chaconas, 2008).
PF32
A PF 32 member is found on every plasmid except lp5 and cp9. This is the only family with any sequence homology to known plasmid maintenance proteins. Because of the presence of a Walker box ATPase domain (Casjens et al., 2000; Zückert and Meyer, 1996) the PF32 family of proteins has been referred to as ParA proteins. However, neither parB nor clearcut parS sequences have been identified on Borrelia plasmids. The purified protein from lp17 does display ATPase activity and the ability to interact with the lp17 PF62 protein (Deneke and Chaconas, 2008). At this time, a role of P32 proteins in partitioning remains to be established; thus the ParA designation should be avoided as premature and potentially inaccurate.
PF49
PF49 members are found on all B. burgdorferi plasmids except lp5 and lp17. The role of PF49 in plasmid maintenance remains unknown. Recent studies have shown that the PF49 member from a cp32 plasmid is involved in modulating the transcription of other cp32 genes, including the Erp family of surface lipoproteins, a nuclease, and a single-stranded binding protein (Burns et al., 2010; Chenail et al., 2012; Jutras et al., 2011). The purified PF49 protein binds upstream of all three regulated genes in vitro. These findings raise the possibility that the role of PF49 members in plasmid maintenance is a gene regulatory role, however, further studies will be required to investigate this possibility. Unfortunately, the characterized cp32 PF49 member was given the confusing misnomer of “BpaB” for Borrelia ParB analogue (Burns et al., 2010). There is no sequence similarity or functional resemblance between the conserved ParB family of proteins and the Borrelia PF49 members; hence the BpaB designation is both confusing and likely incorrect.
PF50
A PF50 member exists on all B. burgdorferi plasmids except for lp5, lp17 and lp21. The role of PF50 in plasmid maintenance remains obscure.
A heterologous system to study B. burgdorferi plasmid cp26 maintenance
As a means of eliminating the concerns about trans complementation of Borrelia plasmid maintenance functions, Tilly, Checroun, and Rosa (Tilly, Checroun et al., 2012) have recently used a heterologous system for studying the Borrelia plasmid maintenance functions in E. coli. Although Borrelia plasmids do not autonomously replicate in E. coli, these authors were successful in studying the maintenance functions of the essential 26 kb circular plasmid (cp26) by cloning them into a mini-F lacking the sopABC partitioning genes (Fig. 1). This new approach to studying Borrelia plasmid maintenance proteins is a very welcome addition to the limited arsenal of genetic tools available to study this complex organism (Rosa et al., 2005). Transformations in B. burgdorferi take about two weeks and require large amounts of DNA. This limitation, coupled with concerns over the possibility of interplasmidic complementation, make a heterologous system extremely attractive.
Fig. 1.
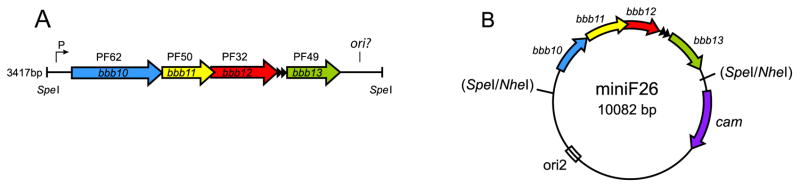
Heterologous system to study B. burgdorferi plasmid maintenance genes. A) The plasmid maintenance region of B. burgdorferi plasmid cp26. The region is composed of the PF62, 50, 32 and 49 members bbb10, bbb11, bbb12 and bbb13, respectively. A possible replication origin (ori?) and parS-type sequence (arrowheads) may also be present. B) The maintenance genes inserted into a sopABC-deficient mini-F. This figure is adapted from (Tilly, Checroun et al., 2012).
The mini-F system has been used previously to study partition functions from other bacterial species (Dubarry et al., 2006; Godfrin-Estevenon et al., 2002). Tilly, Checroun and Rosa (Tilly, Checroun et al., 2012) have now reported that the cp26 maintenance region from B. burgdorferi was capable of stabilizing the partition-deficient mini-F plasmid in the absence of selection, thereby allowing further characterization of the genes required in the absence of any other B. burgdorferi plasmids. A series of deletion mutants in the B. burgdorferi maintenance region was generated and tested in the mini-F system. The results of this analysis showed that PF62, 50 and 49 were required but that PF32 was dispensable. To confirm this finding, it was shown that PF32 on cp26 could be disrupted in B. burgdorferi (with no noticeable change in plasmid maintenance). In contrast, a mutation in PF62 could not be generated, most likely due to loss of cp26 replication resulting in the loss of several essential genes. Interestingly, PF32 is the protein family with the Walker box ATPase domain that has been previously referred to as the Borrelia ParA orthologue. Its expendability both in E. coli and B. burgdorferi was unexpected, casting further doubt about its assignment as a ParA orthologue. Because replication of the mini-F is not driven by B. burgdorferi proteins and PF32 physically interacts with the replication initiator protein PF62, perhaps the function of PF32 proteins is limited to DNA replication and not partitioning. The expendability of PF32 on cp26 in B. burgdorferi may be due to in trans complementation by a PF32 paralog on another plasmid.
Transposon mutagenesis of B. burgdorferi plasmid cp26
To study a variety of B. burgdorferi functions, Lin and coworkers prepared and analyzed a transposon insertion library (Lin et al., 2012). This long-term endeavor in this slow-growing and genetically difficult organism resulted in the sequence-based mapping of almost 4,500 insertion mutants. In cp26, 307 transposon mutants at 250 unique sites were recovered (Fig. 2). Insertions were recovered throughout the plasmid with the exception of three areas: the telomere resolvase gene resT gene, the bbb25-26 region and the replication/maintenance genes encoding PF62 and 50. The resT gene has been previously shown to be essential (Byram et al., 2004; Tourand et al., 2006) as has the bbb25-26 region (Jewett et al., 2007). Transposon insertions in the PF62 and PF50 plasmid maintenance genes were not isolated; however, multiple insertions were recovered in both PF32 and PF49. The results are in agreement with those obtained in the heterologous E. coli system (Tilly, Checroun et al., 2012), except for PF49: insertional disruption of this gene occurred in B. burgdorferi, indicating that it is not required. However, it was required for mini-F stabilization in E. coli. How then do we explain the difference in results between these two systems. Once again, the simplest, but unproven explanation may be that PF49 activity is provided to cp26 by a paralogous gene in another plasmid.
Fig. 2.
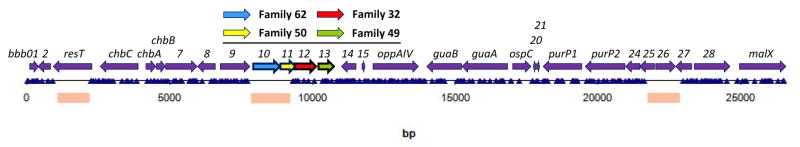
Insertion mutation map of cp26. The positions of transposon insertion mutants are shown by the blue triangles. The genetic map of the plasmid is shown above and the positions of genes that lack insertion mutations is shown below by the pink rectangles. This figure is reprinted from (Lin et al., 2012).
Transposon mutagenesis of B. burgdorferi plasmids
Transposon insertions were also sequenced on the full complement of plasmids carried in the B. burgdorferi strain to make the insertion library (Lin et al., 2012). Fig. 3 shows a summary of the gene disruptions obtained. Several important features have emerged from this collection of data:
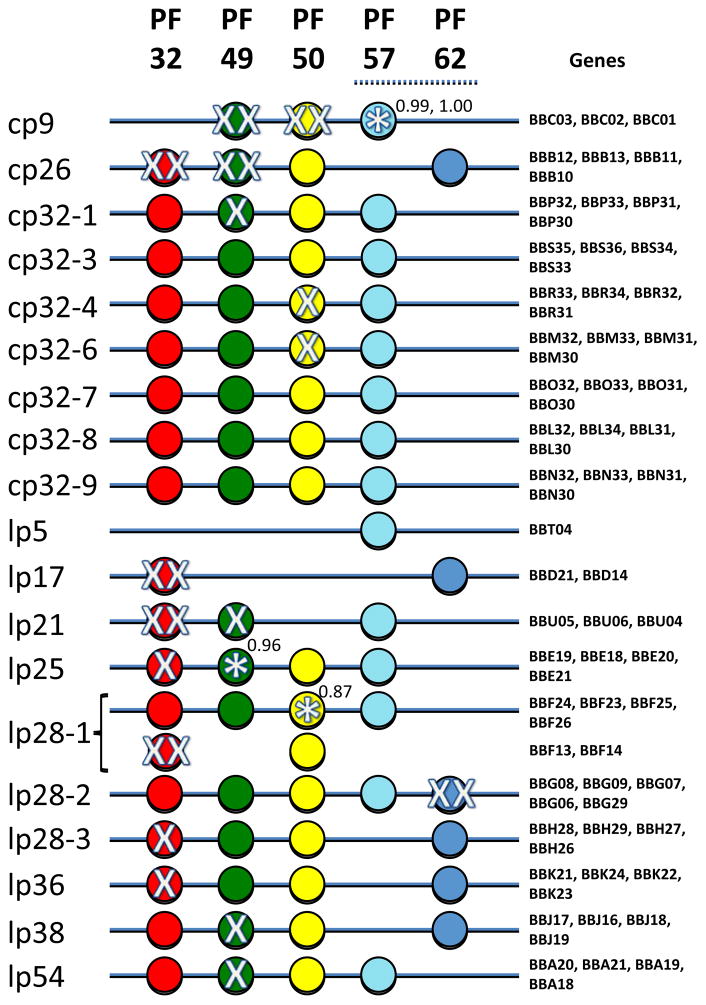
Fig. 3.
Transposon insertion mutations in plasmid maintenance genes. The paralogous family members are listed across the top and represented by colored circles (the position of each circle is not meant to depict the order or relative location). The individual genes for each plasmid are listed on the right hand side of the figure in the same order as for the colored circles. Single insertion mutations in a given gene are indicated by a single X and multiple insertions by XX. An asterisk indicates an insertion near the end of the gene. This figure is reprinted from (Lin et al., 2012).
As expected for a gene encoding a replication initiator protein, no insertions were observed in any PF57 members with the exception of 2 insertions within the last 5 codons of PF57 on cp9. Similarly, no insertions were observed in any PF62 members; the single exception was the PF62 member on lp28-2. This is an orphan PF32 gene (BBG29) that picked up 6 insertions. It is located over 20,000 bp from the cluster of 4 replication/maintenance proteins (including another PF62 paralog) and appears to be an unused remnant from an ancestral fusion with another plasmid.
Insertions seem to be more readily tolerated in some genes when a full set of 4 PF members are not present (cp9, lp17, lp21). The absence of a full set of replication maintenance proteins or sites may perturb the normal requirements, or these plasmids may be complemented by functions from other plasmids.
The cp32 family of plasmids was refractory to insertions in any of the four genes, with a single insertion recovered in PF49 on cp32-1 and single insertions in PF50 on cp32-4 and cp32-6. Hence, all four PF families appear to be required for replication of these plasmids. This result is surprising, however, because the cp32 plasmids have large regions of near sequence identity (including the putative plasmid maintenance genes), so cross-complementation would be expected to occur.
The linear plasmids are exceedingly tolerant of insertions into PF32 while the circular cp32’s did not tolerate even a single insertion. This finding may point to some fundamental difference in the maintenance functions required for the circular and linear plasmid forms, in addition to the ResT requirement for linear replicons. The exceptions to this dichotomy are cp26, which is believed to be derived from a linear plasmid, and cp9, which is a truncated version of a cp32. An alternative explanation is that complementation of the PF32 proteins in trans on linear plasmids has evolved to be a more permissive process.
In summary thus far, the vast majority of B. burgdorferi plasmids carry a full set of four plasmid replication/maintenance genes (exceptions are the smaller plasmids lp21, lp17, lp5 and cp9). In spite of the preponderance of full length copies of all four PF families on these plasmids, a few members of PF32, 49 and 50 appear to be unnecessary for in vitro cultivation based upon insertion mutagenesis. One possible explanation may be that certain PF members can be provided in trans by another plasmid in the cell. Yet another is that in vitro cultivation conditions are not an accurate representation of conditions normally encountered by B. burgdorferi in the mouse host or tick vector and that during a normal lifecycle there is a more stringent requirement for the four PF families in either plasmid replication or some other function (see below). However, the expendability of members of certain PF families but not others during growth in culture remains unexplained.
Plasmid maintenance genes and mouse infection
In the recent paper by Lin and coworkers (Lin et al., 2012), the effects of transposon insertions on the infectivity of B. burgdorferi was analyzed using a signature-tagged mutagenesis approach. Briefly, eleven Himar1 transposon constructs containing a gentamicin resistance cassette were developed so that each contained a different 7-bp ‘tag’ that could be recognized by hybridization or PCR methodologies. Libraries of mutants containing these sequences (Tags 1 through 11) were developed in the strain 5A18NP1, an infectious, moderately transformable derivative of the commonly used strain, B. burgdorferi B31 (Kawabata et al., 2004). Mutant clones containing each of the 11 tags could then be mixed together, inoculated into mice, and detected individually using a tag-specific, Luminex-based PCR detection method (Lin et al., 2012).
An unexpected finding in these studies was that most of the clones with mutations in PF32, PF49, or PF50 plasmid maintenance genes returned low Luminex signals two weeks or four weeks after needle inoculation of the mice. This pattern was observed regardless of whether the transposon was inserted in a plasmid known to be required for full infectivity (e.g. lp25, lp28-1, or cp26) or in a plasmid not required for mouse infection (such as lp17 or lp21). As expected, insertion in the ‘extra’ copy of the gene encoding PF32 on lp28-1 (bbf13) did not reduce the signal. Possible explanations for these results include 1) the plasmid containing the mutation is lost spontaneously because of the lack of gentamicin selection pressure during mouse infection (the mutants are maintained in vitro in gentamicin-containing medium); 2) the plasmids replicate poorly in the mammalian tissue environment when these mutations are present, resulting in loss of signal (despite survival of the organism); and 3) the mutations in PF genes result in loss of fitness in mouse tissue, because of uncharacterized secondary function(s) of the PF products. While preliminary studies point to the first two explanations (T. Lin, L. Gao, S. Peffer, and S. J. Norris, unpublished results), additional studies are needed to address these possibilities.
Concluding remarks
In conclusion, it appears that an ancient ancestor of modern Borrelia organisms evolved a novel plasmid maintenance system that has been preserved in all the currently characterized species. The source of this system is not known; an intriguing possibility is that it was introduced by the bacteriophage(s) that gave rise to the cp32 plasmids (see also (Chaconas and Kobryn, 2010)). The two articles featured in this minirevew (Lin et al., 2012; Tilly, Checroun et al., 2012) support the hypothesis that the PF32, PF49, PF50, PF57 and PF62 protein families play important roles in the mediation of plasmid replication and partitioning. The multiple plasmids present in a given Borrelia strain all share at least a subset of the genes encoding these protein families, but there is significant divergence among the paralogs found on different plasmids. It appears that this divergence confers “replicon-specific” properties that involve interaction of the PF proteins with cognate DNA sequences and perhaps other PF proteins in the same plasmid. Nonetheless, data from the two papers reviewed here suggests that some PF members may be capable of in trans complementation to provide a helping hand to other plasmids. The recently reported miniF heterologous complementation system (Tilly, Checroun et al., 2012) provides an important avenue for ‘sorting out’ the protein domains and DNA sequences that are involved in this process. Information obtained from this and other approaches will be useful in understanding the peaceful coexistence of so many replicons in a small, relatively simple organism. Indeed, it is a marvel that Borrelia species are able to maintain nearly as many replicons as found in humans, despite having 2,000-fold less DNA.
Highlights.
Borrelia species carry more plasmids than reported for any other type of bacteria
The plasmids come in two flavors: circular and linear with hairpin ends
Five parologous protein families confer replication and maintenance functions
Recent work suggests that trans complementation amongst plasmids may sometimes occur
Acknowledgments
The authors would like to thank Kit Tilly and Patti Rosa for helpful comments on the manuscript. G.C. holds a Canada Research Chair in the Molecular Biology of Lyme Borreliosis (http://www.chairs-chaires.gc.ca/home-accueil-eng.aspx) and a Scientist Award from Alberta Innovates – Health Soultions (http://www.ahfmr.ab.ca/). Work in his laboratory is supported by grant MOP 53086 from the Canadian Institutes of Health Research (http://www.cihr-irsc.gc.ca/e/193.html). S. J. N.’s involvement in this work was supported by Grant Number AI 059048 from the United States National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–5. [PMC free article] [PubMed] [Google Scholar]
- Barbour AG. Relapsing Fever. In: Goodman JL, et al., editors. Tick-Borne Diseases of Humans. ASM Press; Washington, D.C: 2006. [Google Scholar]
- Barbour AG, Garon CF. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–11. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Zückert WR. Genome sequencing. New tricks of a tick-borne pathogen. Nature. 1997;390:553–554. doi: 10.1038/37475. [DOI] [PubMed] [Google Scholar]
- Beaurepaire C, Chaconas G. Mapping of essential replication functions of the linear plasmid lp17 of B.burgdorferi by targeted deletion walking. Mol Microbiol. 2005;57:132–42. doi: 10.1111/j.1365-2958.2005.04688.x. [DOI] [PubMed] [Google Scholar]
- Brisson D, et al. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46:515–36. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LH, et al. BpaB, a novel protein encoded by the Lyme disease spirochete’s cp32 prophages, binds to erp Operator 2 DNA. Nucleic Acids Res. 2010;38:5443–55. doi: 10.1093/nar/gkq284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byram R, et al. The essential nature of the ubiquitous 26-kilobase circular replicon of Borrelia burgdorferi. J Bacteriol. 2004;186:3561–9. doi: 10.1128/JB.186.11.3561-3569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, et al. Telomeres of the linear chromosomes of Lyme disease spirochaetes: nucleotide sequence and possible exchange with linear plasmid telomeres. Mol Microbiol. 1997;26:581–96. doi: 10.1046/j.1365-2958.1997.6051963.x. [DOI] [PubMed] [Google Scholar]
- Casjens S, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- Casjens SR, et al. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One. 2012;7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas S. Hairpin telomeres and genome plasticity in Borrelia: all mixed up in the end. Molecular Microbiology. 2005;58:625–635. doi: 10.1111/j.1365-2958.2005.04872.x. [DOI] [PubMed] [Google Scholar]
- Chaconas G. Replication of the B. burgdorferi genome and scrambling of the linear replicons through reverse telomere resolution. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Horizon Scientific Press; Norwich, UK: 2010. [Google Scholar]
- Chaconas G, Kobryn K. Structure, function, and evolution of linear replicons in Borrelia. Annu Rev Microbiol. 2010;64:185–202. doi: 10.1146/annurev.micro.112408.134037. [DOI] [PubMed] [Google Scholar]
- Chaconas G, et al. Telomere resolution in the Lyme disease spirochete. EMBO J. 2001;20:3229–37. doi: 10.1093/emboj/20.12.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenail AM, et al. Borrelia burgdorferi cp32 BpaB modulates expression of the prophage NucP nuclease and SsbP single-stranded DNA-binding protein. J Bacteriol. 2012;194:4570–8. doi: 10.1128/JB.00661-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damman CJ, et al. Characterization of Borrelia burgdorferi BlyA and BlyB proteins: a prophage-encoded holin-like system. J Bacteriol. 2000;182:6791–7. doi: 10.1128/jb.182.23.6791-6797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke J, Chaconas G. Purification and properties of the plasmid maintenance proteins from the Borrelia burgdorferi linear plasmid lp17. J Bacteriol. 2008;190:3992–4000. doi: 10.1128/JB.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubarry N, et al. ParABS systems of the four replicons of Burkholderia cenocepacia: new chromosome centromeres confer partition specificity. J Bacteriol. 2006;188:1489–96. doi: 10.1128/JB.188.4.1489-1496.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulebohn DP, et al. Borrelia burgdorferi linear plasmid 38 is dispensable for completion of the mouse-tick infectious cycle. Infect Immun. 2011;79:3510–7. doi: 10.1128/IAI.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin MS, et al. Tick-borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–68. viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CH, et al. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochete. Mol Microbiol. 2002;43:281–295. doi: 10.1046/j.1365-2958.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- Eggers CH, et al. Transduction by φBB-1, a bacteriophage of Borrelia burgdorferi. J Bacteriol. 2001;183:4771–8. doi: 10.1128/JB.183.16.4771-4778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CH, Samuels DS. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J Bacteriol. 1999;181:7308–13. doi: 10.1128/jb.181.23.7308-7313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Godfrin-Estevenon AM, et al. The parAB gene products of Pseudomonas putida exhibit partition activity in both P. putida and Escherichia coli. Mol Microbiol. 2002;43:39–49. doi: 10.1046/j.1365-2958.2002.02735.x. [DOI] [PubMed] [Google Scholar]
- Hinnebusch J, Barbour AG. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991;173:7233–9. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J, Barbour AG. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992;174:5251–7. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MW, et al. Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Mol Microbiol. 2007;66:975–90. doi: 10.1111/j.1365-2958.2007.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras BL, et al. BpaB and EbfC DNA-binding proteins regulate production of the Lyme disease spirochete’s infection-associated Erp surface proteins. J Bacteriol. 2011;194:778–86. doi: 10.1128/JB.06394-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, et al. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun. 2004;72:7147–54. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobryn K, Chaconas G. ResT, a telomere resolvase encoded by the Lyme disease spirochete. Mol Cell. 2002;9:195–201. doi: 10.1016/s1097-2765(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Kobryn K, Chaconas G. Fusion of hairpin telomeres by the B. burgdorferi telomere resolvase ResT: Implications for shaping a genome in flux. Mol Cell. 2005;17:783–91. doi: 10.1016/j.molcel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Lin T, et al. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, et al. A second allele of eppA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9-2. J Bacteriol. 2000;182:6254–8. doi: 10.1128/jb.182.21.6254-6258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ, et al. Pathobiology of Lyme disease Borrelia. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Horizon Scientific Press; Norwich, UK: 2010. [Google Scholar]
- Radolf JD, et al. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012 doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R, et al. Chromosome replication and segregation in bacteria. Annu Rev Genet. 2012;46:121–43. doi: 10.1146/annurev-genet-110711-155421. [DOI] [PubMed] [Google Scholar]
- Rosa PA, et al. Genetic manipulation of B. burgdorferi. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. [Google Scholar]
- Rosa PA, et al. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3:129–43. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- Samuels DS, Radolf JD. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. p. 547. [Google Scholar]
- Stanek G, et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- Steere AC, et al. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, et al. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid. 2005;53:1–13. doi: 10.1016/j.plasmid.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Stewart PE, et al. Conservation of plasmid maintenance functions between linear and circular plasmids in Borrelia burgdorferi. J Bacteriol. 2003;185:3202–9. doi: 10.1128/JB.185.10.3202-3209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, et al. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001;39:714–721. doi: 10.1046/j.1365-2958.2001.02256.x. [DOI] [PubMed] [Google Scholar]
- Tilly K, Checroun C, et al. Requirements for Borrelia burgdorferi plasmid maintenance. Plasmid. 2012;68:1–12. doi: 10.1016/j.plasmid.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourand Y, et al. Differential telomere processing by Borrelia telomere resolvases in vitro but not in vivo. J Bacteriol. 2006;188:7378–86. doi: 10.1128/JB.00760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourand Y, et al. Characterization and in vitro reaction properties of 19 unique hairpin telomeres from the linear plasmids of the Lyme disease spirochete. J Biol Chem. 2009;284:7264–72. doi: 10.1074/jbc.M808918200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zückert WR, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–98. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]