Abstract
Bacteria and Archaea encode clustered, regularly interspaced, short palindromic repeat (CRISPR) systems to confer adaptive immunity to invasive viruses and plasmids. Recent studies of CRISPR systems revealed that diverse CRISPR-associated (Cas) interference modules often coexist in different organisms but functions of cas genes have not been dissected in any of these systems. The crenarchaeon Sulfolobus islandicus encodes three distinct CRISPR interference modules, including a type IA system and two type IIIB systems: Cmr-α and Cmr-β. To study the genetic determinants of protospacer-adjacent motif (PAM)-dependent DNA targeting activity and mature CRISPR RNA (crRNA) production in this organism, mutants deleting individual genes of the type IA system or removing each of other Cas modules were constructed. Characterization of these mutants revealed that Cas7, Cas5, Cas6, Cas3′ and Cas3” are essential for PAM-dependent DNA targeting activity, whereas Csa5, along with all other Cas modules, is dispensable for the targeting in the crenarchaeon. Cas6 is implicated as the only enzyme for pre-crRNA processing and the crRNA maturation is independent of the DNA targeting activity. Importantly, we show that Cas7 and Cas5 are essential for stabilizing the processing intermediates and mature crRNAs, respectively, and that depleting the helicase or nuclease domain of Cas3 leads to the accumulation of processing intermediates. This demonstrates that in addition to Cas6, other Cas proteins of an archaeal type IA system also contribute to crRNA processing.
Keywords: CRISPR/Cas, Cascade, protospacer-adjacent motifs, crRNA biogenesis, DNA interference, cas mutants, Sulfolobus islandicus
Introduction
Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas) proteins constitute the adaptive and inheritable immune system in almost all archaea and more than 40% of bacteria. The antiviral nature of these systems was predicted based on bioinformatic analyses of bacterial and archaeal genomes and their genetic elements, which predicted that unique DNA sequences (spacers) of CRISPR arrays were derived from invasive genetic elements1-3 and it was further hypothesized that the defense could occur via an RNA interference-like mechanism.4 Subsequently, in vivo CRISPR activity has been studied in different microorganisms demonstrating that immune response by CRISPR/Cas systems exerts through three distinct stages5-10: (1) Pieces of DNA sequence of invading genetic elements are incorporated into a CRISPR locus at a position between the leader and the first spacer, generating new spacers; (2) Transcription of CRISPR arrays from their leaders gives precursor CRISPR RNA molecules (pre-crRNAs) that are processed to yield mature CRISPR RNAs (crRNAs) and (3) crRNAs and Cascade (CRISPR-associated complex for antiviral defense) form ribonucleoprotein complexes, exerting silencing of invasive nucleic acids.
CRISPR/Cas systems are very diverse and their reevaluation has led to the classification of three main classes, type I, II and III, which are further divided into subgroups.7 Recently, Cas1 and Cas2, the only universally conserved Cas proteins,4,7,11 formed a complex with Csa1 and Cas4 in Thermoproteus tenax, which was implicated in acquisition of new spacers, and for this reason they were classified as a new Cas module, CRISPR-associated cluster for integration of new spacers (Cascis).12 Following the first demonstration of spacer acquisition in Streptococcus thermophilus,13 insertion of new spacers between the leader sequence and the first spacer of CRISPR arrays has been shown in Escherichia coli14-16 and Sulfolobus solfataricus.17 Moreover, it has been shown that Cas1 and Cas2 are sufficient to yield spacer insertion in E. coli.14
Small RNA products generated from CRISPR arrays were first detected a decade ago in Archaeoglobus fulgidus18 and later were found to be widespread in microorganisms. CRISPR arrays often consist of a large cluster of spacer-repeat units. Transcription of an entire CRISPR array gives pre-crRNA to be processed into effector crRNAs.19-22 Two distinct mechanisms are known to generate crRNAs. The first is endonuclease Cas6, a processing enzyme for type I and type III CRISPR systems,23-27 which binds to a repeat of a pre-crRNA and cleaves it. In fact, the enzyme remains bound to the RNA after cleavage.23,28 The second is RNase III that does the cleavage under the guidance of a trans-acting tracrRNA in type II systems.29
At the final stage, it is believed that Cascade complexes containing crRNAs recruit Cas3 helicase/nuclease and exert invader silencing,25,30-32 during which a target nucleic acid is recognized by complementarity between the protospacer and the corresponding effector crRNA. DNA targeting activity has been demonstrated for representative systems belonging to each of the main types of CRISPR systems, including the E. coli type IE crRNA-Cascade ribonucleoprotein complex in vitro,33 DNA targeting activity of type II systems in S. thermophilus in vivo34 and in vitro35 and the Staphylococcus epidermidis type IIIA system Csm.36 Furthermore, P. furiosus and S. solfataricus type IIIB CRISPR repeat-associated mysterious proteins (RAMP),11 also named Cmr modules, mediate RNA targeting activity.32,37,38
An important point in the antiviral defense is how CRISPR systems distinguish self vs. non-self DNA. Most known CRISPR systems recognize an invasive genetic element by identifying a protospacer-adjacent motif (PAM) located either at 5′- or at 3′-flanking position to a protospacer of the genetic element.9 PAM motifs were first recognized from genome sequence analyses,20,39 and subsequently experimentally demonstrated by virus infection in bacteria40,41 or by interference plasmid assay in archaea.42-44 The other type of self vs. non-self discrimination involves repeat protection of chromosome-encoded CRISPR arrays from the DNA interference by the Csm module in S. epidermidis.45
All known Sulfolobus strains encode multiple CRISPR interference modules belonging to type IA or type IIIA or IIIB, whereas the number and types of modules carried in each strain vary from one to another.46 In the recently completed genome of S. islandicus REY15A,47 there are two arrays of CRISPR elements with the same repeat, a Cascis locus with five genes, a type IA CRISPR interference system and two distinct type IIIB Cmr systems (Fig. 1). This organism is one of the genetically tractable archaeal models48 with which a novel gene knockout method has been established49 and plasmid interference assays were developed for testing interference activities.42,50 This has enabled us, for the first time, to conduct genetic analyses of cas gene functions in a microbe carrying complex CRISPR/Cas systems. Deletion mutants lacking Cascis, Cmr-α or Cmr-β module and those deleting each of the genes encoding components of the putative IA-Cascade complex were constructed and analyzed pre-crRNA processing and CC PAM motif-mediated targeting. We show that type IA module is responsible for the PAM-dependent interference. Evidence has been provided to demonstrate that other Cas proteins also play a role in modulating pre-crRNA processing by Cas6.
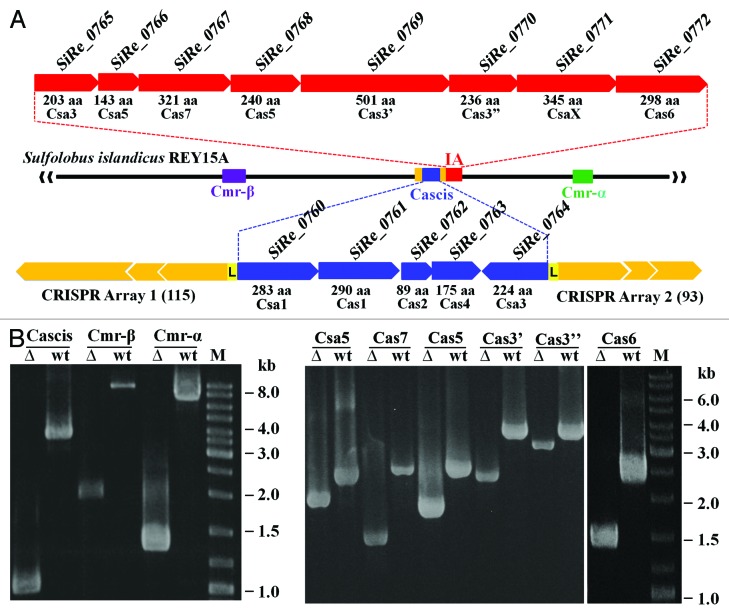
Figure 1.S. islandicus Cas modules and construction knockouts lacking a Cas module or a cas gene. (A) Schematic of CRISPR arrays and Cas modules in S. islandicus REY15A. (B) PCR verification of mutant alleles of Cas modules and cas genes in the mutants constructed with the MID procedure, described previously.66 PCR was conducted with specific primers for each Cas module or cas gene with genomic DNAs extracted from the genetic host S. islandicus E233S (wt) and corresponding deletion mutant (∆). M, DNA size marker. Cascis, CRISPR-associated cluster for integration of new spacers; IA, type IA interference module; Cmr-α and Cmr-β, two type IIIB modules containing 6 and 7 cmr genes, respectively.47
Results
Cascis, Cmr-α and Cmr-β modules are dispensable for pre-crRNA processing and CC PAM motif DNA targeting
Previously, it was shown that CRISPR arrays were transcribed and processed into short RNA molecules in different Sulfolobus species20,44,51 and that Sulfolobus CRISPR systems conferred DNA targeting activity.42 However, as Sulfolobus contain multiple Cas interference modules, functions of each Cas module and individual cas genes in these molecular processes have not been studied. Here, we systematically studied possible functions of four Cas modules in S. islandicus REY15A, i.e., a type IA interference module, a Cas cluster for integration of new spacers (Cascis) and two type IIIB modules Cmr-α and Cmr-β modules (Fig. 1A). Mutant strains lacking each entire module of Cascis, Cmr-α or Cmr-β were constructed, from which total RNAs were prepared and subsequently analyzed by northern hybridization as described in Materials and Methods.
Results of the northern hybridization of RNAs prepared from the wild-type strain were very similar to those of the northern analyses of the mutants (Fig. 2A and B). The radiolabeled repeat oligonucleotide probe hybridized to RNAs of 60–70 nt in size. These RNAs are comparable in size with the mature crRNAs produced by an S. solfataricus Cas6, the primary product of pre-crRNAs was processed by Cas6, yielding spacers carrying 8 nt 5′-handle and 16 nt 3′-handle of the repeat.25 A few processing intermediates were also hybridized by the repeat probe showing the sizes equivalent to the RNAs carrying 3, 5 and 7 spacers (Fig. 2A). When analyzed with an oligonucleotide of array 2 spacer 32 as probe, an RNA of ca. 62 nt as well as RNAs < 60 nt were identified (Fig. 2B). The former could represent a primary processing product of Cas6 cleavage and the latter should be resulted from further processing/maturation of the primary product. Again, the probe identified the same RNA bands in ∆Cascis, ∆Cmr-α and ∆Cmr-β, comparing with the wild-type strain except for the smallest crRNA species (ca. 40 or 41 nt) that is absent from ∆Cmr-α (Fig. 2B), a result consistent with the functional analysis of Cmr-α in this crenarchaeon.50 Furthermore, none of these bands was present in the hybridization of the total RNAs prepared from ∆CRISPR, a strain lacking both arrays of CRISPR, indicating that the hybridization was specific for both probes. Taken together, this indicated that whereas ∆Cmr-α is responsible for the maturation of the smallest crRNA, none of these Cas modules play an important role in pre-crRNA processing in this archaeon.
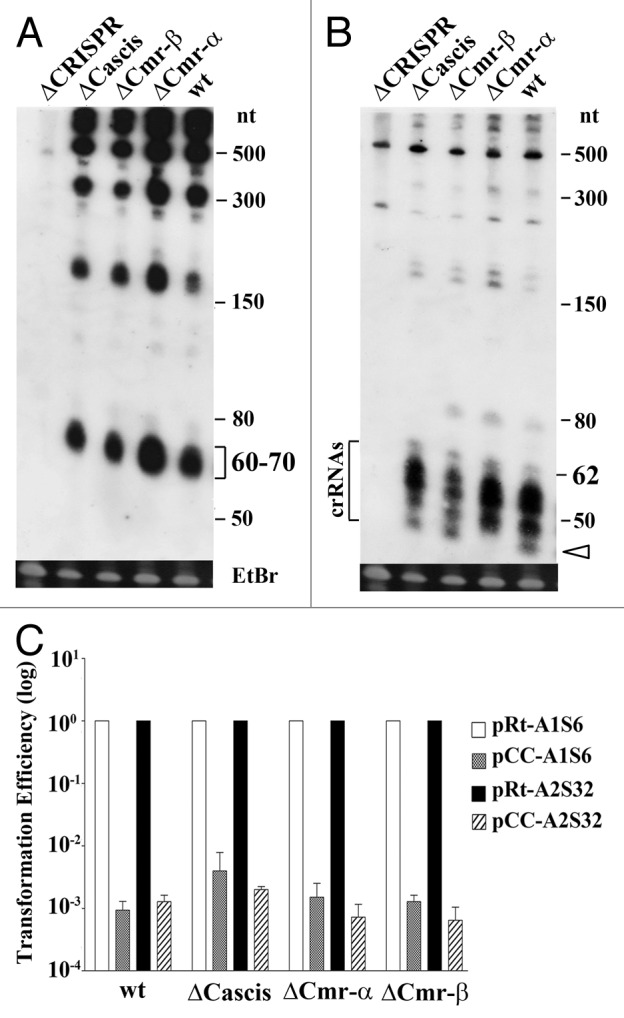
Figure 2. Cascis, Cmr-α and Cmr-β modules are dispensable for pre-crRNA processing and PAM motif-dependent DNA silencing. (A and B) Northern analyses of total RNAs prepared from the S. islandicus wild-type strain (wt) and knockouts of Cas modules using oligonucleotide of the repeat (A) or that of spacer 32 of array 2 (B) as a probe. Band of ca. 60–70 nt corresponds to the primary cleavage products of pre-crRNAs by Cas6. Mature crRNAs (ca. 40–60 nt) of A2S32 are also indicated. Unfilled arrowhead indicates the location of the smallest crRNA. EtBr, RNA staining with ethidium bromide as a loading control. (C) Plasmid interference assay of Cas module knockouts. The genetic host E233S (wt) and Cas module knockouts were used as hosts for plasmid transformation. Two artificial interference plasmids, pCC-A1S6 and pCC-A2S32, were used to test for PAM-dependent DNA interference. In the corresponding reference plasmids, the PAM motif (5′-CCA-3′) was replaced with 5′-GAAAG-3′ (pRt-A1S6) or 5′-ATTGAAAG-3′ (pRt-R2S32). Transformation efficiencies of each interference plasmid were expressed as relative values to the efficiencies of corresponding reference plasmids, the latter of which were set to 1.0.
These strains were then tested for DNA targeting mediated by CC PAM motif using two different interference plasmids, pCC-A2S32 and pCC-A1S6. These plasmids carry the artificial protospacer sequence of array 2 spacer 32 (A2S32) or that of array 1 spacer 6 (A1S6) where each spacer is preceded by the PAM motif (5′-CCA-3′) of Sulfolobus.20 In the corresponding reference plasmids, each PAM motif was replaced with 5 or 8 nt of the repeat sequence, yielding a structure resembling the genetic organization of CRISPR arrays in the chromosome. As shown in Figure 2C, when each mutant was employed as host for transformation, hundreds to thousands fold lower rates of transformation were yielded with pCC-A2S32 and pCC-A1S6 comparing with their reference plasmids, indicating none of these modules is essential for the CC motif-mediated DNA targeting activity.
Functions of Cas6, Cas7, Cas5 and Csa5, components of the aCASCADE complex in S. islandicus
Recently, an archaeal Cascade complex was isolated from S. solfataricus.25 It contained Cas7, Cas5, Cas6 and Csa5 and was loaded with crRNAs of 60–70 nt, which correspond to primary cleavage products by Cas6. Each of these Cascade proteins has a homolog in S. islandicus REY15A showing 82–93% amino acid sequence identity. Since both S. solfataricus and S. islandicus exhibited PAM-dependent DNA targeting activity, we were interested in studying possible functions of these Cas proteins in this process. In-frame deletion mutants were constructed for all four cas genes, giving ∆cas7, ∆cas5, ∆csa5 and ∆cas6. Their complementation plasmids were constructed with a highly efficient expression vector pSeSD52 and transformed into corresponding mutants to yield complementation strains. Both mutants and complementation strains were used for northern analysis of pre-crRNA processing and for the interference plasmid assay.
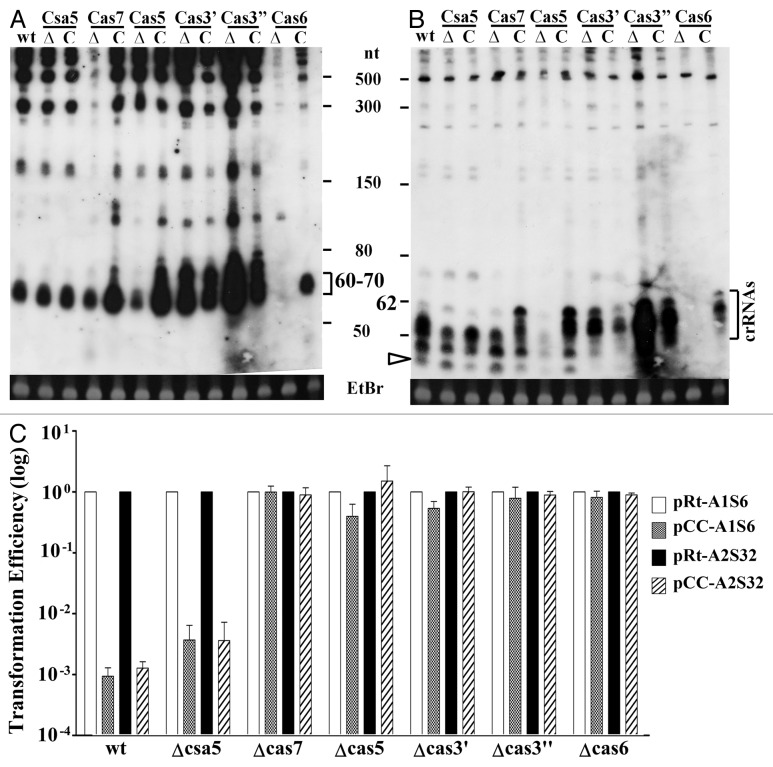
First, we examined pre-crRNA processing in ∆cas6 and its complementation strain. As shown in Figure 3, depleting Cas6 completely inactivated pre-crRNA processing since neither intermediates nor mature crRNAs were present in the mutant strain. These bands appeared again in the complementation strain where the Cas6 protein was expressed from the expression plasmid pSe-cas6. These results indicated that cas6 is essential for crRNA production in S. islandicus and that it is the only enzyme responsible for pre-crRNA processing in this organism.
Figure 3. Functions of Cas proteins of type IA interference module in pre-crRNA processing and PAM-dependent DNA silencing. (A and B) Northern analyses of total RNAs prepared from the S. islandicus wild-type strain (wt), knockouts (∆) of each cas gene of type IA interference module and their complementation strains (C) using oligonucleotide of the repeat (A) or that of spacer 32 of array 2 (B) as a probe. Band of ca. 60–70 nt corresponds to the primary cleavage products of pre-crRNAs by Cas6. Mature crRNAs (ca. 40–60 nt) of A2S32 are also indicated. Unfilled arrowhead indicates the location of the smallest crRNA. EtBr, RNA staining with ethidium bromide as a loading control. (C) Plasmid interference assay of knockout mutants using pCC-A2S32 and pCC-A1S6 as interference plasmids and pRt-A2S32 and pRt-A1S6 as reference plasmids. Transformation efficiencies of each interference plasmid were expressed as relative values to the efficiencies of corresponding reference plasmids, the latter of which were set to 1.0.
Next, we analyzed processing intermediates and crRNAs in ∆csa5, ∆cas5 and ∆cas7 from which different results were obtained. Northern analysis of ∆csa5 and its complementation strain showed the same hybridization pattern and RNA band intensities that were essentially identical to the results of the wild-type strain. Hybridization signals of processing intermediates were absent or greatly weakened in ∆cas7, while crRNAs were maintained at very similar levels. The exactly opposite results were obtained for ∆cas5 where mature crRNAs were hardly detectable but normal levels of processing intermediates accumulated. Furthermore, genetic complementation of mutant deficiency by episomic expression of each corresponding gene from a plasmid restored the hybridization pattern of the wild-type strain (Fig. 3). These results indicated that Cas7 is essential for stabilizing RNA intermediates of pre-crRNA processing and it could function in the absence of Csa5 or Cas5, and that Cas5 is essential for maintaining mature crRNAs whereas Csa5 does not appear to have a role in pre-crRNA processing and maturation.
These mutants were also tested for PAM motif-mediated DNA targeting. As shown in Figure 3C, whereas ∆cas7 and ∆cas5 lost the capability of conferring DNA interference to pCC-A2S32 and pCC-A1S6, depleting Csa5 did not compromise the inference activity.
Taken together, we have demonstrated that only three of the four putative Cascade proteins, i.e., Cas6, Cas7 and Cas5 are essential for the PAM-mediated DNA interference. All these Cas proteins function in pre-crRNA processing in which Cas6 is the primary processing enzyme in vivo whereas Cas7 and Cas5 stabilize processing intermediates and crRNAs, respectively. However, Csa5 does not appear to have a role in either process, suggesting that Csa5 may not represent an integral part of the Cascade that mediates PAM-directed DNA silencing.
Cas3 helicase/nuclease functions both in processing and targeting
Cas3 is required for all known type I CRISPR systems for interference. In some CRISPR systems such as those in Sulfolobus species, Cas3 is divided into two parts: Cas3′ containing a helicase domain and Cas3” carrying a nuclease domain. To reveal their role in DNA interference, ∆cas3′ and ∆cas3” were investigated for silencing of pCC-A2S32 and pCC-A1S6 interference plasmids. As shown in Figure 3C, both mutants showed high transformation efficiencies with all tested plasmids, including both interference and control plasmids, indicating that the interference activity was inactivated in the mutants.
When pre-crRNA processing was studied by northern analysis, both mutants showed increased levels of processing intermediates compared with the wild-type strain and a much elevated level of mature crRNA was observed for Δcas3”(Fig. 3). Interestingly, while genetic complementation of the mutant deficiency by episomic expression of cas3′ or cas3” reversed the strong accumulation of the intermediates, a few new RNA species (60 < crRNAs < 80 nt), which first appeared in ∆cas3′ and ∆cas3”, persisted in their complementing strains. These results suggest that the helicase and nuclease activities of Cas3 function in resolving the processing intermediates and that the nuclease domain could play a role in crRNA degradation.
Discussion
In this work, we have studied functions of different interference modules in pre-crRNA processing and PAM-mediated DNA targeting in S. islandicus REY15A. Mutants lacking each module were constructed and analyzed for mature crRNA and their processing intermediates and for DNA interference. This has revealed that the type IA interference module mediates PAM-dependent DNA targeting and it functions independently from other Cas/Cmr modules that co-exist in S. islandicus REY15A. We also show that depleting Cas6 inactivates pre-crRNA processing and that inactivation of Cas7, Cas5, Cas3′ or Cas3” impairs processing of pre-crRNAs. This unravels an important difference between in vivo and in vitro results since biochemical characterization of a S. solfataricus Cas6 protein, which is 93% identical to this S. islandicus Cas6 in amino acid sequence, demonstrated that Cas6 was capable of generating mature crRNAs by itself.25
A Cascade complex (IA-Cascade) isolated from S. solfataricus was reported consisting of at least four components, Cas7, Cas5, Csa5 and Cas6.25 Characterization of other type I Cascade complexes, including the IE-Cascade of E. coli and the IF Cascade of Pseudomonas aeruginosa, revealed five components (Cse1, Cse2, Cas7, Cas5 and Cas6e) for type IE19,30 and four components Csy1, Csy2, Csy3 and Cas6f (Csy4) for type IF.53 Importantly, all Cascade components and Cas3 are shown to be essential for the DNA-targeting activity in IE and IF systems.19,30,54 Here, we show that Csa5, one of the Cascade components, is dispensable for the PAM-directed DNA targeting in S. islandicus. It is important to point out though that a functional S. solfataricus Cascade complex remains to be demonstrated since the isolated one did not show any cleavage activity.25
Interestingly, although non-essential to DNA interference, Csa5 is encoded in all known archaea carrying a type IA CRISPR system, including all known Sulfolobus species,47,55-58 Methanocaldococcus jannaschii,59 Thermoproteus tenax12 and four Pyrobaculum species,60 whereas Csa5 is absent from all known type IB CRISPR systems, including Haloferax volcanii,43 Clostridium thermocellum and Methanococcus maripaludis.27 Currently, the role of Csa5 in type 1A CRISPR system remains to be demonstrated.
In the E. coli type IE system, cleavage of pre-crRNAs by Cas6e yields mature crRNAs that bind to IE-Cascade to form ribonucleoprotein complexes.33 It has been shown that Cse1 component of the IE-Cascade recognizes the PAM motif of a protospacer of invasive DNAs61 and complementarity between the crRNA and the protospacer then guides the cleavage of foreign DNA by Cas3.31 Currently, the counterpart of Cse1 remains to be identified in the Sulfolobus IA-Cascade complex if this complex functions in analogy to the E. coli IE-Cascade.
Examination of crRNA processing in S. islandicus by northern hybridization showed that the processing was inactivated by Cas6 depletion and the inactivation only occurred in ∆cas6 (Fig. 3). This is consistent with the results that P. furiosus and S. solfataricus Cas6 proteins are capable of generating crRNA in vitro.25,62 Together, these studies demonstrate that Cas6 is both necessary and sufficient for pre-crRNA processing in these archaea.
Furthermore, our study of pre-crRNA processing in the cas gene knockouts of S. islandicus has revealed three interesting features: (1) mature crRNAs are stabilized in ∆cas7, (2) processing intermediates are stabilized in ∆cas5 and (3) processing intermediates are accumulated in ∆cas3′ and ∆cas3.” Interestingly, Cas7 forms protein filaments in S. solfataricus.25 Putting all these facts together, possible mechanistic details are revealed for pre-crRNA processing in S. islandicus. (1) Immediately after transcription, pre-crRNAs are bound by Cas7 protein filaments, protecting the large RNA molecules from degradation; (2) Cas6 proteins bind to repeat sequences of pre-crRNAs and make a cut at each repeat, (3) as both Cas7 and Cas6 proteins remain bound to the processing products, interaction between Cas3′ and Cas3” and the complex will facilitate the remodeling of new smaller complexes and (4) single spacer unit complexes can only be stabilized by recruiting Cas5, which either constitute the Cascade or a basis for generating Cascade complexes. Possibly, Cas5 may also modulate Cas3 activities that could lead to crRNAs degradation.
The proposed strategy is apparently not used in the type I systems of E. coli and P. aeruginosa because relative band intensities of intermediates and crRNAs did not change upon depletion of any component of the Cascade complexes in these organisms.19,54 Neither has it been reported that the E. coli Cas7 forms protein filaments. In fact, these type I systems are further diverged, whereas only Cas6 is essential in pre-crRNA processing in S. islandicus, two protein factors are reported to be essential for in vivo pre-crRNA processing in the bacterial systems, namely Cas5 and Cas6 in E. coli19,63 and Cas6f (Csy4) and Csy2 in P. aeruginosa.54
Then, the question is why it is relevant for S. islandicus to employ such a strategy in crRNA processing. This archaeon contains three distinct interference modules that are presumed to employ different types of mature crRNA for their interference. The type IA system most likely utilizes 60–70 nt crRNAs, which are components of the S. solfataricus Cascade complex.25 The Cmr-β module most likely uses 30–50 nt crRNAs, which are associated with the S. solfataricus type IIIB Cmr reported by Zhang et al.,38 because the two Cmr systems share high-sequence similarity and exhibit the same gene synteny. Although the Cmr-α complex has not been isolated thus far, here we show that depleting the complex eliminates the smallest crRNA, which is ca. 40–41 nt crRNA for A2S32. More recently, interference activity has been demonstrated for the S. islandicus Cmr-α, which confers a protospacer transcript-dependent DNA interference.50 Further investigation of the distinct Cas interference modules in S. islandicus will yield important insights into regulation of pre-crRNA processing and maturation as well as mechanisms of coordinating different interference activities that co-exist intracellularly.
Materials and Methods
Sulfolobus strains, growth conditions and transformation
All experiments were performed with the model organism of Sulfolobus islandicus REY15A originally isolated from an enrichment culture obtained from samples collected from hotsprings in Iceland.64 Its genetic host E233S is double deletion mutant carrying a large deletion in pyrEF genes and a complete deletion of lacS gene.65
All Sulfolobus strains used in this study are listed in Table 1. The strains that carry pyrEF genes either on the chromosome or on a plasmid were grown at 76°C in the SCV (0.2% sucrose, 0.2% casamino acids plus 1% vitamin solution) selective medium, whereas the medium was supplemented with 20 µg/ml uracil (SCVU) when growing pyrEF-deficient strains. Sulfolobus-competent cells were prepared as described previously65 and plasmids were introduced into the cells by electroporation and transformed cells were allowed to form colonies on SCV plates.
Table 1. Strains used in this study.
| Strain | Genotype and features | Source |
|---|---|---|
|
S. islandicus E233S |
∆pyrEF∆lacS |
Deng et al.65 |
| ∆CRISPR |
CRISPR arrays are not detectable by PCR |
Gudbergsdottir et al.42 |
| ∆Cascis |
All genes in Cascis module, including csa3, csa1, cas1, cas2 and cas4 are deleted |
This work |
| ∆Cmr-α |
Deletion of the type IIIB Cmr locus with 6 genes |
This work |
| ∆Cmr-β |
Deletion of the type IIIB Cmr locus with 7 genes |
This work |
| ∆csa5 |
In-frame deletion of csa5 |
This work |
| ∆cas7 |
In-frame deletion of cas7 |
This work |
| ∆cas5 |
In-frame deletion of cas5 |
This work |
| ∆cas3′ |
In-frame deletion of cas3’ |
This work |
| ∆cas3” |
In-frame deletion of cas3” |
This work |
| ∆cas6 | Carrying deletion of the entire cas6 gene plus 277 bp downstream flanking sequence | This work |
Construction of deletion mutants of S. islandicus
A novel gene knockout method named marker insertion and unmarked target gene deletion (MID) developed by Zhang et al.66 was employed to construct cas gene knockouts. In MID manipulation, each plasmid vector contains three homologous sequence arms, i.e., left, right and target gene arms. These homologous arms were carefully designed such that the insertion of a marker cassette would yield a functional target gene at the first step of recombination. Primers for amplification of these arms for each Cas module or cas gene by polymerase chain reaction are listed in Table 3. Ligation of these arms to the E. coli vector pUC19 gave pMID-Cascis, -Cmr-α, -Cmr-β, -cas7, -cas5, -cas6, -csa5, -cas3′ and -cas3.” These knockout plasmids were linearized with Pvu II before transformation. Transformants were obtained on SCV plates, whereas mutant cells were recovered by counter selection of pyrEF with 5′-fluoroorotic acids (5′-FOA). After three rounds of single colony purification, the mutants were analyzed for their mutant alleles by PCR with specific primers for each target gene or gene region and their mutant genotypes were verified (Fig. 1B).
Complementation plasmids were constructed with pSeSD, a highly efficient expression vector recently reported for Sulfolobus.52 The expression is under the control of ParaS-SD, a synthetic promoter that confers up to one-third of the fully induced level of expression in a sucrose medium.52 Each cas gene was amplified by PCR from S. islandicus total DNA with a Pfu DNA polymerase using specific primers listed in Table 3. After cleavage with the enzymes indicated in each PCR primer, the DNA fragments were cloned to pSeSD at the compatible sites, yielding pSe-csa5, -cas5, -cas7, -cas3′ -cas3” and -cas6.
Table 3. Oligonucleotides used in this study.
| Oligonucleotide | Sequence (5′-3′; PAM motif or repeat sequence underlined, restriction site in bold) |
|---|---|
| A1S6fwd-MluI |
CGCGTCCAGTTGGCATAATCCTCAACATACTTTCTTGTGTCGGGTATGC |
| A1S6rev |
GCATACCCGACACAAGAAAGTATGTTGAGGATTATGCCAACTGGA |
| ReA1S6fwd-MluI |
CGCGAAAGGTTGGCATAATCCTCAACATACTTTCTTGTGTCGGGTATGC |
| ReA1S6rev |
GCATACCCGACACAAGAAAGTATGTTGAGGATTATGCCAACCTTT(C) |
| A2S32fwd-MluI |
CGCGTCCCAAGTTCATAGAGCCCTATGGGAATCCCTACTAATCCCGC |
| A2S32rev |
GCGGGATTAGTAGGGATTCCCATAGGGCTCTATGAACTTGGGA |
| ReA2S32fwd-MluI |
CGCGATTGAAAGAGTTCATAGAGCCCTATGGGAATCCCTACTAATCCCGC |
| ReA2S32rev |
GCGGGATTAGTAGGGATTCCCATAGGGCTCTATGAACTCTTTCAAT |
| Csa5fwd-MluI |
CCACGCGTTGGCACAACAAGTAAAAGAAG |
| Csa5rev-StuI |
AAGGCCTTTTCTTCTCACCACCACCTTG |
| Cas7fwd-NdeI |
GTTGTTCCATATGATAAGTGGTTCAGGTAGATTT |
| Cas7rev-MluI |
CGACGCGTCTACTTTTCCTTTAATTTACC |
| Cas5fwd-MluI |
GGACGCGTTGATCTACTCTAAGGTTTTTT |
| Cas5rev-StuI |
GAGGCCTGCTAAAGACAACATATTCTCC |
| Cas3′fwd-NdeI |
CCCAAAGCATATGTTGTCTTTAGCTGACTTCTAT |
| Cas3′rev-MluI |
CGACGCGTTCAATACACACCACCTATTTC |
| Cas′′fwd-NdeI |
AATAAATCATATGATCAAGCCTTGCGCTTATGAG |
| Cas′′rev-MluI |
CGACGCGTTTATAAAGTGGAACCTCCATT |
| Cas6fwd-NdeI |
GGGACCGCATATGCCATTAATTTTCAAGATA |
| Cas6rev-MluI |
GTACGCGTACCTTTAAAGTCTGAGGA |
| Cmr-α-Gfwd-SalI |
AATTGTCGACGCAGGAATGGTGGTAGAGTCT |
| Cmr-α-Grev-MluI |
CCACGCGTCCTCCCCACTTGAATACTACC |
| Cmr-α-Lfwd-NcoI |
AATTCCATGGCAGGAAAGCAGTAGAGAAGGA |
| Cmr-α-Lrev-XhoI |
ATCCTCGAGAACTTGTAACCCTACGTCGTT |
| Cmr-α-Rfwd-XhoI |
ATCCTCGAGACTAAGATGACTAAGGAAGGC |
| Cmr-α-Rrev-SphI |
AATTGCATGCACAAAAACATGAACGTATGAG |
| Cmr-β-Gfwd-SalI |
AATTGTCGACATAGTCTTCATCGGCACATAC |
| Cmr-β-Grev-MluI |
CCACGCGTTCATCTGTAGAGGAGGAAATA |
| Cmr-β-Lfwd-NcoI |
AATTCCATGGTCAGCAGGCAGTAAAGGAAGA |
| Cmr-β-Lrev-XhoI |
ATCCTCGAGCAATGGGTAAGGTGGATAAGA |
| Cmr-β-Rfwd-XhoI |
ATCCTCGAGTTGCATCGGGACTACTGTAAG |
| Cmr-β-Rrev-SphI |
AATTGCATGCAGAGGGTGGGTAAGTGATGAT |
| Cassis-Gfwd-SalI |
AATTGTCGACATGTTCTCCTTCATGCCCGTT |
| Cascis-Grev-MluI |
CCACGCGTGTCCGTTCTAATACCGCTCCT |
| Cascis-Lfwd-NcoI |
AATTCCATGGTTCATCAAGAGGAAAATCGTC |
| Cascis-Lrev-XhoI |
ATCCTCGAGTTCGTTAGAAACACTCGCTAG |
| Cascis-Rfwd-XhoI |
ATCCTCGAGTGTATTCATAAGCCTCATTCC |
| Cascis-Rrev-SphI |
AATTGCATGCCAACGGAAATAGTAGGGAACA |
| Cas3′-Gfwd-SalI |
AATTGTCGACAAGGTAACGAAACTAAAGACT |
| Cas3′-Grev-MluI |
CCACGCGTCACACCACCTATTTCACTATT |
| Cas3′-Lfwd-NcoI |
AATTCCATGGGCTTACACTGAGGACATTGTT |
| Cas3′-Lrev-XhoI |
ATCCTCGAGACCATGACGAGTTATGAAATC |
| Cas3′-Rfwd-XhoI |
ATCCTCGAGTGATCAAGCCTTGCGCTTATG |
| Cas3′-Rrev-SphI |
AATTGCATGCTATCCCCCTAATTGCGTTCAG |
| Cas3”-Gfwd-SalI |
AATTGTCGACGGTGATTGATTCGATTCTGGC |
| Cas3”-Grev-MluI |
CCACGCGTAGTGGAACCTCCATTTAACTC |
| Cas3”-Lfwd-NcoI |
AATTCCATGGGGTGATTGATTCGATTCTGGC |
| Cas3”-Lrev-XhoI |
ATCCTCGAGCTCATAAGCGCAAGGCTTGAT |
| Cas3”-Rfwd-XhoI |
ATCCTCGAGTTATAATATATTTGGAGATAA |
| Cas3”-Rrev-SphI |
AATTGCATGCTGAATCAAACTGTAGAGAATA |
| Cas5-Gfwd-SalI |
AATTGTCGACAGTGATCCCTACTGGTAAAGT |
| Cas5-Grev-MluI |
CCACGCGTAGAAGTCAGCTAAAGACAACA |
| Cas5-Lfwd-NcoI |
AATTCCATGGGTTAGAAAGGCAAAAGGCTCG |
| Cas5-Lrev-XhoI |
ATCCTCGAGTAACTACGGAAAAACCCCAAT |
| Cas5-Rfwd-XhoI |
ATCCTCGAGTTAGCTGACTTCTATAACGAT |
| Cas5-Rrev-SphI |
AATTGCATGCGTCTTGAGCACCAATACTTTT |
| Cas6-Gfwd-SalI |
AATTGTCGACTTGCCATCAGTTGGCTTAATT |
| Cas6-Grev-MluI |
CCACGCGTCCTTAACCTTTAAAGTCTGAG |
| Cas6-Lfwd-NcoI |
AATTCCATGGGTTAGAAAGGCAAAAGGCTCG |
| Cas6-Lrev-XhoI |
ATCCTCGAGTGAAACCGAGATGGGATATAA |
| Cas6-Rfwd-XhoI |
ATCCTCGAGCCTCAGACTTTAAAGGTTAAG |
| Cas6-Rrev-SphI |
AATTGCATGCGGAGGGGAGATGTTCCGATGC |
| Cas7-Gfwd-SalI |
AATTGTCGACAAGCCTTAGCTCATGCCTAT |
| Cas7-Grev-MluI |
CCACGCGTTCTTGCCCCTCTACTTTTCCT |
| Cas7-Lfwd-NcoI |
AATTCCATGGTCCAGGTTTATGAAAAATCTT |
| Cas7-Lrev-XhoI |
ATCCTCGAGATCATTTCTTCTCACCACCAC |
| Cas7-Rfwd-XhoI |
ATCCTCGAGGTAGAGGGGCAAGAGAGATTT |
| Cas7-Rrev-SphI |
AATTGCATGCCTCAACTTCAACCTCCTTGCT |
| Csa5-Gfwd-SalI |
AATTGTCGACGAGGCTGTGACTAAGGTTCTA |
| Csa5-Grev-MluI |
CCACGCGTTATCATTTCTTCTCACCACCA |
| Csa5-Lfwd-NcoI |
AATTCCATGGGGAGGGATGAGATTAATGATT |
| Csa5-Lrev-XhoI |
ATCCTCGAGCTTCTTTTACTTGTTGTGCCA |
| Csa5-Rfwd-XhoI |
ATCCTCGAGAGAAATGATAAGTGGTTCAGG |
| Csa5-Rrev-SphI | AATTGCATGCTACATGAAACCTCCAATATCA |
Plasmid interference assay of PAM-mediated DNA targeting
Artificial interference plasmids were constructed with two spacers, spacer 6 of CRISPR array 1 (A1S6) and spacer 32 of array 2 (A2S32). Oligonucleotides containing the complete sequences of the two spacers that were preceded with 5′-CCA-3′ or with the last five or eight nucleotides of the repeat were synthesized from TAG Copenhagen (Table 3). Each pair of primers was annealed with each other by heating to 95°C for 10 min and then cooling down gradually to room temperature, yielding double-stranded DNAs with one blunt end and one protruding end, the latter of which matched the DNA ends after Mlu I digestion. These DNA fragments were cloned to pSeSD at Stu I and Mlu I sites individually, yielding plasmids containing an artificial protospacer (interference plasmids) and those containing a repeat-spacer module (reference plasmids) (Table 2).
Table 2. Plasmids used in this study.
| Plasmid | Features | Source |
|---|---|---|
| pSeSD |
A Sulfolobus-E. coli shuttle vector carrying an expression cassette controlled under a synthetic strong promoter ParaS-SD |
Peng et al.52 |
| pCC-A1S6 |
An artificial interference plasmid; pSeSD carrying the interference module of the sequence of spacer 6 of array 1 preceded by the 5′-CCA-3′ motif |
This work |
| pRt-A1S6 |
Reference plasmid of pCC-A1S6; the PAM motif (5′-CCA-3′) was replaced with the last 5 nt of the repeat sequence (5′-GAAAG-3′) |
This work |
| pCC-A2S32 |
An artificial interference plasmid; pSeSD carrying the interference module of the sequence of spacer 32 of array 2 preceded by the 5′-CCA-3′ motif |
This work |
| pRt-A2S32 |
Reference plasmid of pCC-A2S32; the PAM motif (5′-CCA-3′) was replaced with the last 8 nt of the repeat sequence (5′-ATTGAAAG-3′) |
This work |
| pSe-Csa5 |
pSeSD carrying the S. islandicus csa5 gene |
This work |
| pSe-Cas7 |
pSeSD carrying the S. islandicus cas7 gene |
This work |
| pSe-Cas5 |
pSeSD carrying the S. islandicus cas5 gene |
This work |
| pSe-Cas3′ |
pSeSD carrying the S. islandicus cas3′ gene |
This work |
| pSe-Cas3” |
pSeSD carrying the S. islandicus cas3” gene |
This work |
| pSe-Cas6 | pSeSD carrying the S. islandicus cas6 gene | This work |
RNA preparation and northern analysis
Total RNAs were extracted using the Trizol reagent (Invitrogen) following the protocol provided by the manufacturer. Thirty µg RNA was loaded on a 6% polyacrylamide-SDS gel for each sample and fractionated. RNAs in the gel were then transferred onto a nylon membrane and immobilized. Procedures for pre-hybridization, end labeling of complementary nucleotides and hybridization in northern analysis were described earlier20 and the probes used in this work included a probe of the repeat sequence (5′-CTTTCAATTCTATAGTAGATTAGCNNNN-3′) for detecting intermediates of pre-crRNA processing and a probe of spacer 32 of array 2 (5′- GCGGGATTAGTAGGGATTCCCATAGGGCTCTATGAACT-3′) hybridizing to mature crRNAs.
Acknowledgments
This work was supported by the Danish Council for Independent Research: Technology and Production Sciences (11-106683) and the Natural Science Foundation of China (grant no. 31128011, no. 31100050 and no. 31100096). We thank our colleagues at the laboratories in Copenhagen and in Wuhan for fruitful discussions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Conceived and designed the experiments: Q.S., Y.X.L., W.P. and N.P. Performed experiments: W.P., H.L. and S.H. Analyzed data: Q.S., Y.X.L., W.P., N.P., H.L. and S.H. Wrote the paper: Q.S. and W.P.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/23798
References
- 1.Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–82. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 2.Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–63. doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- 3.Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–61. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 4.Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct. 2006;1:7. doi: 10.1186/1745-6150-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–70. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 6.Terns MP, Terns RM. CRISPR-based adaptive immune systems. Curr Opin Microbiol. 2011;14:321–7. doi: 10.1016/j.mib.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–77. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett RA, Vestergaard G, Shah SA. Archaeal CRISPR-based immune systems: exchangeable functional modules. Trends Microbiol. 2011;19:549–56. doi: 10.1016/j.tim.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Westra ER, Swarts DC, Staals RH, Jore MM, Brouns SJ, van der Oost J. The CRISPRs, they are a-changin’: how prokaryotes generate adaptive immunity. Annu Rev Genet. 2012;46:311–39. doi: 10.1146/annurev-genet-110711-155447. [DOI] [PubMed] [Google Scholar]
- 10.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–8. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 11.Haft DH, Selengut J, Mongodin EF, Nelson KE. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol. 2005;1:e60. doi: 10.1371/journal.pcbi.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plagens A, Tjaden B, Hagemann A, Randau L, Hensel R. Characterization of the CRISPR/Cas subtype I-A system of the hyperthermophilic crenarchaeon Thermoproteus tenax. J Bacteriol. 2012;194:2491–500. doi: 10.1128/JB.00206-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–12. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 14.Yosef I, Goren MG, Qimron U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 2012;40:5569–76. doi: 10.1093/nar/gks216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarts DC, Mosterd C, van Passel MW, Brouns SJ. CRISPR interference directs strand specific spacer acquisition. PLoS One. 2012;7:e35888. doi: 10.1371/journal.pone.0035888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, Semenova E. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun. 2012;3:945. doi: 10.1038/ncomms1937. [DOI] [PubMed] [Google Scholar]
- 17.Erdmann S, Garrett RA. Selective and hyperactive uptake of foreign DNA by adaptive immune systems of an archaeon via two distinct mechanisms. Mol Microbiol. 2012;85:1044–56. doi: 10.1111/j.1365-2958.2012.08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang TH, Bachellerie JP, Rozhdestvensky T, Bortolin ML, Huber H, Drungowski M, et al. Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus. Proc Natl Acad Sci USA. 2002;99:7536–41. doi: 10.1073/pnas.112047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–4. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lillestøl RK, Shah SA, Brügger K, Redder P, Phan H, Christiansen J, et al. CRISPR families of the crenarchaeal genus Sulfolobus: bidirectional transcription and dynamic properties. Mol Microbiol. 2009;72:259–72. doi: 10.1111/j.1365-2958.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- 21.Hale C, Kleppe K, Terns RM, Terns MP. Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. RNA. 2008;14:2572–9. doi: 10.1261/rna.1246808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatoum-Aslan A, Maniv I, Marraffini LA. Mature clustered, regularly interspaced, short palindromic repeats RNA (crRNA) length is measured by a ruler mechanism anchored at the precursor processing site. Proc Natl Acad Sci USA. 2011;108:21218–22. doi: 10.1073/pnas.1112832108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carte J, Pfister NT, Compton MM, Terns RM, Terns MP. Binding and cleavage of CRISPR RNA by Cas6. RNA. 2010;16:2181–8. doi: 10.1261/rna.2230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garside EL, Schellenberg MJ, Gesner EM, Bonanno JB, Sauder JM, Burley SK, et al. Cas5d processes pre-crRNA and is a member of a larger family of CRISPR RNA endonucleases. RNA. 2012;18:2020–8. doi: 10.1261/rna.033100.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lintner NG, Kerou M, Brumfield SK, Graham S, Liu H, Naismith JH, et al. Structural and functional characterization of an archaeal clustered regularly interspaced short palindromic repeat (CRISPR)-associated complex for antiviral defense (CASCADE) J Biol Chem. 2011;286:21643–56. doi: 10.1074/jbc.M111.238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–8. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter H, Zoephel J, Schermuly J, Maticzka D, Backofen R, Randau L. Characterization of CRISPR RNA processing in Clostridium thermocellum and Methanococcus maripaludis. Nucleic Acids Res. 2012;40:9887–96. doi: 10.1093/nar/gks737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gesner EM, Schellenberg MJ, Garside EL, George MM, Macmillan AM. Recognition and maturation of effector RNAs in a CRISPR interference pathway. Nat Struct Mol Biol. 2011;18:688–92. doi: 10.1038/nsmb.2042. [DOI] [PubMed] [Google Scholar]
- 29.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–7. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jore MM, Lundgren M, van Duijn E, Bultema JB, Westra ER, Waghmare SP, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–36. doi: 10.1038/nsmb.2019. [DOI] [PubMed] [Google Scholar]
- 31.Wiedenheft B, Lander GC, Zhou K, Jore MM, Brouns SJ, van der Oost J, et al. Structures of the RNA-guided surveillance complex from a bacterial immune system. Nature. 2011;477:486–9. doi: 10.1038/nature10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell. 2009;139:945–56. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westra ER, van Erp PB, Künne T, Wong SP, Staals RH, Seegers CL, et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell. 2012;46:595–605. doi: 10.1016/j.molcel.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 35.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–5. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hale CR, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol Cell. 2012;45:292–302. doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Rouillon C, Kerou M, Reeks J, Brugger K, Graham S, et al. Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Mol Cell. 2012;45:303–13. doi: 10.1016/j.molcel.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mojica FJ, Díez-Villaseñor C, García-Martínez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–40. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 40.Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 2011;39:9275–82. doi: 10.1093/nar/gkr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semenova E, Jore MM, Datsenko KA, Semenova A, Westra ER, Wanner B, et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad Sci USA. 2011;108:10098–103. doi: 10.1073/pnas.1104144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudbergsdottir S, Deng L, Chen Z, Jensen JV, Jensen LR, She Q, et al. Dynamic properties of the Sulfolobus CRISPR/Cas and CRISPR/Cmr systems when challenged with vector-borne viral and plasmid genes and protospacers. Mol Microbiol. 2011;79:35–49. doi: 10.1111/j.1365-2958.2010.07452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer S, Maier LK, Stoll B, Brendel J, Fischer E, Pfeiffer F, et al. An archaeal immune system can detect multiple protospacer adjacent motifs (PAMs) to target invader DNA. J Biol Chem. 2012;287:33351–63. doi: 10.1074/jbc.M112.377002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng L, Kenchappa CS, Peng X, She Q, Garrett RA. Modulation of CRISPR locus transcription by the repeat-binding protein Cbp1 in Sulfolobus. Nucleic Acids Res. 2012;40:2470–80. doi: 10.1093/nar/gkr1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–71. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garrett RA, Shah SA, Vestergaard G, Deng L, Gudbergsdottir S, Kenchappa CS, et al. CRISPR-based immune systems of the Sulfolobales: complexity and diversity. Biochem Soc Trans. 2011;39:51–7. doi: 10.1042/BST0390051. [DOI] [PubMed] [Google Scholar]
- 47.Guo L, Brügger K, Liu C, Shah SA, Zheng H, Zhu Y, et al. Genome analyses of Icelandic strains of Sulfolobus islandicus, model organisms for genetic and virus-host interaction studies. J Bacteriol. 2011;193:1672–80. doi: 10.1128/JB.01487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.She Q, Zhang C, Deng L, Peng N, Chen Z, Liang YX. Genetic analyses in the hyperthermophilic archaeon Sulfolobus islandicus. Biochem Soc Trans. 2009;37:92–6. doi: 10.1042/BST0370092. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Tian B, Li S, Ao X, Dalgaard K, Gökce S, et al. Genetic manipulation in Sulfolobus islandicus and functional analysis of DNA repair genes. Biochem Soc Trans. 2013;41:405–10. doi: 10.1042/BST20120285. [DOI] [PubMed] [Google Scholar]
- 50.Deng L, Garrett RA, Shah SA, Peng X, She Q. A novel interference mechanism by a type IIIB CRISPR-Cmr module in Sulfolobus. Mol Microbiol. 2013 doi: 10.1111/mmi.12152. In press. [DOI] [PubMed] [Google Scholar]
- 51.Lillestøl RK, Redder P, Garrett RA, Brügger K. A putative viral defence mechanism in archaeal cells. Archaea. 2006;2:59–72. doi: 10.1155/2006/542818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng N, Deng L, Mei Y, Jiang D, Hu Y, Awayez M, et al. A synthetic arabinose-inducible promoter confers high levels of recombinant protein expression in hyperthermophilic archaeon Sulfolobus islandicus. Appl Environ Microbiol. 2012;78:5630–7. doi: 10.1128/AEM.00855-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiedenheft B, van Duijn E, Bultema JB, Waghmare SP, Zhou K, Barendregt A, et al. RNA-guided complex from a bacterial immune system enhances target recognition through seed sequence interactions. Proc Natl Acad Sci USA. 2011;108:10092–7. doi: 10.1073/pnas.1102716108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cady KC, O’Toole GA. Non-identity-mediated CRISPR-bacteriophage interaction mediated via the Csy and Cas3 proteins. J Bacteriol. 2011;193:3433–45. doi: 10.1128/JB.01411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.She Q, Singh RK, Confalonieri F, Zivanovic Y, Allard G, Awayez MJ, et al. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc Natl Acad Sci USA. 2001;98:7835–40. doi: 10.1073/pnas.141222098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawarabayasi Y, Hino Y, Horikawa H, Jin-no K, Takahashi M, Sekine M, et al. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain7. DNA Res. 2001;8:123–40. doi: 10.1093/dnares/8.4.123. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Brügger K, Skovgaard M, Redder P, She Q, Torarinsson E, et al. The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota. J Bacteriol. 2005;187:4992–9. doi: 10.1128/JB.187.14.4992-4999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reno ML, Held NL, Fields CJ, Burke PV, Whitaker RJ. Biogeography of the Sulfolobus islandicus pan-genome. Proc Natl Acad Sci USA. 2009;106:8605–10. doi: 10.1073/pnas.0808945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beloglazova N, Petit P, Flick R, Brown G, Savchenko A, Yakunin AF. Structure and activity of the Cas3 HD nuclease MJ0384, an effector enzyme of the CRISPR interference. EMBO J. 2011;30:4616–27. doi: 10.1038/emboj.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernick DL, Cox CL, Dennis PP, Lowe TM. Comparative genomic and transcriptional analyses of CRISPR systems across the genus Pyrobaculum. Front Microbiol. 2012;3:251. doi: 10.3389/fmicb.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sashital DG, Wiedenheft B, Doudna JA. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol Cell. 2012;46:606–15. doi: 10.1016/j.molcel.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22:3489–96. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pougach K, Semenova E, Bogdanova E, Datsenko KA, Djordjevic M, Wanner BL, et al. Transcription, processing and function of CRISPR cassettes in Escherichia coli. Mol Microbiol. 2010;77:1367–79. doi: 10.1111/j.1365-2958.2010.07265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Contursi P, Jensen S, Aucelli T, Rossi M, Bartolucci S, She Q. Characterization of the Sulfolobus host-SSV2 virus interaction. Extremophiles. 2006;10:615–27. doi: 10.1007/s00792-006-0017-2. [DOI] [PubMed] [Google Scholar]
- 65.Deng L, Zhu H, Chen Z, Liang YX, She Q. Unmarked gene deletion and host-vector system for the hyperthermophilic crenarchaeon Sulfolobus islandicus. Extremophiles. 2009;13:735–46. doi: 10.1007/s00792-009-0254-2. [DOI] [PubMed] [Google Scholar]
- 66.Zhang C, Guo L, Deng L, Wu Y, Liang Y, Huang L, et al. Revealing the essentiality of multiple archaeal pcna genes using a mutant propagation assay based on an improved knockout method. Microbiology. 2010;156:3386–97. doi: 10.1099/mic.0.042523-0. [DOI] [PubMed] [Google Scholar]