Abstract
Periodontal disease is characterised by proteolytic processes involving enzymes that are released by host immune cells and periodontal bacteria. These enzymes, when detectable in whole saliva, may serve as valuable diagnostic markers for disease states and progression. Because the substrate specificities of salivary proteases in periodontal health and disease are poorly characterised we probed these activities using several relevant substrates: 1) gelatin and collagen type IV; 2) the Arg/Lys–rich human salivary substrate histatin-5; and 3) a histatin-derived synthetic analog benzyloxycarbonyl-Arg-Gly-Tyr-Arg-methyl cumaryl amide (Z-RGYR-MCA). Substrate degradation was assessed in gel (zymography) and in solution. Whole saliva supernatant enzyme activities directed at gelatin, quantitated from the 42 kDa, 92 kDa and 130 kDa bands in the zymograms, were 1.3, 1.4 and 2.0 fold higher, respectively, in the periodontal patient group (p<0.01), consistent with enhanced activities observed towards collagen type IV. On the other hand, histatin 5 degraded equally fast in healthy and periodontal patients' whole saliva supernatant samples (p>0.10). Likewise, the hydrolysis rates of the Z-RGYR-MCA substrate were the same in the healthy and periodontal patient groups (p>0.10). In conclusion, gelatinolytic/collagenolytic activities but not trypsin-like activities in human saliva differentiate health from periodontal disease, and may thus provide an adjuvant to diagnosis for monitoring of disease activity.
Keywords: Oral, Saliva, Diagnostic, Periodontal, Enzymes
Introduction
It is well recognised that all molecular interactions on oral soft and hard tissues occur in human whole saliva (WS). WS is a highly complex fluid derived mainly from salivary glands, but also contains significant amounts of non-exocrine components such as gingival crevicular fluid, oral microorganisms, desquamated oral epithelial cells, and host and bacteria-derived proteases (Bosch et al., 2003; Dawes 2003). Particularly the presence of gingival crevicular fluid, a serum-like transudate, has increased the interest in saliva as a diagnostic fluid for diseases unrelated to glandular disorders. The complexity of whole saliva (WS) also forms a challenge to diagnostic applications, not in the least due to the high proteolytic activity in WS (de Jong et al., 2011; Henson and Wong 2010; Schipper et al., 2007; Siqueira and Dawes 2011; Thomadaki et al., 2011). Without preventive measures, this may lead to the degradation of biomarkers of interest. At the same time, WS enzymatic activities could also be exploited for the diagnosis of diseases that involve changes in proteolytic activity. One of such diseases is periodontal disease.
Periodontal disease involves inflammation of the supporting tissues of the teeth, resulting in progressive destruction of the periodontal ligament and alveolar bone. Its most common form is periodontitis, a condition typically of chronic nature, which is initiated by specific microorganisms. The establishment and progression of periodontal disease involves breakdown of collagen and other matrix proteins proteases released by leukocytes and tissue cells (e.g., collagenases, matrix metallo-proteinases, hyaluronidase) as well as bacteria-derived enzymes (e.g., gingipain R and gingipain K produced by Porphyromonas gingivalis). Since WS is the fluid that baths the periodontal tissues, its composition may reflect disease processes ongoing in the periodontal pocket (Giannobile et al., 2009). While the levels of such markers may be elevated in gingival fluid and dental plaque directly surrounding the periodontal pocket, from a practical perspective WS fluid is a more attractive source for biomarker detection. As such, WS may serve as the ideal bodyfluid for monitoring periodontal inflammation (Spielmann and Wong 2011; Zhang et al., 2009). Indeed, in an experimental model for gingivitis gingival crevicular fluid-derived albumin levels in human saliva increased with time, and decreased upon resumption of oral hygiene (Oppenheim, 2008). Furthermore, increased saliva concentrations of proteolytic enzymes such as neutrophil collagenase MMP-8 and gelatinase MMP-9 (Ramseier et al., 2009; Sorsa et al., 2006, 2010, 2011), as well as increased saliva enzyme activities of MMP-2, MMP-9 and elastase have been reported to correlate with periodontal inflammation (Ingman et al., 1994; Makela et al., 1994; Nieminen et al., 1993).
While connective tissue degradation by proteases has been a well-documented aspect of periodontal disease, and it is also known that WS protease levels are elevated, the protease specificities and protein targets of the WS protease mixture are poorly characterised. For instance, it is unclear if also salivary proteins are targeted by the periodontitis-associated enzymes. The pattern of breakdown of natural salivary proteins as well as tissue proteins could be informative of the level and type of proteolysis, which may have adjuvant diagnostic and/or prognostic value.
The present study aims to gain an insight into salivary enzyme activities and specificities in periodontal health and disease. We compared the overall proteolytic activities in whole saliva supernatant (WSS) from these two groups using four different enzyme substrates. The first substrate was denatured collagen (gelatin), which was used to detect matrix metal protease (MMP) enzyme activities in saliva. The second substrate was histatin 5, which is a natural Arg/Lys-rich salivary protein with properties that are deemed protective in periodontal disease. For instance, histatin 5 inhibits inflammatory cytokine expression by human gingival fibroblasts, induced by Porphyromonas gingivalis, and neutralises leukotoxin from the periodontal pathogen Aggregatibacter actinomycetemcomitans (Imatani et al., 2000; Murakami et al., 2002). The third substrate was Z-RGYR-MCA, which was used to probe specifically for trypsin-like enzymes. Such proteases are typically released by periodontal bacteria such as P. gingivalis, and cleave the peptide bond C-terminal to an arginine (R) residue. Lastly, we investigated collagen type IV as an enzyme substrate, which is one of the collagen types found in the periodontal ligament (Romanos and Bernimoulin 1990). To our knowledge this is the first systematic study into the activities and specificities of WS enzymes in periodontal health and disease, investigating both host-derived and salivary protease substrates.
Materials and Methods
Enzymatic substrates
Synthetic histatin 5 and benzyloxycarbonyl-Arg-Gly-Tyr-Arg-methyl cumaryl amide (Z-RGYR-MCA) were obtained from the American Peptide Company, Sunnyvale, CA. Synthetic histatin 5 was dissolved in water to 9.6 mg/ml. The concentration was determined by measurement of the absorbance at 215 nm (ε = 20 ml.mg−1cm−1). Z-RGYR-MCA was dissolved in deionised milliQ water to a final concentration of 10 mM. Natural histatin 5 was isolated from human parotid saliva by the zinc precipitation method as described (Flora et al., 2001). Collagen Type IV was from human placenta (Bornstein and Traub Type IV) and was obtained from Sigma-Aldrich. It was dissolved in 0.1 M acetic acid to a final concentration of 3.3 mg/ml.
Human subjects
The participating subjects were recruited at Boston University Medical Center and consented to the collection of saliva in accordance with approved protocols of the Institutional Review Board at Boston University and according to ethical principles, including the World Medical Association Declaration of Helsinki. A total of 25 orally healthy and 25 periodontitis patients were enrolled. Two healthy subjects' samples were lost on processing, and thus the experiments reported here were conducted with samples from 23 healthy subjects and 25 periodontal patients. The healthy subjects ranged in age from 25 to 41 years of age, displayed no signs of periodontal disease as evidenced from pocket depths < 5mm and absence or minimal bleeding on probing, with no history of periodontal disease. Periodontal patients were scheduled to undergo periodontal surgery at the Periodontology clinic of the Henry M. Goldman School of Dental Medicine. Inclusion criteria for the periodontitis patients were: moderate to severe periodontal disease in at least 9 teeth with pocket depths ≥5mm in at least two different quadrants, or periodontal treatment in the last 6 months. All subjects presented with good overall health.
Whole saliva collection and processing
Subjects were asked to not eat or drink 1 hr prior to WS collection. Prior to collection, donors were asked to rinse their mouth with water. A volume of 20 ml of masticatory stimulated whole saliva (WS) was obtained from donors chewing on parafilm (Fisher Scientific, Pittsburg, PA). The saliva produced was collected into graduated cylindrical tubes placed on ice. Immediately after collection, the samples were cleared from particulate matter by centrifugation at 27,000×g for 30 min at 4°C. The supernatant, referred to hereafter as WSS, was removed and frozen at −80°C.
Histatin 5 proteolysis in WSS
Synthetic histatin 5 (American Peptide Company, Sunnyvale, CA) was dissolved in water and added to WSS samples to a final concentration of 0.2 mg/ml. Samples were incubated at 37°C. After 0 h, 0.5 h, 1.5 h and 3 h incubation time intervals, 100 μl sample aliquots were removed, and boiled for 5 minutes. After sample cooling EDTA was added to a final concentration of 2.5 mM followed by drying at 60°C in a Vacufuge (Eppendorf, Hauppauge, NY).
Cationic Polyacrylamide Gel Electrophoresis (PAGE)
Cationic PAGE was performed essentially as described (Baum et al., 1977). The dried 100 μl sample aliquots were dissolved in 20 μl of basic gel sample buffer, containing 40% (w/v) sucrose and 0.04% (w/v) methyl green. The separating gel of the cationic gel contained 15% w/v acrylamide, 0.04% w/v bisacrylamide, 25% v/v glacial acetic acid, 0.12M KOH, 0.12% v/v TEMED, 0.04% w/v ammonium persulfate and 0.0007% w/v riboflavin 5' phosphate (pH 2.7). The stacking gel solution contained 2.5% w/v acrylamide, 0.625% w/v bisacrylamide, 60mM KOH, 0.125% v/v TEMED, 20% sucrose and 0.001% riboflavin 5' phosphate (pH 5.9). The polymerisation was accelerated by exposing the gels to a light source (60 Watts) for about 40 minutes. The anode running buffer contained 0.37 M glycine (pH 4.0, adjusted with glacial acetic acid) and the cathode running buffer contained 4.1% v/v glacial acetic acid (pH 4.3, adjusted with KOH). Gel electrophoresis was carried out at a constant voltage of 120 V for about 1.5 h. Gels were stained for 18 hr in a solution containing 0.1% Coomassie Brilliant Blue R-250, 10% v/v acetic acid and 40% v/v methanol. Gels were destained in the same solution without dye.
Z-RGYR-MCA hydrolysis
An aliquot of 5 μl of Z-RGYR-MCA from a 10 mM stock solution was added to each well of a black microtiter plate (Fisher Scientific, Pittsburgh, PA), followed by the addition of a 95 μl aliquot of WSS from each of the 48 healthy and periodontitis patients. Prior to mixing with substrate, the WSS samples had been incubated for 15 minutes at 37°C for temperature adjustment. Each subject's saliva was tested in triplicate. Substrate hydrolysis resulting in the release of the MCA group from cleavage after RGYR↓ was followed fluorimetrically at 37°C using a Genios spectrophotometer (Tecan, Männedorf, Switzerland) in the kinetic mode, at a λex=340nm and a λem=465nm, using a gain setting of 41. Measurements were conducted every 5 minutes during the initial phase period which was determined to be 0 to 60 minutes.
Zymography
Gelatin zymography
For gelatin zymography, precast 10% gelatin zymogram gels, 1.0mm × 10 well, were obtained from Invitrogen Novex, Carlsbad, CA. An aliquot of 15 μl of 1:10 diluted WSS were mixed with 10 μl of zymogram sample buffer containing 0.5M Tris-HCl pH 6.8, Glycerol, 10% (w/v) SDS, 0.1% bromophenol blue. In the first experiment, the 23 healthy subjects and 25 periodontally diseased subjects were loaded in order of disease status as follows: the healthy subjects in consecutive order, followed by the periodontal patients in consecutive order. In the second experiment, the samples were loaded in order of patient ID number, yielding an alternating healthy/perio pattern. Gel electrophoresis was carried out at a constant voltage of 100 V, for approximately 2 h and 15 min. The running buffer contained 2.9% (w/v) TrisBase, 14.4% (w/v) Glycine and 1% (w/v) SDS, pH 8.3. After completion of gel electrophoresis, each gel was washed twice for 30 min with 100 ml of Invitrogen Novex Zymogram Renaturing Buffer. After completion of these two cycles, the gels were washed three times for 20 min with 100 ml of Novex Zymogram Developing Buffer. Finally, the gels were placed in 100 ml of developing solution and incubated at 37°C for 24 hours. Gel staining and destaining was achieved in 10% (v/v) acetic acid and 40% (v/v) methanol with and without Coomassie Brilliant Blue R-250 0.1% (w/v), respectively.
Collagen Zymography
The collagen zymogram gels contained 0.055% collagen type IV, 8% w/v acrylamide, 0.4% w/v bisacrylamide, 0.1% w/v SDS and 375 mM Tris-HCl, pH 8.8. The stacking gel contained 4% w/v acrylamide, 0.2% w/v bisacrylamide, 0.1% w/v SDS and 126 mM Tris-HCl, pH 6.8. Polymerisation was initiated upon addition of 0.05% w/v ammonium persulfate and 0.1% v/v TEMED. Gel polymerisation was accelerated by exposure to a light source. The running buffer contained 2.9% (w/v) TrisBase, 14.4% (w/v) glycine and 1% (w/v) SDS, pH 8.3. Electrophoresis was carried out at 4°C and a constant voltage of 100 V, for approximately 2 hr and 15 min. Procedures for gel renaturing, developing, staining and destaining were performed as described above.
Histatin 5 zymography
Histatin zymography was conducted as described (Sun et al., 2009) and is essentially the same as the method for the collagen zymogram, except that natural histatin was incorporated in the gel as the enzymatic substrate. The final concentration of histatin 5 in the gel was 0.08% (w/v).
Densitometric Analysis
Relative quantitation of histatin 5 in cationic gels
Cationic gels containing the histatin 5/WSS incubation mixtures were scanned using a Versadoc Imager (BioRAD, Hercules, CA), followed by densitometric analysis of the histatin 5 protein bands using Quantity One software (Biorad, Hercules, CA). All values were corrected for background intensity. The pixel intensity of the histatin 5 band at t=0 min (immediately after addition to WSS) was set to 100%. Pixel intensities at each time point were plotted in Microsoft Excel and the half life time (t½) of histatin 5 degradation were determined from these plots.
Relative quantitation of protease bands in gelatin zymograms
Zymogram gels were scanned with the Versadoc Imager (BioRad) and the image was reversed such that cleared protease bands appeared as black bands on a white background. Images were manually adjusted to exhibit comparable levels of contrast by reference to the border of the gel. Densitometric analysis was performed of the three most prominent bands in the 37–150 kDa area, specifically a 42 kDa band, a 92 kDa band and a 130 kDa band. The pixel intensities (AU) in the respective bands/gel areas were established by using the Versadoc Software (Bio-Rad, Hercules, CA), and data were exported and averaged in Excel.
Statistical Analysis
Half-life times (t½) for histatin 5 degradation as well as pixel intensities in the gelatin zymogram bands in WSS were compared between the healthy and periodontal patients with the Student's t-test. To assure that these analyses were robust (i.e., unbiased by distribution or influential data points) non-parametric analyses were used in parallel (Wilcoxon Ranked-Sign test). In all instances parametric and non-parametric analyses yielded comparable results, and only results of t-tests are therefore mentioned in the text. Analyses were performed using SPSS for windows version 19.0 (SPSS IBM Inc, Chicago, IL).
Results
Gelatin proteolysis
WSS aliquots of the 23 healthy and the 25 periodontal subjects were thawed and loaded onto gelatin zymogram gels, either in order of periodontal health status (Supplemental Figure 1) or in order of subject ID (Figure 1). Visual inspection showed clear differences between the healthy and the periodontal group in the 37–150 kDa area of the gels. Prominent bands representing proteins with gelatinolytic activities, exhibited molecular weights of ~130 kD, ~92 kD and ~42 kD (Figure 1; indicated with arrows). By densitometric analysis of the reversed images of the gels, the pixel intensities of these bands were determined to be for the healthy group 991.6±243.4, 934.8±258.4, 278.9±120.3 (Mean±SD), respectively, and for the periodontal group 1320.7±278.5, 1319.5±235.1, 552.3±249.6 (Mean±SD), respectively. The group differences were statistically significant for all three bands (p‹0.001), indicating a stronger gelatin proteolysis in WSS of the periodontal patient group.
Fig. 1.
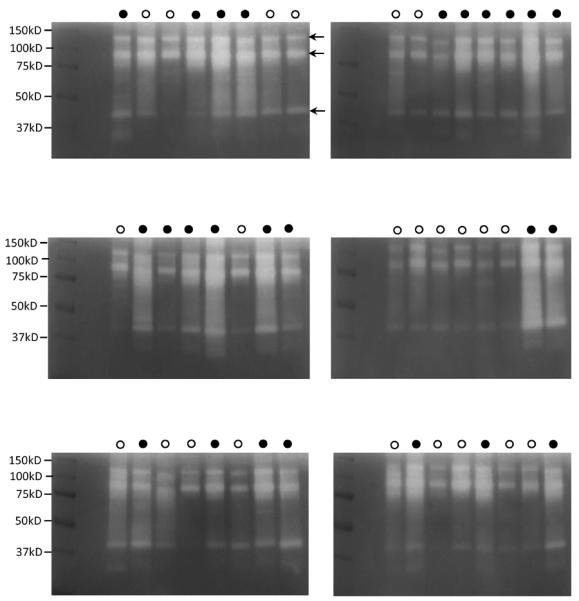
Gelatin zymography of whole saliva supernatant (WSS) of healthy and periodontal patients. An aliquot of 15 μl of 1:10 diluted WSS was loaded on gelatin zymogram gels. Samples were loaded according to the subject's ID number. Open circles: healthy subjects; closed circles: periodontal patients. Arrows point to prominent proteins displaying enzymatic activities with approximate molecular weights of 130 kD, 92 kD band and 42 kD.
Histatin 5 proteolysis
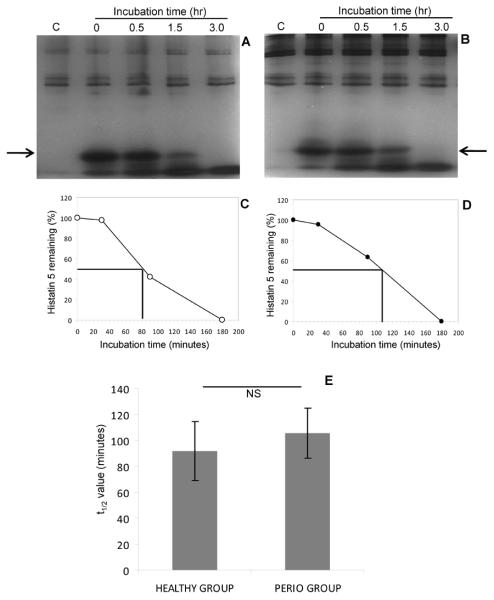
WSS of each of the 48 participating subjects was mixed with histatin 5. After 0 hr, 0.5 hr, 1.5 hr and 3 hr incubation, aliquots were removed and analyzed by cationic PAGE. Gels of a representative healthy and periodontitis patient are plotted in Figure 3A and 3B, and the gels of the remaining 22 healthy and 24 periodontal patients are shown in Supplemental Figures 2A and 2B. Unadulterated WSS of each subject was loaded in the far left lane. From these patterns it can be observed that subjects varied with respect to the overall proteins content in the high molecular weight region. Endogenous histatin 5 levels (arrow) were negligible, consistent with the high sensitivity of histatins to proteolytic fragmentation (Baum et al., 1976; Campese et al., 2009). When synthetic histatin 5 was added to WSS it was rapidly cleaved. From the degradation curves, the half-life time (t½) of the added histatin 5 was calculated (Figures 3C and 3D, respectively). The average t½ for the healthy group (n=23) was [Mean ± SD] 92.0±45.7 min and for the periodontal group (n=25) [Mean ± SD] 105±38.4 min (Figure 3E). There was no statistically significant difference between the healthy and periodontal groups (p>0.10; NS). Nonparametric tests (Wilcoxon ranked-sign test) yielded identical results.
Fig. 3.
Degradation of histatin 5 in WSS of healthy subjects and periodontal patients. The degradation of histatin 5 (H5) was visualised by cationic PAGE. A, representative gels of a healthy subject; B, representative gel of a periodontal patient; C, degradation curve of histatin 5 in WSS of the healthy subject; D, degradation curve of histatin 5 in WSS of the periodontal patient; E, average t1/2 values for histatin degradation in WSS of all 23 healthy and 25 periodontal patients.
Z-RGYR-MCA hydrolysis
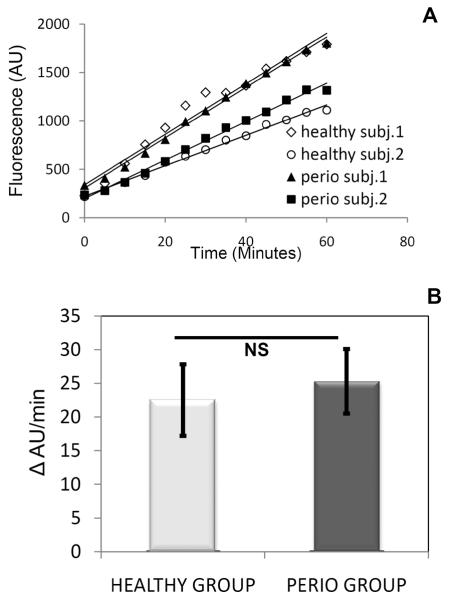
We also used a chemically synthesised substrate, Z-RGYR-MCA to probe for overall trypsin-like activities in the WSS samples. The RGYR sequence is naturally present in salivary histatins. This substrate will only be cleaved by enzymes recognising R (arginine) in the P1 position. Upon addition of Z-RGYR-MCA to WSS of the 48 subjects, the release of the MCA group, reflecting enzymatic activity, was measured fluorimetrically over a 60 minute time period. The fluorescence increase over time, representing Vi (initial rate), was determined for all subjects (data not shown). Graphs obtained for two representative subjects from the healthy and periodontal group are shown in Figure 4. The average Z-RGYR-MCA hydrolysis rate in all subjects was 22.51±10.6 and 25.30±9.61 AU/min for healthy and periodontal patients, respectively (p>0.10; NS), indicating no differences between groups, consistent with observations made with the argine-rich substrate histatin 5.
Fig. 4.
Rate of hydrolysis of the synthetic enzyme substrate Z-RGYR-MCA in WSS of healthy subjects and periodontal patients. A, Initial rates of degradation of Z-RGYR-AMC in WSS from two representative healthy subjects and two representative periodontal patients; B, Average rates (bars) and standard deviations (vertical lines) of degradation of Z-RGYR-MCA in all 23 healthy and 25 periodontal patients.
Comparison of collagen type IV and histatin 5 zymography
The above results suggest that gelatinolytic activities are elevated in periodontal disease whereas activities towards histatin 5 or other arginine-rich salivary protein substrates are not increased, However, the comparisons were made using different assay systems: gelatin degradation was assessed in gel (zymography) and histatin degradation was assessed in solution. We wished therefore to compare the differences side by side in a comparable assay format: collagen type IV zymography and histatin 5 zymography. Collagen type IV was chosen instead of gelatin since it more closely resembles connective tissue proteins observed in the periodontal pocket. WSS from four representative healthy and four representative periodontal patients were loaded onto the gels (Figure 5). In the collagen IV zymogram (Figure 5A), the patterns of the healthy and periodontal patients resembled those observed with gelatin zymography where the periodontal patients showed a higher activity. On the other hand, the histatin 5 zymogram (Figure 5B) did not show differences in activity between both groups, in accordance with the activities observed towards histatin 5 in solution. In fact, one of the healthy subjects (Figure 5B, lane 9) showed extensive degradation of histatin 5 whereas some of the periodontal patients demonstrated remarkably low levels of degradation.
Fig. 5.
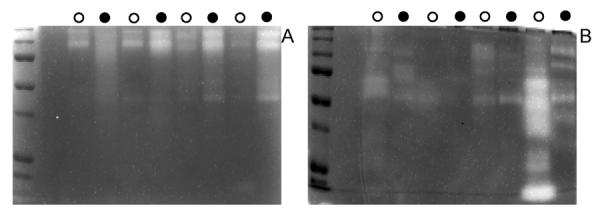
Histatin 5 zymography (A) and collagen zymography (B) of WSS of 4 representative healthy (open circles) and 4 representative periodontal patients (closed circles). For the histatin zymogram 30 μl of undiluted WSS was loaded whereas for the collagen zymogram a 15 μl aliquot of a 1:4 diluted WSS sample was loaded. The experiment was performed in duplicate. Representative gels are shown.
Discussion
A plethora of chemical reactions and molecular interactions take place in WS. The present study focused on the interactions between enzymes and their tissue and salivary protein substrates. The objective of our study was two-fold. First, to confirm that collagenolytic activies are increased during periodontal inflammation and detectable in human WSS. Second, to investigate if enzymes elevated during periodontal inflammation would include enzymes with trypsin-like activities, such as gingipain R and G released by P. gingivalis, and if salivary protein substrates would be targeted.
In periodontal WSS we found increased protease activities toward gelatin or collagen, which was substantial, readily detectable, and statistically significant for all three prominent enzyme bands in the gelatin zymograms. Visual analyses of the gelatin zymograms showed particularly strong increases at three bands (42 kDa, 92 kDa, 130 kDa), which was quantitatively confirmed by densiometric analyses. The 42 kDa band likely represents activated MMP-1 (Bildt et al., 2008; Sorsa et al., 2006), the 92 kDa band represents MMP-9 (Makela et al., 1994; Teng et al., 1992) and the 130 kDa band a complexed form of MMP-2 (Bildt et al., 2008). The increased gelatinolytic activities is consistent with reported increased levels of salivary MMP-8 and MMP-9 in periodontal patients, as determined by quantitative PCR, antibody arrays (Ramseier et al., 2009) and immunoblotting (Ding et al., 1994; Gursoy et al., 2010; Sorsa et al., 2010), and elevated WS gelatinolytic activities as assessed by zymography (Gangbar et al., 1990; Makela et al., 1994).
Healthy individuals show high WS proteolytic activities as evidenced by the extensive fragmentation of salivary proteins, including histatins, statherins and non-glycosylated members of the proline-rich protein family (Helmerhorst et al., 2008; Messana et al., 2008; Vitorino et al., 2010). However, these overall activities had not previously been compared for periodontal disease patients and healthy individuals. Therefore, the second objective of the study was to determine if trypsin-like activities, typically associated with periodontal microbial enzymes, were increased during periodontal inflammation. Histatin 5 was a protease substrate of particular interest since it is rich in both lysine and arginine residues and exhibits antimicrobial activities relevant in periodontal disease. For example, periodontal pathogens such as P. gingivalis produce gingipain R and gingipain K, cleave specifically after arginine and lysine residues, respectively (Sheets et al., 2008). Notwithstanding, we found no difference between healthy and periodontally diseased groups in WSS enzyme activities directed towards histatin 5, neither when it was exposed to the enzymes in solution or in gel. We likewise noted no differences in WSS protease activities between healthy and periodontal groups towards histatin-derived synthetic enzymatic substrates Z-RGYR-AMC (this study) or Z-FHEK-AMC (data not shown). The fact that such trypsin-like activities were not elevated in periodontal WSS samples suggests that increased colonisation with pathogens such as P. gingivalis in periodontal disease cannot be readily ascertained by enzyme activity measurement as utilised in the present study.
A limitation of our study is that clinical correlates such as smoker status, gingival/plaque index, and detailed systemic health and disease parameters were not collected from the enrolled subjects. Unfortunately such information could not anymore be obtained retrospectively in a reliable fashion due to the time that had elapsed since sample collection. While the literature provides no indication that smoking is a major determinant of proteolytic activity, it remains possible that poor oral hygiene (e.g., as assessed by plaque index) may contribute to the group differences, and further explorations should aim to elucidate the exact determinants of the gelatinolytic results. Notwithstanding, this activity showed the expected increase in the periodontal patient group, and given the noted differences in gelatinolytic activities, the subsequent assessment of histatin and RGYR-cleaving activities was justified. Importantly, the present study showed, for the first time, that proteases present in WS of periodontal patients tend not to degrade proteins randomly, but show some level of substrate specificity. The specificity observed, directed towards connective tissue proteins but not salivary substrates may explain the observation that in patients with periodontal disease destruction of the periodontal ligaments is rampant, while overall, other salivary defense mechanisms are seemingly unaffected. Furthermore, this pattern of results strongly indicates that host-derived collagenolytic enzymes, but not bacteria-derived trypsin-like enzymes in human saliva are of diagnostic value in periodontal disease. Implementation of the noted differences in MMP-protease activities in the clinic has been proposed (5) and these activities may represent one of the best diagnostic markers for active periodontal disease to date. In the future, WS proteolytic parameters in conjunction with microarray-based microbial signatures could increase the discriminating power of such diagnostic assays for periodontal disease and its progression.
Supplementary Material
Fig. 2.
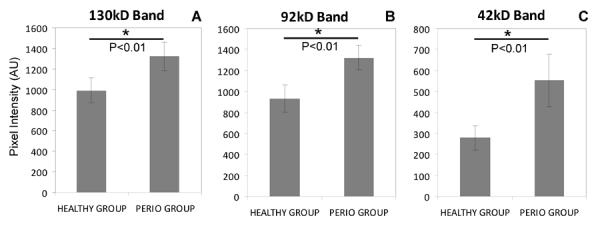
Densitometric analysis of the 37–150 kD region in gelatin zymography of healthy and periodontal patients. A, B, C, pixel analysis of the 130 kD, 92 kD band and 42 kD band, respectively.
References
- Baum BJ, Bird JL, Millar DB, Longton RW. Studies on histidine-rich polypeptides from human parotid saliva. Arch Biochem Biophys. 1976;177:427–36. doi: 10.1016/0003-9861(76)90455-0. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Bird JL, Millar DB, Longton RW. Isolation and partial characterization of an histidine-rich polypeptide from parotid saliva of the monkey, Macaca nemestrina. Comp Biochem Physiol A. 1977;56:115–20. doi: 10.1016/0300-9629(77)90171-2. [DOI] [PubMed] [Google Scholar]
- Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von den Hoff JW. Collagenolytic fragments and active gelatinase complexes in periodontitis. Journal of periodontology. 2008;79:1704–11. doi: 10.1902/jop.2008.080021. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Turkenburg M, Nazmi K, Veerman EC, de Geus EJ, Nieuw Amerongen AV. Stress as a determinant of saliva-mediated adherence and coadherence of oral and nonoral microorganisms. Psychosom Med. 2003;65:604–12. doi: 10.1097/01.psy.0000074759.71084.ab. [DOI] [PubMed] [Google Scholar]
- Campese M, Sun X, Bosch JA, Oppenheim FG, Helmerhorst EJ. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Archives of oral biology. 2009;54:345–53. doi: 10.1016/j.archoralbio.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C. Estimates, from salivary analyses, of the turnover time of the oral mucosal epithelium in humans and the number of bacteria in an edentulous mouth. Archives of oral biology. 2003;48:329–36. doi: 10.1016/s0003-9969(03)00014-1. [DOI] [PubMed] [Google Scholar]
- de Jong EP, van Riper SK, Koopmeiners JS, Carlis JV, Griffin TJ. Sample collection and handling considerations for peptidomic studies in whole saliva; implications for biomarker discovery. Clin Chim Acta. 2011;412:2284–8. doi: 10.1016/j.cca.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Liede K, Leppa S, Ingman T, Sepper R, Konttinen YT, Sorsa T. Gingival crevicular fluid and salivary matrix metalloproteinases of heavy smokers as indicators of periodontal health. Annals of the New York Academy of Sciences. 1994;732:453–5. doi: 10.1111/j.1749-6632.1994.tb24783.x. [DOI] [PubMed] [Google Scholar]
- Flora B, Gusman H, Helmerhorst EJ, Troxler RF, Oppenheim FG. A new method for the isolation of histatins 1, 3, and 5 from parotid secretion using zinc precipitation. Protein expression and purification. 2001;23:198–206. doi: 10.1006/prep.2001.1493. [DOI] [PubMed] [Google Scholar]
- Gangbar S, Overall CM, McCulloch CA, Sodek J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: correlation with periodontal disease activity in adult and juvenile periodontitis. J Periodontal Res. 1990;25:257–67. doi: 10.1111/j.1600-0765.1990.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontology 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy UK, Kononen E, Pradhan-Palikhe P, Tervahartiala T, Pussinen PJ, Suominen-Taipale L, Sorsa T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37:487–93. doi: 10.1111/j.1600-051X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Helmerhorst EJ, Sun X, Salih E, Oppenheim FG. Identification of Lys-Pro-Gln as a novel cleavage site specificity of saliva-associated proteases. The Journal of biological chemistry. 2008;283:19957–66. doi: 10.1074/jbc.M708282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods in molecular biology (Clifton, N.J. 2010;666:21–30. doi: 10.1007/978-1-60761-820-1_2. [DOI] [PubMed] [Google Scholar]
- Imatani T, Kato T, Minaguchi K, Okuda K. Histatin 5 inhibits inflammatory cytokine induction from human gingival fibroblasts by Porphyromonas gingivalis. Oral microbiology and immunology. 2000;15:378–82. doi: 10.1034/j.1399-302x.2000.150607.x. [DOI] [PubMed] [Google Scholar]
- Ingman T, Sorsa T, Lindy O, Koski H, Konttinen YT. Multiple forms of gelatinases/type IV collagenases in saliva and gingival crevicular fluid of periodontitis patients. J Clin Periodontol. 1994;21:26–31. doi: 10.1111/j.1600-051x.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Makela M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. Journal of dental research. 1994;73:1397–406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- Messana I, Cabras T, Pisano E, Sanna MT, Olianas A, Manconi B, Pellegrini M, Paludetti G, Scarano E, Fiorita A, Agostino S, Contucci AM, Calò L, Picciotti PM, Manni A, Bennick A, Vitali A, Fanali C, Inzitari R, Castagnola M. Trafficking and postsecretory events responsible for the formation of secreted human salivary peptides: a proteomics approach. Mol Cell Proteomics. 2008;7:911–26. doi: 10.1074/mcp.M700501-MCP200. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Xu T, Helmerhorst EJ, Ori G, Troxler RF, Oppenheim FG. Inhibitory effect of synthetic histatin 5 on leukotoxin from Actinobacillus actinomycetemcomitans. Oral microbiology and immunology. 2002;17:143–9. doi: 10.1034/j.1399-302x.2002.170302.x. [DOI] [PubMed] [Google Scholar]
- Nieminen A, Nordlund L, Uitto VJ. The effect of treatment on the activity of salivary proteases and glycosidases in adults with advanced periodontitis. Journal of periodontology. 1993;64:297–301. doi: 10.1902/jop.1993.64.4.297. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG. Saliva diagnostics - historical perspectives and present. In: Wong DT, editor. Salivary Diagnostics. 1st Edition Wiley-Blackwell; Ames, Iowa: 2008. pp. 79–93. [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. Journal of periodontology. 2009;80:436–46. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos GE, Bernimoulin JP. Collagen as a basic element of the periodontium: immunohistochemical aspects in the human and animal. 1. Gingiva and alveolar bone. Parodontol. 1990;1:363–75. [PubMed] [Google Scholar]
- Schipper RG, Silletti E, Vingerhoeds MH. Saliva as research material: biochemical, physicochemical and practical aspects. Archives of oral biology. 2007;52:1114–35. doi: 10.1016/j.archoralbio.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Sheets SM, Robles-Price AG, McKenzie RM, Casiano CA, Fletcher HM. Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis. Front Biosci. 2008;13:3215–38. doi: 10.2741/2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Dawes C. The salivary proteome: challenges and perspectives. Proteomics. 2011;5:575–9. doi: 10.1002/prca.201100046. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of medicine. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Mantyla P, Tervahartiala T, Pussinen PJ, Gamonal J, Tuomainen AM, Lauhio A, Pussinen PJ, Mäntylä P. MMP activation in diagnostics of periodontitis and systemic inflammation. J Clin Periodontol. 2010;38:817–9. doi: 10.1111/j.1600-051X.2011.01753.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, Lauhio A, Pussinen PJ, Mäntylä P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2011;63:108–13. doi: 10.1016/j.phrs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–54. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Salih E, Oppenheim FG, Helmerhorst EJ. Activity-based mass spectrometric characterization of proteases and inhibitors in human saliva. Proteomics. 2009;3:810–20. doi: 10.1002/prca.200800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YT, Sodek J, McCulloch CA. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J Periodontal Res. 1992;27:544–52. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Thomadaki K, Helmerhorst EJ, Tian N, Sun X, Siqueira WL, Oppenheim FG. Whole-saliva proteolysis and its impact on salivary diagnostics. Journal of dental research. 2011;90:1325–30. doi: 10.1177/0022034511420721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino R, Alves R, Barros A, Caseiro A, Ferreira R, Lobo MC, Bastos A, Duarte J, Carvalho D, Santos LL, Amado FL. Finding new posttranslational modifications in salivary proline-rich proteins. Proteomics. 2010;10:3732–42. doi: 10.1002/pmic.201000261. [DOI] [PubMed] [Google Scholar]
- Zhang L, Henson BS, Camargo PM, Wong DT. The clinical value of salivary biomarkers for periodontal disease. Periodontology 2000. 2009;51:25–37. doi: 10.1111/j.1600-0757.2009.00315.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.