Abstract
Objective
Alcohol abuse occurs frequently in those with HIV infection. Alcohol has been linked to poor response to HIV treatment and more rapid progression of HIV. One possible contributor to such observations is drug interactions between alcohol and antiretroviral medications (ARV). This study examined drug interactions between antiretroviral therapies (ART) containing either efavirenz or ritonavir with alcohol.
Methods
HIV-infected individuals not currently receiving ART participated in a randomized, double-blind, placebo-controlled, study in which alcohol (or placebo) was administered and followed by blood sampling for pharmacokinetics, subjective, cardiovascular, and neuropsychological responses obtained at pre-determined times. ART was then initiated and alcohol (or placebo) sessions were repeated after at least two weeks of observed ART.
Results
Blood alcohol concentrations (BAC) were lower following ART in a pattern consistent with decreased bioavailability. No effect of alcohol on ritonavir or efavirenz pharmacokinetics was observed. A pharmacodynamic interaction between alcohol and efavirenz was observed as evidenced by no change in intoxication or drowsiness before and after efavirenz ART despite lower BAC.
Conclusions
These results show the effectiveness of implementing ART and its role in diminution of BAC which could be associated with decreased risk of physiological toxicities related to alcohol consumption relative to those with untreated HIV infection. A potential pharmacodynamic interaction between alcohol and efavirenz was observed as demonstrated by a lack of decline in ratings of intoxication and drowsiness despite decreased BAC. Alcohol consumption did not alter the pharmacokinetics of ritonavir or efavirenz.
Keywords: alcohol, efavirenz, ritonavir, HIV infection, drug interactions
Introduction
Alcohol use and abuse occur frequently in those with HIV/AIDS. Alcohol has been linked to poor response to HIV disease treatment, as well as more rapid progression of HIV (Samet et al., 2004; Chander et al., 2006; Braithwaite et al., 2007). The underlying etiology of these effects is not well understood. One possible contributor to such observations is drug-drug interactions between alcohol and antiretroviral medications (ARV). Whether drug interactions between antiretroviral therapy (ART) medications and alcohol occur is unknown, but, based on the clinical pharmacology of alcohol and major classes of HIV therapeutics, there is reason to believe that there could be interactions of clinical consequence.
The principal metabolic route for alcohol is through alcohol dehydrogenase (ADH) which mediates the conversion of alcohol to acetaldehyde. Acetaldehyde is then converted to acetate by other enzymes and the final products in this metabolic pathway are carbon dioxide and water (Bosron et al., 1993). Alcohol also is metabolized in the liver by the enzyme cytochrome P450 (CYP 450) 2E1 and to a lesser extent by CYP 450 3A4 (Lieber, 2004), enzymes whose activity may be increased with chronic drinking (Lieber, 1994).
Two commonly used HIV therapeutics in expert-recommended preferred ART regimens have robust effects on CYP 450 3A4 drug metabolizing enzymes and are likely to affect pharmacokinetics and potentially, pharmacodynamics of drugs such as alcohol that are metabolized by these enzymes. Ritonavir is an inhibitor of CYP 450 3A4 (Kumar et al., 1996) and it might potentially increase effects of alcohol when these drugs are administered concomitantly. Ritonavir is metabolized principally by CYP 3A4 and 2D6 (Li and Chan, 1999). Efavirenz is a potent inducer of CYP 3A4 (Physician’s Desk Reference, 2007) which might decrease blood alcohol levels, potentially decreasing alcohol effects. Efavirenz is principally metabolized by CYP 2B6 and CYP 3A4 (Ward et al., 2003). Drug interactions might place patients at risk for toxicities or possibly for ineffective antiretroviral plasma concentrations, as well as for non-adherence to prescribed regimens.
In the current study alcohol administration was undertaken in volunteers with HIV infection who were eligible for, but were not taking ART and repeated following stabilization on ART. We report on pharmacokinetics, subjective, neuropsychological, and physiological effects of alcohol alone, ART components alone, and alcohol and ART given concurrently.
Methods
Methods for this study have been described in detail previously (McCance-Katz et al., 2012). Briefly, volunteers with untreated HIV disease participated in this study which was reviewed and approved by the Institutional Review Board at the University of California, San Francisco (UCSF) and is registered at ClinicalTrials.gov (NCT00879047). Two participants were ART naïve. The remaining participants had been previously treated with ART, but had discontinued therapy for reasons unrelated to the current study and had been off ART for at least one month prior to enrollment. All participants were over age 21, had a confirmed diagnosis of HIV disease, 18 of 20 had at least one previous course of ART, a BMI <30, and no acute medical illness or current substance use disorder that, in the view of the study team following medical history and physical examination, would interfere with the ability to undertake the study or would require immediate treatment. Physiological dependence on alcohol was exclusionary. Subjects could take concomitant medications for co-occurring medical disorders if those medications were known not to be strong inducers or inhibitors CYP 3A4 or 2E1 activity. Upon completion of the first set of study sessions (prior to ART initiation) (described below), participants were discharged from the clinical research center inpatient unit to be followed in the Addiction Medicine Research Clinic where the combination ART regimen prescribed by their clinician was initiated (Table 1). Observed ART dosing occurred at the clinic on weekdays. On weekends, participants took their ART at home, but called in each day to report medication ingestion. If they did not call in the morning, they received a reminder call from study staff to assist the participant with medication adherence.
Table 1.
Antiretroviral medications co-administered with either efavirenz or ritonavir as part of effective antiretroviral therapy
| Efavirenz | Ritonavir |
|---|---|
| Emtricitabine | Emtricitabine |
| Tenofovir disoproxil fumarate | Tenofovir disoproxil fumarate |
| Atazanavir Darunavir |
The study design was within-subject (ritonavir sample n=10, efavirenz sample n=10) and participants were randomly assigned in a double-blind fashion to receive either alcohol at a dose of 1 g/kg dissolved in a total volume of 16 ounces of a fruit-flavored drink or an alcohol placebo where alcohol was sprayed at the surface of the drink, but no other alcohol was contained in the drink. The dose of alcohol administered was one that had been calculated and demonstrated to produce peak blood alcohol concentrations of approximately 100 mg/dL in healthy subjects (McCance-Katz et al., 1993; McCance-Katz et al., 1998). Two sets of study sessions occurred; one set in which alcohol or placebo was administered on separate days and a second set of alcohol administration studies following at least 2 weeks of ART. The study sessions took place at the UCSF Clinical Research Center (CRC) at San Francisco General Hospital.
On study days, participants received a standardized breakfast prior to alcohol or placebo administration. Study sessions began in the morning, with baseline physiological and subjective assessments at −60 and −15 minutes before alcohol administration. For the second set of alcohol or placebo alcohol administration sessions, ART was administered immediately following ingestion of the alcohol drink. Blood sampling for all sessions occurred at baseline prior to alcohol administration and over the next 8 hours at time points of 15 min, 30 min, 1, 1.5, 2, 3, 4, 5, 6 and 8 hours. In the post-ART sessions, blood samples were also obtained at 12 and 24 hours for antiretroviral drug measurement. Subjective and cardiovascular responses, as well as neuropsychological measures (Digit Symbol Substitution Test (DSST), a global measure of proficiency in information processing, concentration, and fine motor coordination (Wechsler, 1958)), were collected at baseline and at pre-determined times following alcohol and ART administration.
Alcohol Assay
Plasma alcohol concentrations were determined using gas chromatography with flame ionization detection with isopropanol as an internal standard.
Pharmacokinetics Analysis
Alcohol and ARV pharmacokinetics were calculated following administration of each alone, and again following alcohol and ARV co-administration. Area under the concentration-time curve (AUC expressed for alcohol as AUC 0-8 (time from alcohol administration to 8 hours post administration) or for ARV as AUC 0-24 (time from ARV administration to 24 hours post administration)) (as shown in Table 3) was calculated using the trapezoidal rule. Maximum plasma concentration (Cmax), time of Cmax (Tmax), and minimum plasma concentration (Cmin, expressed as C8 (alcohol concentration at 8 hours post-administration) or C24 (efavirenz concentration at 24 hours post administration)) (as shown in Table 3), were determined directly by inspection from the concentration-time curve. The elimination rate was determined from the slope of the linear portion of the descending arm of the concentration-time curve.
Table 3.
| a. Effect of ritonavir on alcohol pharmacokinetics | |||
|---|---|---|---|
| Pharmacokinetic Parameter | Pre-Ritonavir (n = 10) | Post-Ritonavir (n = 10) | p value |
| AUC0-8 (mg-h/dL) | 573 (45) | 485 (46) | 0.08 |
| Cmax (mg/dL) | 134 (6) | 118 (8) | 0.01 |
| Tmax (h) | 1.0 (0.5–1.5) | 1.125 (0.25–2.0) | n.s. |
| C8 | 7.9 (3.9) | 1.6 (1.5) | 0.09 |
| Elimination rate (mg/dL-h) | 21.2 (0.6) | 20.7 (0.8) | 0.50 |
| b. Effect of efavirenz on alcohol pharmacokinetics | |||
| Pharmacokinetic Parameter | Pre-Efavirenz (n = 10) | Post-Efavirenz (n = 10) | p value |
| AUC0-8 (mg-h/dL) | 572 (51) | 492 (34) | 0.03 |
| Cmax (mg/dL) | 131 (6) | 115 (5) | 0.06 |
| Tmax (h) | 1.0 (0.5–2.0) | 1.5 (0.5–3.0) | n.s. |
| C8 | 7.2 (5.5) | 2.7 (1.8) | 0.30 |
| Elimination rate (mg/dL-h) | 22.7 (1.0) | 24.1 (1.4) | 0.36 |
| c. Effect of alcohol on ritonavir pharmacokinetics | |||
| Pharmacokinetic Parameter | Placebo (n = 10) | Ethanol (n = 10) | p value |
| AUC0-24 (mg-h/L) | 9.72 (1.61) | 9.59 (1.65) | 0.83 |
| Cl/F (L/h) | 12.6 (1.5) | 13.5 (2.0) | 0.45 |
| Cmax (mcg/mL) | 1.21 (0.20) | 1.01 (0.19) | 0.17 |
| Tmax (h) | 5 (1 – 6) | 5 (2 – 8) | n.s. |
| Half-life (h) | 5.4 (0.5) | 6.5 (0.6) | 0.18 |
| d. Effect of alcohol on efavirenz pharmacokinetics | |||
| Pharmacokinetic Parameter | Placebo (n = 10) | Ethanol (n = 10) | p value |
| AUC0-24 (mg-h/L) | 71.8 (10.1) | 73.8 (10.1) | 0.51 |
| Cl/F (L/h) | 10.7 (1.9) | 9.7 (1.3) | 0.28 |
| Cmax (mcg/mL) | 5.5 (0.5) | 5.3 (0.4) | 0.58 |
| Tmax (h) | 3 (1-4) | 4 (1.5 -6) | n.s. |
| C24 (mcg/mL) | 2.1 (0.4) | 2.3 (0.4) | 0.21 |
| Half-life (h) | 30 (3.8) | 47 (11.4) | 0.12 |
Note: Values are the mean (standard error of the mean), except that Tmax is given as median (range). The paired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
Statistical Analysis
Student’s paired t-test was used to test the significance of the differences in pharmacokinetic parameters for alcohol alone and alcohol following ART and for ARV alone and ARV following alcohol consumption (within-subject analyses). The Wilcoxon test was used for the within-subject comparison of the values of Tmax. AUC (with baseline subtracted) was calculated for cardiovascular, subjective and neuropsychological responses (values were divided by the total length of the study period and are thus interpreted as an average elevation from baseline over the study time) and these were compared by repeated measures ANOVA as each subject was studied under all four conditions. Comparisons of subject characteristics were made by single factor ANOVA. Because of non-normality of the data for HIV viral load, the non-parametric Wilcoxon signed-rank test, which is not affected by lack of normality, was applied to analyze differences in HIV viral load at baseline and post-study. A difference was considered statistically significant if the p value was ≤ 0.05 (two-tailed).
Results
Characteristics of the study samples are shown in Table 2. Study participants included Caucasian and racial minorities (African-American and Latino/Hispanic) in nearly even numbers. Alcohol use was moderate and not different between the two samples (ritonavir, efavirenz). Alcohol use disorders (alcohol abuse and nonphysiological alcohol dependence) were present in only two participants. Concomitant illicit drug use disorders included mainly stimulant use disorders (cocaine and methamphetamine abuse). HIV viral load in the samples was variable and ranged from undetectable to 5.0 log copies/mL with evidence of a strong antiviral response in study participants as shown by the decrement in viral load following initiation of a clinician-selected ART regimen (ritonavir-containing ART group: p=0.004 and efavirenz-containing ART group: p= 0.004) (Table 2). Baseline AST and ALT were slightly higher in the ritonavir subjects and increased modestly after initiation of ritonavir, although no differences were statistically significant. This may relate to the fact that 3 of the 4 study participants with HCV co-infection were in the ritonavir sample. The increases observed in these liver enzymes were moderate (≤ 2 times the upper limit of the normal range) and without associated adverse events.
Table 2.
Participant demographics and characteristics
| Ritonavir (n=10) | Efavirenz (n=10) | |
|---|---|---|
| Age (yrs) | 43.0 (2.6)1 | 43.5 (2.7) |
| Female | 1 (10%)2 | 1 (10%) |
| Race: | ||
| African-American | 2 (20%) | 4 (40%) |
| Caucasian | 4 (40%) | 6 (60%) |
| Hispanic | 4 (40%) | 0 (0%) |
| Current Substance Use Disorders: | ||
| Cocaine Abuse | 1 (10%) | 2 (20%) |
| Methamphetamine Abuse | 0 (0%) | 1 (10%) |
| Cannabis Abuse | 1 (10%) | 1 (10%) |
| Alcohol Abuse | 1 (10%) | 1 (10%) |
| Nicotine Dependence | 1 (10) | 0 (0%) |
| Current Alcohol Use (drinks/week) | 7.7 (2.8) | 6.9 (2.4) |
| Current Nicotine Use (cigarettes/day) | 4.6 (2.0) | 0.7 (0.4) |
| Laboratory Values Pre ART: | ||
| ALT (U/L) | 44 (11.2) | 27 (3.3) |
| AST (U/L) | 45 (8.0) | 30 (3.4) |
| HIV Viral Load (copies/mL) | ||
| Mean | 72,948 | 33,423 |
| Median | 17,674 | 15,225 |
| Range | 109 - 394,578 | 0 - 168,067 |
| CD4 Count (mm3) | ||
| Mean | 312 | 376 |
| Median | 303 | 372 |
| Range | 119 - 592 | 85 - 838 |
| Laboratory Values Post ART: | ||
| ALT (U/L) | 68 (26.0) | 32 (6.0) |
| AST (U/L) | 61 (19.1) | 28 (3.2) |
| HIV Viral Load (copies/mL) | ||
| Mean | 799 | 149 |
| Median | 125 | 64 |
| Range | 0 - 4985 | 0 - 720 |
| CD4 Count (mm3) | ||
| Mean | 396 | 367 |
| Median | 382 | 294 |
| Range | 106 - 733 | 133 - 697 |
| Hepatitis B (lifetime) | 1 (10%) | 5 (50%) |
| Hepatitis C | 3 (30%) | 1 (10%) |
|
| ||
| Other Diagnoses | Chronic Pain | |
| Neuropathy | ||
| Hypertension | ||
| Hyperlipidemia | ||
| Dysthymia | ||
mean (SE)
number (percent of sample)
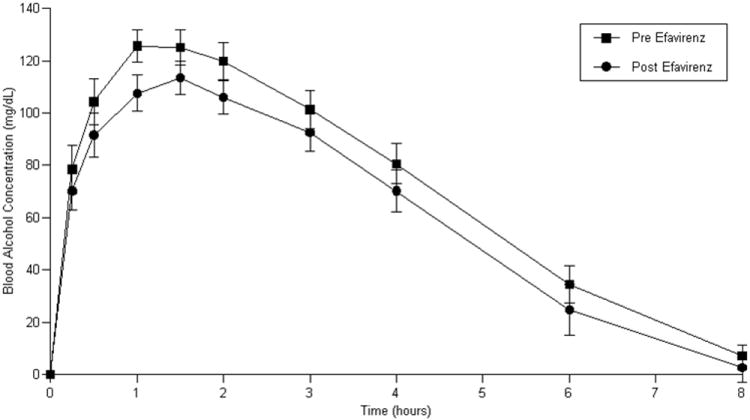
Blood alcohol concentrations in study participants were decreased after initiation of and adherence to ART relative to alcohol concentrations when untreated for HIV infection as indicated by alcohol AUC 0-8 (area under the concentration-time curve from time zero to 8 hours post alcohol ingestion) (ritonavir-containing ART group: (p = 0.08); efavirenz-containing regimen (p=0.03)) and reductions in Cmax (maximum alcohol concentration following alcohol ingestion) (Cmax ritonavir-containing ART group: (p = 0.01); efavirenz-containing ART group: (p=0.06). (Figure 1 a and b, Table 3 a and b).
Figure 1.
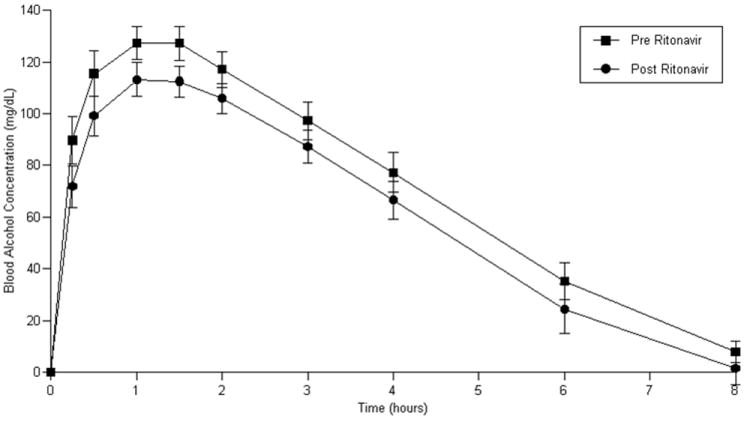
a: Blood alcohol concentrations prior to and following ritonavir-containing ART
b: Blood alcohol concentrations prior to and following efavirenz-containing ART
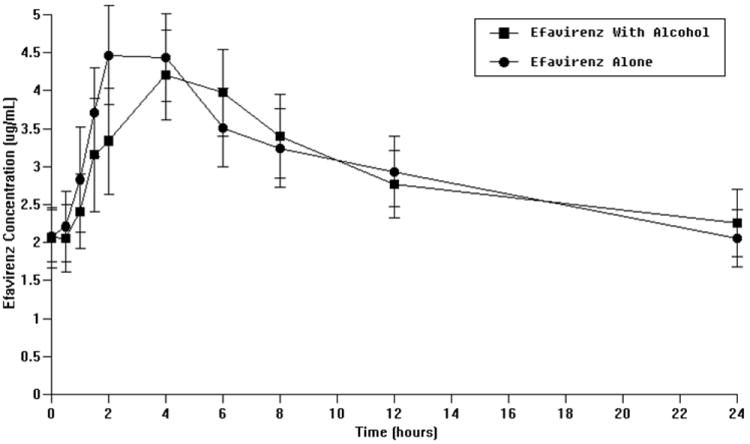
No significant differences in ritonavir or efavirenz plasma concentrations or pharmacokinetics over a 24 hour dosing interval (as shown by AUC 0-24) were observed (Figure 2 a and b, Table 3 c and d, respectively). Of note, most Cmin (minimum plasma concentration following ritonavir administration) results for ritonavir were below the limit of detection and, therefore, are not reported.
Figure 2.
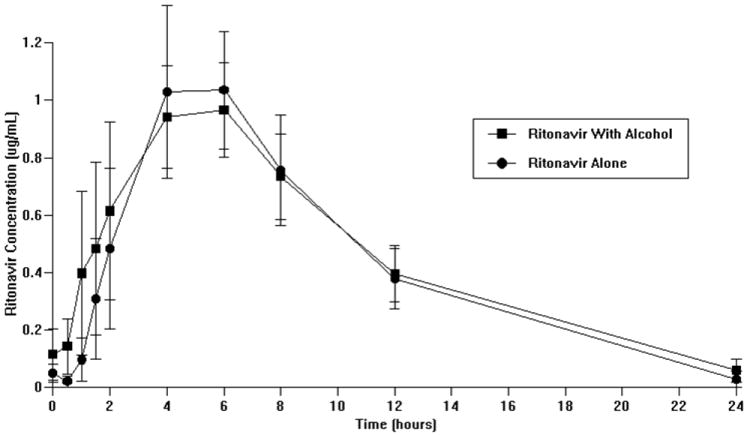
a: Effect of alcohol on ritonavir plasma concentrations
b: Effect of alcohol on efavirenz plasma concentrations
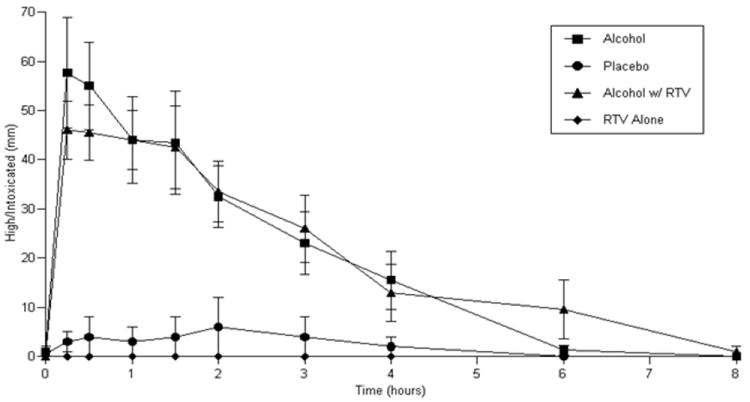
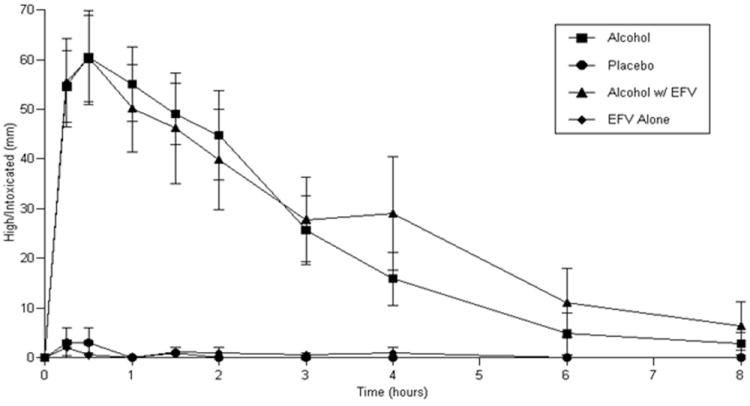
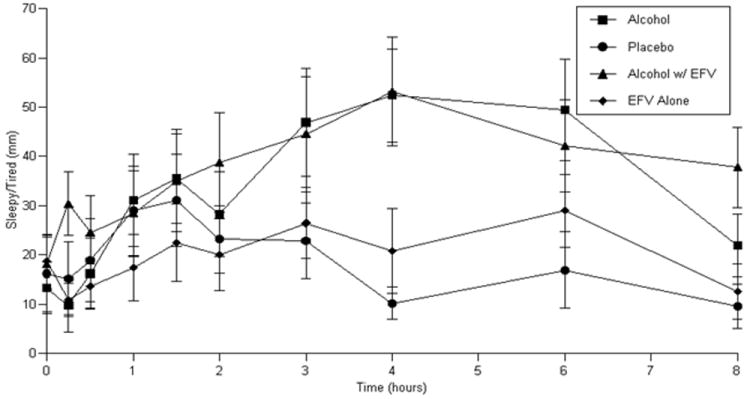
Visual analog scales showed signficant effects for ‘High/Intoxicated’ (ritonavir-containing ART group: p<0.0001, efavirenz-containing ART group: p<0.0001) with study conditions in which alcohol was administered showing the greatest effects (Figure 3 a and b). However, there was no significant difference in ‘High/Intoxicated’ with alcohol administration either with or without ART administration. ‘Sleepy/Tired’ ratings were significantly increased in those receiving efavirenz-containing ART (p=0.0037) with the greatest effects occurring under conditions in which alcohol was administered. ‘Sleepy/Tired’ AUC increased by 25.6 (8.0) mm·h (mean (standard error)) for alcohol administration (Figure 3c). Alcohol administration with efavirenz produced an increase in AUC of 22.6 (10.1) mm·h (Figure 3c). There was no significant difference in ‘Sleepy/Tired’ ratings for the comparison of the alcohol alone condition and the alcohol-efavirenz-containing ART condition. ‘Sleepy/Tired’ ratings for placebo alcohol given with efavirenz-containing ART were significantly lower than when alcohol was given with efavirenz-containing ART (p=0.0149). There were no other significant differences in subjective effects observed for ‘happy/feel good’, ‘sad’, ‘nervous’, ‘concentration’, ‘liking for alcohol’, or ‘craving for alcohol’. Significant effects were observed on Digit Symbol Substitution Test performance (ritonavir-containing ART group: p=0.0214; efavirenz-containing ART group: p=0.0137). Decreased performance was observed during study sessions in which alcohol was administered relative to sessions in which placebo alcohol was administered with an average decline in DSST score of 7.3 (2.5) for alcohol administration and 4.9 (2.0) for alcohol administration given with ritonavir-containing ART. Similarly, an average decline in DSST score of 3.8 (2.5) was observed in the efavirenz-containing ART group for alcohol alone administration and a decline of 6.1 (2.5) for alcohol administration when given with efavirenz-containing ART. There were no statistically significant differences in DSST performance for alcohol alone vs. alcohol with either ritonavir or efavirenz-containing ART.
Figure 3.
a-c: Subjective responses during alcohol/ART administration sessions:
a. ‘High/Intoxicated’ ratings in those receiving ritonavir-containing ART
b: ‘High/Intoxicated’ ratings in those receiving efavirenz-containing ART
c: ‘Sleepy/Tired’ ratings in those receiving efavirenz-containing ART
Statistically significant, but clinically insignificant, increases in heart rate were observed with alcohol and ART administration (ritonavir-containing ART group: p=0.0021; efavirenz-containing ART group: p=0.0002) relative to placebo alcohol administration. Baseline heart rate at the time of study sessions was 70 (1.7) bpm. Peak heart rate under alcohol alone conditions prior to ART was 91 (2.9) bpm, under ART alone was 82 (3.3) bpm and following ART plus alcohol was 94 (2.9) bpm. Similarly for diastolic blood pressure in the efavirenz sample, a statistically significant (p=0.012), but clinically insignificant decrease was observed and was related to a small decline in diastolic blood pressure under the efavirenz-alcohol administration condition as compared to efavirenz-placebo alcohol administration (p=0.0345). No other significant effects of alcohol or alcohol in combination with ART were observed on systolic or diastolic blood pressure (data not shown) or cardiac electrical function.
Discussion
This study was undertaken to explore the possible drug-drug interactions between alcohol and frequently administered ARV (ritonavir or efavirenz) that might be associated with altered pharmacokinetics when consumed simultaneously. Importantly, while the focus of the study was on two HIV therapeutics that might potentially alter alcohol pharmacokinetics, the study participants’ ART regimens included other ARV co-administered with either ritonavir or efavirenz (Table 1). Blood alcohol concentration (BAC) was reduced in study participants after initiation of ART, regardless of which ART regimen was received. As we have discussed previously (McCance-Katz et al., 2012), the most likely explanation for the decreased BAC was decreased alcohol bioavailability after HIV treatment. No effect of alcohol on the pharmacokinetics of either ritonavir or efavirenz was observed. Further, the effectiveness of ART was demonstrated by the substantial and significant decline in HIV viral load observed in participants. Aside from expected differences in neuropsychological function and ‘High/Intoxicated’ related to alcohol administration, as well as increases in drowsiness in those receiving efavirenz-containing ART, there were no other significant differences in subjective responses observed.
We recently reported on the increased bioavailability of alcohol in untreated HIV (McCance-Katz et al., 2012) and we speculated that the mechanism of this finding is related to the intense inflammatory response of gut-associated lymphoid tissue (GALT) to HIV which has been shown to be associated with damage to the intestinal epithelium. Such injury might diminish alcohol and aldehyde dehydrogenases in the gut wall contributing to first pass metabolism of alcohol or may contribute to increased alcohol absorption. We showed HIV viral load decreases of 2 logs for both ritonavir and efavirenz-containing ART (Table 1) which could have been associated with decreased inflammatory responses of GALT with a resulting decrease in alcohol bioavailability; although longitudinal and larger sample size studies would be necessary to fully test this hypothesis. The ability of efavirenz to induce CYP 3A4 (Physician’s Desk Reference, 2007) might contribute to the decreased alcohol AUC and peak alcohol concentrations observed in those receiving efavirenz. Since CYP 3A4 is a minor pathway for alcohol metabolism (Lieber, 2004), medications that inhibit or induce this enzyme function might result in small corresponding changes in overall metabolism of alcohol. Mean elimination rate did decrease slightly with ritonavir, a CYP3A4 inhibitor, and increased slightly with efavirenz, a CYP3A4 inducer. However, results were not significant. Much larger studies would be needed to confirm these apparent effects. It is notable that the reduction in alcohol exposure after initiation of ART with either ritonavir or efavirenz resulted in alcohol pharmacokinetics more consistent with those observed in healthy subjects receiving the same dose of alcohol used in this study (McCance-Katz et al., 1993; McCance-Katz et al., 1998).
Additional ARVs co-administered to study participants with either efavirenz or ritonavir-boosted protease inhibitors as components of expert-recommended preferred ART regimens (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2013) included the nucleoside reverse transcriptase inhibitors, tenofovir disoproxil fumarate and emtricitabine. These medications are known to have no significant effect on CYP 450 metabolic enzymes. Neither tenofovir disoproxil fumarate nor its active metabolite are substrates of CYP 3A4 (Gilead Sciences, 2012) and tenofovir disoproxil fumarate is eliminated via glomerular filtration and renal tubular secretion (Fung et al., 2002). An oral dose of emtricitabine is primarily excreted unchanged in the urine (Molina et al., 2004). ART regimens that included ritonavir were also administered with either of the protease inhibitors, darunavir (n=2) or atazanavir (n=8). Darunavir is a substrate of CYP 3A4 and is given with ritonavir as part of an ART regimen with a resulting increase darunavir plasma concentrations due to the ability of ritonavir to inhibit CYP 3A4 (Janssen Therapeutics, 2012). Atazanavir is a substrate of CYP 3A4 and has been shown to inhibit CYP 3A4 in vitro (Perloff et al., 2005). The hypothesized effect of ritonavir on alcohol was that it would inhibit alcohol metabolism producing increases in BAC. Atazanavir might be expected to contribute to ritonavir inhibition of CYP 3A4 which could also have been associated with increased BAC. Contrary to this hypothesis, however, the observed effect shown in this study was for lower BAC with ritonavir-containing ART.
Subjective and neuropsychological responses indicate that a potential pharmacodynamic interaction between alcohol and efavirenz may occur. Subjective responses showed significant increases in ‘High/Intoxicated’ not unexpected for alcohol administration at the given dose which was calculated to produce mild intoxication (i.e.: a peak BAC of approximately 100 mg/dL) (McCance-Katz et al., 1993; McCance-Katz et al., 1998). The addition of an efavirenz-containing ART regimen did not significantly alter alcohol associated responses for ‘High/Intoxicated’. However, it is notable that alcohol exposure as measured by alcohol AUC was significantly decreased in the efavirenz sample following ART. Similarly, for the sample receiving efavirenz-containing ART, alcohol administration was associated with significantly greater ratings of drowsiness (measured as ‘Sleepy/Tired’) when given alone and with efavirenz. Despite decreased alcohol AUC following administration of efavirenz-containing ART, there was no concomitant decline in ratings of ‘Sleepy/Tired’ (Figure 3c). Additionally, a significant increase in ratings of ‘Sleepy/Tired’ was observed when alcohol and efavirenz-containing ART were administered simultaneously as compared to administration of efavirenz-containing ART alone (p=0.0149). Neuropsychological performance as measured by the Digit Symbol Substitution Test declined with alcohol alone administration and with alcohol plus efavirenz relative to sessions with placebo alcohol administration; however there was no significant difference in neuropsychological performance under either alcohol condition (i.e.: with or without efavirenz).
Efavirenz has recently been reported to be abused for its intoxicating effects (Scuitto, 2013); thus the presence of a pharmacodynamic interaction with alcohol as observed in this study is not inconsistent with such reports. These observations are important because they indicate that alcohol and efavirenz-containing ART regimens may be associated with increased alcohol effects for the same dose of alcohol. Intoxication and drowsiness can be impairing and these effects may be increased as those with HIV infection stabilize on efavirenz--a response that patients would be unlikely to expect. Therefore, patients should be informed that consumption of what might be usual amounts of alcohol for them could result in greater alcohol effects with efavirenz.
Others have shown the adverse effects of alcohol use on HIV disease progression and clinical outcomes (Samet et al., 2004; Chander et al., 2006; Braithwaite et al., 2007). Further, it has been reported that individuals with HIV infection may be nonadherent to ART when consuming alcohol due to beliefs regarding adverse interactions between alcohol and ART or because their providers have advised them not to consume alcohol while taking ART (Cook et al., 2001; Hendershot et al., 2009; Kalichman et al., 2012). The findings from this study provide information that should dispel some of the concerns regarding adverse alcohol/ARV interactions and can provide information that clinicians can utilize in their treatment of those with HIV infection that may be helpful in improving adherence to ART. These findings also illuminate some sources of alcohol risk in this population that underlines the need for clinician awareness and provision of treatment for alcohol misuse and use disorders when detected.
There are limitations to this study. The study samples, while adequate to detect pharmacokinetic interactions for specified ARV, were small in relation to the ability to make inference regarding all HIV medications prescribed by clinicians. Further, we were required to exclude those prescribed medications for other medical or psychiatric conditions that might alter CYP 3A4 function, thus limiting generalizability. Additional studies of frequently utilized ARV with alcohol might further extend the findings of this study and inform clinical care.
Conclusions
In summary, these results show the effectiveness of implementing ART and its role in diminution of BAC which could be associated with decreased risk of physiological toxicities related to alcohol consumption relative to alcohol consumers with untreated HIV infection. The findings in this study show a potential pharmacodynamic interaction between alcohol and efavirenz which should be confirmed in larger, clinical studies. Finally, there is no evidence that alcohol consumption significantly impacts the pharmacokinetics of the HIV therapeutics studied indicating that no alteration in either ritonavir or efavirenz dosing is needed in patients who consume alcohol.
Acknowledgments
The authors thank Vincent Samson and Suneel Agerwala for expert technical assistance with this study.
Supported by grants NIAAA grants RO1-AA018001 (EMK), NIDA K24 DA 023359 (EMK), and NIH/NCRR UCSF-CTSI UL1 RR024131 (University of California San Francisco)
References
- 1.Bosron WF, Ehrig T, Li TK. Genetic factors in alcohol metabolism and alcoholism. Semin Liver Dis. 1993;13(2):126–135. doi: 10.1055/s-2007-1007344. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite RS, Conigliaro J, Roberts MS, et al. Estimating the impact of alcohol consumption on survival for HIV + individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook RL, Sereika SM, Hunt SC, et al. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16(2):83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung HB, Stone EA, Piacenti FJ. Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection. Clin Ther. 2002;24(10):1515–1548. doi: 10.1016/s0149-2918(02)80058-3. [DOI] [PubMed] [Google Scholar]
- 6. [March 12, 2013];Gilead Sciences: Emtriva Package Insert. 2012 [Gilead web site]. Available at: http://www.gilead.com/pdf/emtriva_pi.pdf.
- 7.Hendershot CS, Stoner SA, Pantalone DW, et al. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [March 12, 2013];Janssen Therapeutics: Prezista Package Insert. 2013 [Prezista web site]. Available at: http://www.prezista.com/sites/default/files/pdf/us_package_insert.pdf.
- 9.Kalichman SC, Amaral CM, White D, et al. Alcohol and adherence to antiretroviral medications: interactive toxicity beliefs among people living with HIV. J Assoc Nurses AIDS Care. 2012;23(6):511–520. doi: 10.1016/j.jana.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalichman SC, Grebler T, Amaral CM, et al. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med. 2013;28(3):399–405. doi: 10.1007/s11606-012-2231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar GN, Rodrigues AD, Buko AM, et al. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 12.Li X, Chan WK. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv Drug Deliv Rev. 1999;39:81–103. doi: 10.1016/s0169-409x(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 13.Lieber CS. Metabolic consequences of ethanol. Endocrinologist. 1994;4(2):127–139. [Google Scholar]
- 14.Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev. 2004;36:511–529. doi: 10.1081/dmr-200033441. [DOI] [PubMed] [Google Scholar]
- 15.McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either drug alone—a multiple dose study. Biol Psychiatry. 1998;44:250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- 16.McCance-Katz EF, Lum PJ, Beatty G, et al. Untreated HIV infection is associated with higher blood alcohol levels. J Acquir Immune Defic Syndr. 2012;60(3):282–238. doi: 10.1097/QAI.0b013e318256625f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCance-Katz EF, Price LH, McDougle CJ, et al. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior and the role of cocaethylene. Psychopharmacology. 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- 18.Molina JM, Peytavin G, Perusat S, et al. Pharmacokinetics of emtricitabine, didanosine, and efavirenz administered once daily as treatment for HIV-infected adults (pharmacokinetic substudy of the ANRS 091 trial) HIV Med. 2004;5:99–104. doi: 10.1111/j.1468-1293.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 19.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [March 14, 2013]. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 20.Perloff ES, Duan SX, Skolnik PR. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 21.Physician’s Desk Reference. Efavirenz (Sustiva) 2007:930–938. [Google Scholar]
- 22.Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 23.Scuitto J. Teens in South Africa smoke anti-retroviral drug efavirenz for cheap high. [January 15, 2013];2009 Apr 8; [AIDS Health Foundation web site]. Available at: http://www.aidshealth.org/archives/news/no-turning-back-teens-abuse.
- 24.Ward BA, Gorski JC, Jones DR, et al. The cytochrome P450 2B6 (CYP 2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP 2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. The Measurement and Appraisal of Adult Intelligence. Baltimore: Williams and Wilkins; 1958. [Google Scholar]


