Abstract
Purpose
Regular surveillance decreases the risk of recurrent cancer in colorectal cancer (CRC) survivors. However, studies suggest that receipt of follow-up tests is not consistent with guidelines. This systematic review aimed to: (1) examine receipt of recommended post-treatment surveillance tests and procedures among CRC survivors, including adherence to established guidelines, and (2) identify correlates of CRC surveillance.
Methods
Systematic searches of Medline, PubMed, PsycINFO, CINAHL Plus, and Scopus databases were conducted using terms adapted for each database’s keywords and subject headings. Studies were screened for inclusion using a 3-step process: (1) lead author reviewed abstracts of all eligible studies; (2) coauthors reviewed random 5% samples of abstracts; and (3) two sets of coauthors reviewed all “maybe” abstracts. Discrepancies were adjudicated through discussion.
Results
Thirty-four studies are included in the review. Overall adherence ranged from 12–87%. Within the initial 12 to 18 months post-treatment, adherence to recommended office visits was 93%. Adherence ranged from 78–98% for physical exams, 18–61% for colonoscopy, and 17–71% for CEA testing. By 2 to 3 years post-treatment, cumulative adherence ranged from 70–88% for office visits, 89–93% for physical exams, 49–94% for colonoscopy, and 7–79% for CEA testing. Between 18–28% of CRC survivors received greater than recommended overall surveillance; overuse of physical exams (42%), colonoscopy (24–76%), and metastatic disease testing (1–29%) was also prevalent. Studies of correlates of CRC surveillance focused on socio-demographic and disease/treatment characteristics, and patterns of association were inconsistent across studies.
Conclusions
Deviation from surveillance recommendations includes both under- and overuse. Examination of modifiable determinants is needed to inform interventions targeting appropriate and timely receipt of recommended surveillance.
Keywords: Surveillance, guidelines, adherence, colorectal cancer, survivors
1. Introduction
Colorectal cancer (CRC) is the third most common cancer among both men and women in the U.S. [1]. Although a majority (about two-thirds) of CRC patients present with local or regional disease for which tumor resection with curative intent is the treatment of choice [2], 28 to 50% of patients will develop recurrent disease [3–7]. Because CRC survivors are at high-risk for recurrence, the goals of post-treatment surveillance are to detect early stage recurrences that are amenable to another curative resection or to detect polyps and precancerous lesions at a pre-invasive stage, thereby reducing mortality [2, 8].
Clinical practice guidelines from various oncological, surgical, and gastroenterological organizations recommend routine post-treatment surveillance of CRC survivors [9–13]. Although the frequency of and interval between surveillance tests and procedures have been debated and modified in recent years, the majority of current guidelines recommend regular provider office visits [9, 12–13], colonoscopy at 1 year post-resection with follow-up colonoscopy every 3–5 years [10–11, 13], and carcinoembryonic antigen (CEA) testing for the first 2–5 years post-resection [9, 12–13]. There is less consistency regarding recommendations for metastatic disease testing (e.g., x-ray, CT scan, ultrasound), and some organizations have modified their guidelines in recent years to recommend previously non-recommended procedures (i.e., CT scans) [9–13]. Results from several systematic reviews and meta-analyses demonstrate a modest but statistically significant survival benefit from more intense versus minimal surveillance (defined as any versus no follow-up to varying combinations of and intervals for follow-up tests and procedures) [14], including earlier detection of asymptomatic and local recurrences [6–7], successful reoperation rates [6], and a 4 to 33% decrease in overall mortality [5–6, 14].
Regardless of the CRC surveillance guidelines used, receipt of CRC follow-up tests and procedures is quite variable [2, 15–44]. Moreover, relatively little is known about correlates of CRC surveillance, with the available evidence focused on patient-level socio-demographic, disease, and treatment factors [2, 15–18, 21–28, 30–31, 33–34, 37–38, 40–43, 45–47], as opposed to psychosocial, provider- and/or system factors that may help inform the development of interventions to promote surveillance.
The documented variability in receipt of surveillance among CRC survivors despite evidence for its effectiveness highlights the need for a systematic review of this topic in order to establish the magnitude of the problem and to identify factors that can be targeted in interventions to increase CRC surveillance. Previous reviews of surveillance follow-up care which included CRC survivors were either too broad (i.e., examined factors related to health service utilization among survivors of a number of cancer sites) [48] or too specific in focus (i.e., ethnic disparities in colonoscopy use) [49]. Accordingly, the specific aims of this systematic review were to: (1) examine receipt of recommended post-treatment surveillance tests and procedures (i.e., office visits, physical exams, colonoscopy, CEA testing, metastatic disease testing) among CRC survivors, including adherence to established guidelines when possible, and (2) identify correlates of CRC surveillance. We also assessed completeness of reporting on selected characteristics relevant to internal and external validity based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist [50].
2. Methods
Search Strategy
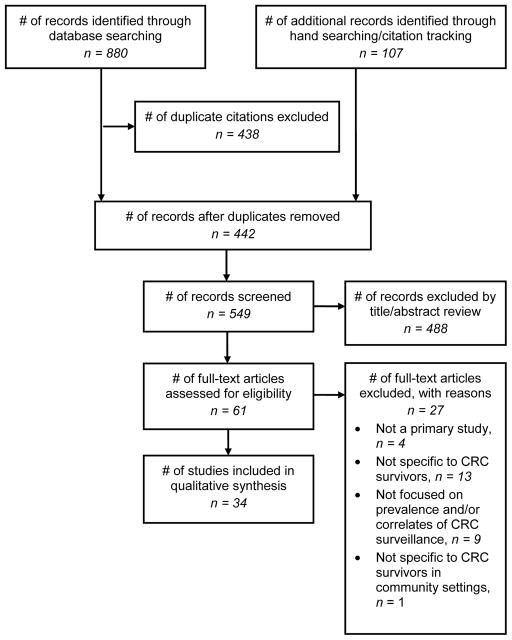
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was used to guide the content and reporting of this systematic review [51]. Using various interfaces, electronic searches of 4 databases were conducted: Medline (via Ovid; 1946 to January Week 2 2013; In-Process & Other Non-Indexed Citations January 22, 2013; searched January 23, 2013); PubMed (National Library of Medicine; searched January 23, 2013); PsycINFO (via Ovid; 1967 to January Week 3 2013; searched January 23, 2013); and CINAHL Plus with Full Text (via Ebsco; 1982 to present; searched January 23, 2013). General concepts that comprised the search included: colorectal cancer, survivors, follow-up, and surveillance/screening methods. Although the term “surveillance” is the one most often used when referring to screening tests in persons with a previous diagnosis of cancer, there is not absolute consistency; therefore, both “surveillance” and “screening” were utilized as search terms. All search terms were adapted for each database’s unique keywords and subject headings with the assistance of a health sciences librarian experienced in developing systematic review search strategies. Strategies were pretested and refined through an iterative process which involved screening citations for relevance to our eligibility criteria. The final strategies for each database searched are presented in the Appendix. In addition to the documented search strategies, reference lists from eligible articles were hand searched for additional studies. Relevant articles were also searched in Scopus (via Elsevier) to determine whether they had been cited by other studies that previous searches had not found. Figure 1 illustrates the flow of information through the different phases of the systematic review.
Fig. 1.
Selection of studies for systematic review
Inclusion and Exclusion Criteria
Studies were considered eligible if they: (1) were written in English; (2) were published or in-press in a peer-reviewed journal; (3) reported data from a primary study (i.e., not a review, editorial, or commentary); (4) included CRC survivors; and (5) reported data on the prevalence and/or correlates of CRC surveillance tests and procedures (i.e., office visits, physical exams, colonoscopy, CEA testing, metastatic disease testing). Inclusion criteria were assessed in the order specified above; the first “no” criterion was the documented reason for exclusion and the remaining criteria were not assessed. According to the Cochrane group, a single failed criterion is sufficient cause to exclude a study from the review [52]. As this represents the first comprehensive systematic review of the topic, no exclusions were made on the basis of year of publication. Studies which did not clearly specify that physician/office visits were for the purpose of surveillance were excluded from this review as were studies that examined prevalence of adherence to CRC surveillance guidelines in clinical trial populations.
Selection of Studies
Studies were screened using a 3-step process. First, the titles and abstracts of all potentially eligible studies were screened by the lead author who assigned a rating of “no” or “maybe” as to whether each study should be included in the review. Next, each coauthor independently reviewed a random 5% sample of abstracts. The random 20% sample of abstracts double-screened for inclusion was considered an adequate representation of eligible abstracts (similar to double-coding or double-checking in original research). Discrepancies in ratings across co-authors occurred in < 5% of all abstracts reviewed; all discrepancies were adjudicated through discussion until consensus was reached. Finally, the titles and abstracts of all studies rated “maybe” over the first 2 screening steps were reviewed by 2 pairs of co-authors. This process was conducted to ascertain final agreement regarding studies whose full-text should be reviewed for potential inclusion. Both pairs of authors were in 100% agreement as to the appropriateness of all “maybe” studies for subsequent full-text review.
Data Extraction
Using an abstraction form created for this review, relevant information was extracted from all eligible studies. To assess Aim 1, data on receipt of recommended post-treatment surveillance tests and procedures, including authors’ operational definitions of adherence (i.e., type and frequency of surveillance tests and procedures) and the basis for their adherence definition (i.e., recommending organization and year of recommendation) were extracted. Adherence to surveillance was judged according to the particular guideline(s) mentioned in the individual studies. To assess Aim 2, data on correlates of CRC surveillance were extracted. Data were also abstracted on relevant study characteristics such as study design, data sources, sample size, subject eligibility criteria, and study follow-up period. All eligible studies were independently read and coded by two reviewers using the abstraction form. Discrepancies in coding occurred in < 5% of all studies; all discrepancies were adjudicated through discussion until consensus was reached between the two coders.
Completeness of Reporting on Selected Characteristics of Internal and External Validity
Using the STROBE checklist [50], studies were assessed for completeness of reporting on 20 selected aspects of internal and external validity related to the study population, design and analysis, and generalizability. Each characteristic was assigned a rating of “yes, explicitly reported by study authors”; “inferred by raters but not explicitly reported by study authors”; or “no, not reported by study authors”. To assess the study population, ratings were assigned as to whether the authors reported setting/location of the study, data collection dates, participant eligibility criteria, source of participants, method of selection into the study, number of potentially eligible participants, number of participants included in the study, number of participants with complete follow-up, and number of analyzed participants. To assess design and analysis characteristics, ratings were assigned as to whether the authors defined their outcome variables; identified sources of data and methods of assessment; provided information on how study sample size was determined; reported descriptive information regarding participant characteristics, potential confounders, and length of follow-up; and reported unadjusted and/or adjusted estimates. To assess generalizability, ratings were assigned as to whether authors discussed study limitations, including potential bias, as well as issues of external validity.
3. Results
Database searches, hand searching of reference lists, and citation tracking yielded 548 unique articles. After title/abstract review, 61 articles remained eligible for full-text review and, of these, 34 met criteria for inclusion (Table 1). The 34 articles represented 32 unique studies conducted in the United States (n = 24), Canada (n = 5), and France (n = 3). Studies were published between 1999 and 2013. The study samples were drawn from local/regional clinical populations (e.g., HMO members, Veterans Administration patients), regional/provincial cancer registries, or population-based (e.g., Medicare beneficiaries). Studies primarily used large administrative databases (e.g., SEER-Medicare, TRICARE military health system claims data, Canadian Ministry of Health) and medical records to examine receipt of post-treatment CRC surveillance tests and procedures. One [17] study used provider surveys to supplement missing administrative data while another [30] study used patient survey data solely. CRC surveillance guidelines included varying combinations of office/physician/clinical visits, physical/clinical exams, colon examination (i.e., colonoscopy, sigmoidoscopy, and/or barium enema), CEA testing, and other metastatic disease testing (e.g., x-ray, CT scan, ultrasound). Most studies assessed receipt of colonoscopy. Different definitions and measurements were used to assess adherence to CRC surveillance. Twenty-two [2, 15–18, 21–26, 28, 30–31, 33–34, 37–38, 40–43) of the 34 studies assessed both prevalence and correlates of adherence to CRC surveillance. Nine [19–20, 27, 29, 32, 35–36, 39, 44] studies reported only prevalence data while 3 [45–47] studies reported only correlates data.
Table 1.
Receipt of recommended post-treatment surveillance tests and procedures among CRC survivors
| Primary author (year) | Data source | Sample size/ characteristics | Eligibility criteria | Year(s) of diagnosis | Follow-up period | Outcome variable(s) | Results | Operational definition of adherence/basis for definition a |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Boehmer (2010) | Medical records | n = 253; ≤54 to ≥75y; 58% male; 54% White; safety net patients | Non-metastatic CRC; treated w/curative intent; no recurrence during study period | 2003–2007 | 5y post-dx | % COL 1/3y post-tx | 27/56% | COL 1 & 3y post-tx (referenced 2008 ACS, USMTFCC) |
|
| ||||||||
| Borie (2004) | Regional cancer registry along with medical records | n = 231; mean age 64y (standard group) to 69y (minimal group); 56% male; French sample | CRC treated with potentially curative surgery; able to be classified into 1 of 2 (standard vs. minimal) follow-up groups | 1992 | 5y post-tx | Mean number PE (standard/minimal) | 20/7 | Standard follow-up: CEA every 4–6m for 3y, then once a year for 2y; PE every 3m for 2y, then every 6m for 3y; 1 COL every 3y; US every 4–6m for 3y, then once a year for 2y; annual CXR |
| Mean number US (standard/minimal) | 6/2 | |||||||
| Mean number COL (standard/minimal) | 5/2 | |||||||
| Mean number CXR (standard/minimal) | 4/1 | Minimal follow-up: CEA & US once a year for 3y; PE every 6m for 5y; 1 COL every 3y; CXR once a year for 2y (based on 1998 French Consensus Guidelines) |
||||||
| Mean number CEA (standard/minimal) | 12/3 | |||||||
|
| ||||||||
| Boulin (2005) | Regional cancer registry along with medical records and provider surveys as necessary | n = 409; mean age 71y; 55% male; 30% advanced stage CRC; French sample | Diagnosed with CRC Stage A, B, or C or Stage D with complete resection of liver metastasis; alive without recurrence ≥ 6m post-curative surgery | 1998 | 3y post-tx | % below/within/over standard for clinical exams | 35/23/42% | Clinical exam every 3m for first 2y; every 6m for next 3y |
| % below/within/over standard for abdominal US | 65/35/1% | Abdominal US every 3–6m for first 3y; yearly for next 2y | ||||||
| % below/within/over standard for CXR | 52/19/29% | CXR yearly for 5y | ||||||
| % below/within/over standard/inappropriate time for COL | 20/27/24/29% | COL after 3y (or 1y if ≥ 3 adenomas with one > 1 cm diameter or presenting villous component | ||||||
|
Optional testing:
% CEA within 3y |
56% | Optional: CEA (based on 1998 French Consensus Guidelines) |
||||||
|
Cluster analysis:
% “minimal/moderate/ intensive” surveillance |
47/24/29% | |||||||
|
| ||||||||
| Brawarsky (2013) | SEER-Medicare | n = 38,889; 56% female; 88/7/5% White/Black/ Hispanic; median age 75y (White), 74y (Black/ Hispanic) | 66 to 85y; White, Black, or Hispanic; diagnosed with first CRC; stage I-III; treated with surgery; alive at end of study period | 1993–2005 | Through Dec 2007 | % COL 15m post-tx | 61% | At least 1 COL 15m post-surgery |
| % PC visits 2y post-tx | 77% | At least 2 PC visits 2y post-surgery | ||||||
| % CEA 2y post-tx | 68% | At least 2 CEA 2y post-surgery (stage II/III) | ||||||
| % overall surveillance | 43% | Overall surveillance: COL and PC visits for stage I or COL, PC, and CEA for stage II/III (based on 2012 NCCN) |
||||||
|
| ||||||||
| Cardella (2008) | Medical records | n = 96; median age 64y; 60% male; median time post-tx 34m; Canadian sample | 19 to 75y; curative intent resection of CRC; non-metastatic disease; able to go home and assume active daily living; alive and disease-free through end of study period | 2000–2002 | Through October 2004 | % clinic visits over follow-up period | 70% | Clinic visits every 6m for first 3y, followed by 12m intervals until 5y post-tx |
| % CEA over follow-up period | 49% | |||||||
| % abdominal imaging over follow-up period | 62% | CEA, CXR, and abdominal imaging (CT or abdominal US) at each clinic visit | ||||||
| % COL over follow-up period | 94% | COL 1y post-tx and every 3y thereafter (basis for adherence definition not reported) |
||||||
|
| ||||||||
| Cheung (2008) | Academic and comm cancer center registries along with medical records | n = 341 (244 academic, 97 community); overall median age 62y; 58% male; 57% stage III; Canadian sample | Stage II or III CRC; referred to either cancer center for follow-up after curative resection; able to be monitored for ≥5y post-dx; not enrolled in clinical trials | 1999–2001 | 5y post-dx | Median CV (academic/ community) | 11/9 | Over the first 5y period of surveillance: 8–14 CV |
| Median CEA (academic/ community) | 9/9 | 8–30 CEA 1 COL |
||||||
| Median COL (academic/ community) | 2/2 | Not routinely recommended: CBC, LFT, CXR, chest CT, abdominal CT, pelvic CT (based on 1999, 2000 ASCO) |
||||||
| % below CV recommendations (academic/community) | 23/23% | |||||||
| % above CV recommendations (academic/community) | 17/0% | |||||||
| % below CEA recommendations (academic/community) | 41/29% | |||||||
| % above CEA recommendations (academic/community) | 0/0% | |||||||
| % below COL recommendations (academic/community) | 15/3% | |||||||
| % above COL recommendations (academic/community) | 67/76% | |||||||
|
Non-recommended testing: % non-recommended CBC/LFT/CXR/chest CT/ abdominal CT/pelvic CT in academic center |
94/91/70/42/9 3/91% | |||||||
| % non-recommended CBC/LFT/CXR/chest CT/abdominal CT/pelvic CT in community center | 99/100/37/14/ 38/34% | |||||||
|
| ||||||||
| Cooper (1999) | SEER-Medicare | n = 5716; mean age 75y; 51% female; 6% African-American | Medicare beneficiaries; local or regional CRC; underwent surgical resection; alive 6m post-dx; complete follow-up data | 1991 | Through 1994 | %/mean COL | 58%/2.8 | NR/NR |
| %/mean abdominal CT | 29%/2.8 | |||||||
| %/mean pelvic CT | 23%/2.6 | |||||||
| %/mean CXR | 66%/4.2 | |||||||
| %/mean abdominal US | 15%/14.8 | |||||||
| %/mean liver enzymes | 74%/4.7 | |||||||
| %/mean CEA | 38%/2.3 | |||||||
| %/mean overall procedures | 87%/12.9 | |||||||
|
| ||||||||
| Cooper (2000) | SEER-Medicare | n = 5716; mean age 75y; 51% female; 6% African-American | Medicare beneficiaries; local or regional CRC; underwent surgical resection; alive 6m post-dx; complete follow-up data | 1991 | Through 1994 | % 1 COL/>1 COL 7–12m post-dx | 22/14% | NR/NR |
| % 1 COL/>1 COL 13–18m post-dx | 23/15% | |||||||
| % 1 COL/>1 COL 19–24m post-dx | 15/10% | |||||||
| % 1 COL/>1 COL 25–30m post-dx | 16/10% | |||||||
| % 1 COL/>1 COL 31–36m post-dx | 12/8% | |||||||
| Cooper (2006) | SEER-Medicare | n = 62,882; 55% female; 86% White | Medicare beneficiaries; ≥65y; treated w/curative intent; alive 1 & 3y post-dx | 1992–2002 | 3y post-dx | % COL 12/18/36m post-dx | 26/54/70% | COL 1 or 3y post-dx (referenced 1997 AGA; 1999, 2000 ASCO; 2001, 2003 ACS; 2003 AGA; 2004 ASCRS) |
|
| ||||||||
| Cooper (2008) | SEER-Medicare | n = 9,426; 55% female; 87% White | Medicare beneficiaries; ≥66y; treated w/curative intent; alive 3.5y post-dx | 2000–2001 | 42m post-dx | % OV/COL/CEA 42m post-dx | 92/74/47% | ≥2 OV/y; ≥1 COL w/in 3y; ≥2 CEA in y1 & y2 (referenced 1989 AGA, ASGE; 1999, 2000, 2005 ASCO; 2004 ASCRS; 2006 ACS, ASGE, USMTFCC; 2008 NCCN) |
| % testing below/at/above recommended levels | 60/17/23% | |||||||
|
| ||||||||
| Ellison (2003) | SEER-Medicare | n = 52,105; 53% female; 86% White | Medicare beneficiaries; ≥65y; stage I-III CRC; treated w/ curative intent | 1986–1996 | Through 998 | % CE 18m/3y/5y post-tx | 57/67/74% | NR/NR |
|
| ||||||||
| Elston Lafata (2001) | Medical records | n = 251; mean age 65y; 62% male; 63% White; HMO members | ≥40y; stage I-III CRC; treated w/ curative intent | 1990–1995 | 8y post-dx | % CE 18m/3y/5y post-tx | 55/65/77% | CE (COL, SIG & BE, BE only) 1y post-tx, every 3–5y thereafter (referenced 1992 ASCRS; 1996 NCCN; 1997 AGA; 1999 ASCO) |
| % CEA 18m/3y/5y post-tx | 71/79/87% | |||||||
| Elston Lafata (2005) | Medical records | n = 100; 56% male; 68% White; HMO members | Enrolled ≥ 1y or ≥1 PCP visit pre-dx; ≥30y; new primary CRC; alive ≥6m post-dx | 1990–1995 | 5y post-dx | % PE 18m post-tx/within 18m of initial | 78/85% | 2 PE/y, 1 CE (COL, SIG, or BE) in y1 & y3-y5 |
| % CE 18m post-tx/within 18m of initial | 61/62% | 4 CEA/yr (based on 1999 ASCO; 2001, 2002 NCCN) |
||||||
| % CEA 18m post-tx/within 18m of initial | 17/12% | |||||||
|
| ||||||||
| Foley (2011) | State cancer registry-Medicaid | n = 1,044; <65 to ≥75y; 68% female; 57% White | Medicaid beneficiaries; stage I to III CRC; alive ≥18m post-dx | 1999–2002 | 18m post-tx | % COL/CEA 3–18m post-tx | 42/25% | COL 1y post-tx (referenced 1999 ASCO; 2008 ACS, USMTFCC, ACR) |
|
| ||||||||
| Fox (2013) | Military Health System claims data (TRICARE) | n = 345; 73% ≥50y; 55% male; 62% Southern US region | 18 to 61y; underwent curative treatment for CRC; enrolled in TRICARE Prime managed care program; ≥ 1 health claim per year | 2005–2007 | Through Sept 2010 | % PE 1y/2y/3y follow-up | 95/93/89% | 1y follow-up: 2 PE, 2 CEA, 1 COL |
| % PE each year | 52% | |||||||
| % CEA 1y/2y/3y follow-up | 59/49/43% | 2y follow-up: 2 PE, 2 CEA | ||||||
| % 1+ COL in 3y | 69% | 3y follow-up: 2 PE, 2 CEA (based on 2009 NCCN) |
||||||
| % all recommended care each year | 26% | |||||||
|
High-cost imaging:
% PET 1y/2y/3y/overall |
16/14/11/24% | |||||||
| % CT 1y/2y/3y/overall | 60/54/50/78% | |||||||
| % MRI 1y/2y/3y/overall | 18/15/15/35% | |||||||
|
| ||||||||
| Haggstrom (2009) | Cross-sectional survivor survey | n = 416; 52% ≥65y; 52% male; 70% White | No treatment in past 6m; no evidence of recurrence | 1999–2001 | April 2003–Nov 2004 | % 1/2/≥3 OV in past 12m | 27/34/39% | Regular history and physicals during the first 3 years post-tx (referenced 2005 ASCO) |
|
| ||||||||
| Hilsden (2004) | Provincial cancer registry linked with ministry of health admin databases and medical records | n = 3918; 46% ≥ 70y; 56% male; 85% rural residence; Canadian sample | ≥30y; first CRC treated w/curative resection; survived ≥270d post-surgery; eligible for Alberta insurance plan at dx; able to be linked to admin databases | 1983–1995 | Through March 2000 | % COL within 5y post-tx | 51% | NR/NR |
|
| ||||||||
| Hu (2011) | SEER-Medicare | n = 7,348; 60% female; 89% White | Medicare beneficiaries; ≥66y; stage I-III CRC; treated w/ curative intent w/in 3m of dx; alive 3.5y post-tx | 2000–2002 | Through 2005 | % OV 1/2/3y post-tx | 93/88/84% | ≥2 OV/y for 3y; ≥2 CEA/y for 2y; ≥1 COL w/in 3y (referenced 1999 ASCO; 2010 NCCN) |
| % CEA 1/2y post-tx | 42/29% | |||||||
| % COL 1/2/3y post-tx | 59/68/74% | |||||||
| % testing at recommended levels | 25% | |||||||
|
| ||||||||
| Jackson (2010) | Medical records | n = 2,492; 98% male; 72% White; VA patients | Stage I-III CRC; treated w/ curative intent | 2003–2006 | Through March 2006 | % COL 7–18m post-tx | 44% | COL 7–18m post-tx (based on 2003 NCCN) |
|
| ||||||||
| Knopf (2001) | SEER-Medicare | n = 52,283; 53% female | Medicare beneficiaries; ≥65y; stage I-III CRC; treated w/curative intent | 1986–1996 | Through 1998 | % CE 1y/1–4y/ 4–7y/7+y post-dx | 46/48/40/31% | CE (COL, SIG, or BE) 1y post-tx & every 3–5y thereafter (referenced 1989 AGA, ASGE; 1996 NCCN; 1997 ACS, AGA; 1999 ASCO) |
| % testing below/above recommended levels | 17/18% | |||||||
|
| ||||||||
| Parsons (2012) | SEER-Medicare | n = 17,906; 59% female; 85% White | Medicare beneficiaries; ≥ 66y; stage III CRC; treated w/curative intent | 1992–2007 | 3y post-tx | % COL/CEA 3y post-tx | 49/72% | COL w/in 3 y post-tx |
| Any CEA w/in 3 y post-tx (referenced 2003 GCP; 2005 ASCO; 2006 ACS, USMTFCC; 2007 NCCN) | ||||||||
|
| ||||||||
| Pollack (2009) | SEER-Medicare along with UPIN registry and AMA Physician file | n = 16,671; 86% 65+y; 56% female; 86% White; 84% metropolitan residence | First CRC dx at ≥ 60y; survived ≥ 5y; no recurrence or multiple cancers | 1992–1997 | Through Dec 2003 | % PV with cancer specialist | 28% | ≥ 1 PV during 6th to 12th year post-dx (basis for adherence definition not reported) |
| % PV with hematologist/oncologist | 26% | |||||||
| % PV with radiation oncologist | 2% | |||||||
| % PV with surgical oncologist | 1% | |||||||
| % PV with gynecologic oncologist | 0.2% | |||||||
| % PV with cancer-related specialist | 37% | |||||||
| % PV with general surgeon | 8% | |||||||
| % PV with colorectal surgeon | 6% | |||||||
| % PV with gastroenterologist | 26% | |||||||
| % PV with PCP | 73% | |||||||
| % PV with medical specialist | 66% | |||||||
|
| ||||||||
| Ramsey (2007) | SEER-Medicare | n = 28,209; 52% female; 86% White | Medicare beneficiaries; ≥ 65y; stage I-III CRC; treated w/ curative intent | 1986–1996 | Through 2003 | % testing below/at/above recommended levels | 47/25/28% | ≥ 1 CE (COL, SIG, or BE) over study period (referenced 2006 ACS, USMTFCC) |
|
| ||||||||
| Rolnick (2005) | Medical records | n = 881; 48% ≥ 70y; 57% male; 75% White; HMO members | ≥ 40y; stage 0-III CRC; White or African-American | 1990–2000 | 5y post-tx | % CE 1/3/5y post-tx | 18/60/67% | CE (COL or SIG & BE) 1, 3, & 5y post-dx (referenced 1989 AGA, ASGE; 1996 NCCN; 1997 AGA; 1999, 2000 ASCO; 2003 AGA) |
|
| ||||||||
| Rulyak (2004) | Medical records linked with SEER | n = 1,002; <50 to 80+y; 50% male; 93% White; HMO members | Stage 0-III new primary CRC | 1993–1999 | 8.7y post-dx | % CE 18m post-dx/within 18m of initial/5 y post-dx | 61/38/80% | NR/NR |
|
| ||||||||
| Rulyak (2007) | Medical records linked with SEER | n = 1002; 78% ≥ 60y at dx; 50% male; 93% White; HMO members | Stage 0-III; treated w/curative intent; survived ≥ 6m post-dx | 1993–1999 | Through Dec 2001 | % ≥ 1 CE during study period | 65% | NR/NR |
| Mean exams during study period | 1.4 | |||||||
| % CE 18m/5y post-dx | 61/80% | |||||||
|
| ||||||||
| Salloum (2012) | Medical records linked to tumor registry | n = 2,297; mean age 68.6y; 51% female; 81% White; HMO members | ≥ 18y; enrolled in HMO plan ≥ 1y pre-dx; treated w/curative intent | 2000–2008 | 8y post-dx | % PE 18m post-tx/within 18m of initial | 98/91% | 2 PE 18m post-tx; 1 CE (COG, SIG, or BE) |
| % CE 18m post-tx/within 18m of initial | 55/16.7% | 18m post-tx (based on 2011 NCCN; 1999 ASCO) |
||||||
|
| ||||||||
| Salz (2010) | Medical records | n = 1,423 56% ≥ 65y; 56% male; 67% White; multi-region cohort including VA & managed care patients | Stage I-III CRC; treated w/curative intent w/in 1m of dx; alive 14m post-tx w/out recurrence | 2003–2005 | 15m post-dx | % COL 14m post-tx | 49% | COL 14m post-tx (based on 2003 ACS; 2006 ACS, NCCN, USMTFCC) |
|
| ||||||||
| Singh (2013) | SEER-Medicare | n = 70,419; 36% ≥ 80y; 55% female; 85% White | ≥ 66y; stage I-III CRC; no history of IBD; Medicare beneficiaries enrolled in Part A & B; not HMO members | 1992–2005 | 1992–2003 (second COL cohort), 1992–2002 (third COL cohort) | % early surveillance COL after first/second normal COL | 32/27% | COL 1y post-tx, 3- and 5y later |
| Median time between first and second COL | 29m | “Early surveillance”: COL 3m to 2y after previous COL | ||||||
| Median time between second and third COL | 33m | (referenced 1997 AGA, 2000 ASCO, 2004 ASCRS, 2006 USMTFCC) | ||||||
|
| ||||||||
| Sisler (2012) | Provincial cancer registry linked with pop health research data | n = 250; median age = 70y; 53% male; Canadian sample | Stage II and III new CRC; treated with definitive surgery; alive 42m post-dx | 2004 | July 2004–June 2008 | % COL within 3y period | 80% | At least 1 COL in 3y study period; at least 1 liver imaging (CT, US, or MRI of the abdomen) in each 1y interval; at least 3 CEA tests in each 1y interval (based on 2005 ASCO) |
| % liver imaging (CT, US, or MRI) in each 1y interval | 47% | |||||||
| % CEA in each 1y interval | 22% | |||||||
| % adherent to all 3 tests over study period | 12% | |||||||
|
| ||||||||
| Spratlin (2008) | Provincial cancer registry along with medical records | n = 152; mean age 66y; 66% male; 57% stage II; Canadian sample | Resected stage II/III CRC w/clear surgical margins; discharged to community; no second malignancy ≤5y post-dx | 2001 | 3y post-dx | % minimum CEA follow-up | 7% | CEA every 4m for ≥2y (based on clinical practice and informed by 2000 ASCO) |
Adherence to surveillance was judged according to the particular guideline(s) mentioned in the individual studies. Individual studies’ definitions of adherence varied. The majority of studies explicitly stated that they based their definition of adherence on a specific guideline(s) (n = 12), yet other studies only referenced published guidelines (n = 12). A few studies did not provide any basis for the definitions of adherence (n = 7).
ABBREVIATIONS: CRC, colorectal cancer; d, days; m, months; y, years; admin, administrative; comm, community; pop, population; post-dx, post-diagnosis; post-tx, post-treatment; NR, not reported; OV, office visits; PE, physical examination; CE, colon examination (i.e., colonoscopy, sigmoidoscopy, and/or barium enema; majority of exams, 77–99%, were colonoscopy); COL, colonoscopy; SIG, sigmoidoscopy; BE, barium enema; CEA, carcinoembryonic antigen test; CBC, complete blood counts; CT, computed tomography; CXR, chest x-rays; LFT, liver function tests; US, ultrasound; OMD, other metastatic disease testing (e.g., x-ray, ultrasound, CT scan, MRI ); ACR, American College of Radiology; AMA, American Medical Association; AGA, American Gastroenterological Association; ASCO, American Society of Clinical Oncology; ASCRS, American Society of Colon and Rectal Surgeons; ASGE, American Society for Gastrointestinal Endoscopy; CanCORS, Cancer Care Outcomes Research and Surveillance Consortium; HMO, health maintenance organization; IBD, inflammatory bowel disease; NSABP, National Surgical Adjuvant Breast and Bowel Project; NCCN, National Comprehensive Cancer Network; SSO, Society of Surgical Oncology; USMTFCC, United States Multi-Society Task Force on Colorectal Cancer; GCP, Gastrointestinal Consortium Panel; UPIN, Unique Physician Identification Number.
Prevalence of Adherence
Office visits
During the initial 12 to 18 months post-surgery, 93% of CRC survivors adhered to recommended office visits [2]. At 2 to 3 years post-treatment, 70–88% of survivors adhered to office visits [2, 18–19] and at 3 ½ years post-treatment, 92% adhered [24]. Data reported by Cheung et al. [20] indicated that 23% of both academic and community-based Canadian CRC survivors received less than the recommended number of 8–14 office visits over a 5-year period of surveillance whereas 17% of survivors from an academic cancer center (and none from a community cancer center) received more than the recommended number. Median number office visits over 5 years among academic and community-based survivors were 11 and 9, respectively. In the sole cross-sectional survey, Haggstrom et al. [30] found that 27% of U.S. CRC survivors reported 1 office visit, 34% reported 2 office visits, and 39% reported 3 or more office visits in the past 12 months. Using SEER-Medicare data in tandem with the American Medical Association Masterfile and the Unique Physician Identification Number Registry, Pollack et al. [35] found that 26% of long-term CRC survivors had physician visits with hematologists/oncologists while 73% had visits with a primary care provider during the 6th to 12th year since diagnosis.
Physical/clinical exams
Approximately 78–98% of CRC survivors adhered to recommended physical/clinical exams during the initial 12 to 18 months post-surgery [27, 29, 40] and 89–93% adhered within 2 to 3 years post-diagnosis and treatment [29]. Data reported by Boulin et al. [17] indicated that 35% of CRC survivors in France fell below and 42% fell above the recommendations for physical/clinical exams. Moreover, mean physical/clinical exams over a 5-year period of surveillance in France were 7 and 20 in minimal and standard follow-up groups, respectively [16].
Colonoscopy
During the initial 12 to 18 months post-surgery, 18–61% of CRC survivors adhered to colon examination, variously defined as colonoscopy, sigmoidoscopy, and/or barium enema [2, 15, 18, 22–23, 25–28, 32–33, 37–41]. Adherence to recommended colonoscopy ranged from 49–94% within 2 to 3 ½ years post-diagnosis and treatment [2, 15, 19, 21, 23–26, 29, 34, 37, 43] and 51–80% within 5-years post-diagnosis and treatment [25–26, 31, 37–39]. Examining colonoscopy use in discrete time intervals post-diagnosis, Cooper et al. [22] reported that 22% of CRC survivors underwent colonoscopy 7 to 12 months post-diagnosis, 15% at 19 to 24 months post-diagnosis, and 12% at 31 to 36 months post-diagnosis. In a similar study utilizing SEER-Medicare, Knopf et al. [33] reported that 48% of CRC survivors received colonoscopy 1–4 years post-diagnosis, 40% received colonoscopy 4–7 years post-diagnosis, and 31% received colonoscopy 7+ years post-diagnosis.
Mean number of colonoscopies over approximately 3-year periods of surveillance among U.S. Medicare beneficiaries ranged from 1.4 to 2.8 [21, 39]. Likewise, mean colonoscopies over a 5-year period of surveillance in France were 2 and 5 in minimal and standard follow-up groups, respectively [16]. Median number of colonoscopies among Canadian CRC survivors from both academic and community cancer centers was 2 over the course of 5 years [20]. In this study, Cheung et al. [20] found that 15% of academic and 3% of community-based survivors received less than whereas 67% of survivors from an academic cancer center and 76% of survivors from a community cancer center received more than the recommended single colonoscopy over the 5-year period of surveillance. Similar results were obtained in a French study where Boulin et al. [17] reported that 20% of CRC survivors fell below and 24% were above the recommendations for colonoscopy.
Several studies examined early receipt of colonoscopy after colonoscopy with normal results and found that 17–62% of CRC survivors received a second colonoscopy within 18 months of initial colonoscopy [27, 38, 40, 42] and 27% of survivors received a third colonoscopy within 2 years of a second normal colonoscopy [42]. These results were comparable to those obtained in a French sample where 29% of CRC survivors received colonoscopy at a period inconsistent with recommended guidelines [17].
CEA testing
During the initial 12 to 18 months post-surgery, 17–71% adhered to CEA testing [2, 26–29]. Adherence to CEA testing ranged from 7–79% within 2 to 3 ½ years post-diagnosis and treatment [2, 17–19, 21, 24, 26, 29, 34, 44] and 87% at 5-years post-diagnosis and treatment [26].
Approximately 41% of Canadian CRC survivors from academic cancer centers and 29% of survivors from community cancer centers received less than the recommended 8–30 CEA tests over a 5-year period of surveillance, with a median number of 9 CEA tests over 5 years among survivors in both settings [20]. In France, mean number of CEA tests were 3 and 12 in minimal and standard follow-up groups, respectively [16]. Within a 3-year surveillance period of U.S. Medicare beneficiaries, the mean number of CEA tests was 2.3 [21].
Metastatic disease testing
Fewer studies examined adherence to metastatic disease testing among CRC survivors, perhaps an artifact of inconsistent guidelines in this area. In France, Boulin et al. [17] found that 65% of CRC survivors fell below French 1998 consensus guidelines which recommended abdominal ultrasound every 3–6 months for the first 3 years; 52% of survivors fell below recommended guidelines for chest x-ray annually for 5 years. Smaller proportions of survivors were over these recommended standards for abdominal ultrasound and chest x-ray, 1% and 29%, respectively. Mean number of ultrasounds over a 5-year period of surveillance were 2 and 6 in minimal and standard follow-up groups of French CRC survivors respectively, whereas mean number of chest x-rays were 1 and 4 in minimal and standard follow-up groups, respectively [16].
Among studies guided by U.S. professional organizations (ASCO, NCCN) prior to their recommendation of CT imaging, receipt of non-recommended testing ranged from 42% (chest CT) to 94% (complete blood count) among Canadian CRC survivors from an academic cancer center and 37% (chest CT) to 100% (liver function tests) among survivors from a community cancer center [20]. Receipt of imaging among U.S. military personnel and families ranged from 24% (PET scans) to 78% (CT scans) over three years [29]. Since the introduction of CT imaging recommendations into ASCO guidelines in 2005, the prevalence of liver imaging using CT, ultrasound, or MRI among Canadian survivors was 47% in each yearly interval of a 3-year surveillance period [43]. Similar rates of metastatic disease testing, ranging from 15% for abdominal ultrasound to 74% for liver enzymes, were observed among both Canadian and U.S. populations [19, 21]; however, no specific guidelines were cited or identified as the basis for adherence.
Overall surveillance
Although the specific surveillance recommendations and timing of such recommendations varied by study, a number of studies examined the proportion of CRC survivors who received all recommended surveillance tests and procedures. Estimates of adherence to overall surveillance ranged from 12 to 87% [2, 18, 21, 24, 29, 36, 43], with a mean of 12.9 surveillance tests/procedures received by CRC survivors over a 3-year period [21]. Between 17–60% of CRC survivors received surveillance testing below recommended levels and 18–28% received testing above recommended levels [24, 33, 36]. Cluster analysis of CRC survivors in France identified three groups of patients with similar surveillance patterns described as “minimal” (47%), “moderate” (24%), and “intensive” (29%) surveillance [17].
Correlates of Adherence
Correlates of CRC surveillance focused largely on non-modifiable (e.g., socio-demographic and disease/treatment) factors (Table 2). Studies generally indicated that CRC survivors who were older, non-White, with more comorbidities and preoperative complications were less likely to receive CRC surveillance [2, 16–18, 21–26, 28, 30–31, 33–34, 37–38, 40–43, 46]. In contrast, CRC survivors who were insured, of higher-income residential zip codes, with colonic site of disease, who underwent preoperative colonoscopy, received adjuvant chemotherapy, or had physician visits/contact (e.g., oncologist, PCP) were generally more likely to receive CRC surveillance [2, 15, 17, 21–23, 26, 28, 30–31, 37–38, 41, 43, 45–46]. Significant variation in receipt of CRC surveillance was also observed across specific U.S. SEER registries [21–22, 24] and healthcare sites [40–41].
Table 2.
Correlates of recommended post-treatment surveillance tests and procedures among CRC survivors
| Variables | Correlates | No Effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OV/ PV/ CV | CE/ PE | COL | CEA | MDT | Any surva | Overall survb | Early survc | Minimal survd | Moderate surve | Intense survf | ||
| Socio-Demographic Factors | ||||||||||||
| Older age | − | − | −− | −− | − | − | −− | − | + | − | ** | |
| Non-White race/ ethnicity | + | +−− | − | − | − | ** | ||||||
| Gender | ** | |||||||||||
| Male | − | − | − | |||||||||
| Female | + | +− | + | |||||||||
| Married | + | + | * | |||||||||
| Education | * | |||||||||||
| No/unknown education | − | |||||||||||
| Higher household income | + | + | ** | |||||||||
| Higher income of residential zip code | + | + | ||||||||||
| Have health insurance | + | + | ||||||||||
| Urban environment | − | − | * | |||||||||
| Neighborhood poverty level | * | |||||||||||
| SEER location | +gh | +h | +h | +i | +j | *k | ||||||
| Living in primary care shortage area | − | |||||||||||
| Concentration of specialists in county | * | |||||||||||
| Disease/Treatment Characteristics | ||||||||||||
| Colonic site of disease | ++ | +− | + | ** | ||||||||
| Regional stage | + | − | −− | + | + | − | + | + | + | + | ** | |
| Unstaged CRC | − | |||||||||||
| Tumor location (proximal vs. distal) | * | |||||||||||
| Adequate (≥12) lymph node evaluation | * | |||||||||||
| Tumor poorly differentiated | + | ** | ||||||||||
| Polypectomy prior to CRC diagnosis | * | |||||||||||
| Underwent preoperative colonoscopy | + | |||||||||||
| Underwent emergency surgery | * | |||||||||||
| Underwent resection w/reanastomosis | + | * | ||||||||||
| Preoperative complications | − | |||||||||||
| Received chemotherapy | + | + | + | − | + | + | * | |||||
| Received radiation | + | ** | ||||||||||
| Type of physician visits/contact | * | |||||||||||
| PCP | + | + | + | * | ||||||||
| Medical oncologist | − | + | + | + | * | |||||||
| Gastroenterologist | − | |||||||||||
| Internist | * | |||||||||||
| Surgeon | + | + | * | |||||||||
| Unspecified post-resection visits | + | |||||||||||
| More recently trained provider | + | |||||||||||
| Received follow-up care instructions | * | |||||||||||
| Higher co-morbidity index | −− | − | +− | − | − | + | ** | |||||
| Increased time since dx | −− | + | ** | |||||||||
| Site of care | +l | +m | ||||||||||
| Center characteristics | −n | *o | ||||||||||
ABBREVIATIONS: CRC, colorectal cancer; OV/PV/CV, office visits/physician visits/clinic visits; CE/PE, clinical exam/physical exam; COL, colonoscopy; CEA, carcinoembryonic antigen test; MDT, metastatic disease testing (e.g., x-ray, ultrasound, CT scan, MRI, enzymes); surv, surveillance; PCP, primary care provider; dx, diagnosis.
NOTE: +, ≤ three studies report positive associations; ++, > three studies report positive associations; −, ≤ three studies report negative associations; −−, > three studies report negative associations;
≤ three studies report no effect; and
> three studies report no effect.
Reflects receipt of any one surveillance test/procedure among studies that reported multiple tests/procedures.
Reflects receipt of overall recommended surveillance as specified by each individual study.
Reflects receipt of early surveillance, defined as colonoscopy within 3 months to 2 years after colonoscopy with normal results [42].
Reflects receipt of minimal (vs. standard) surveillance as specified by each individual study.
Reflects receipt of moderate (vs. minimal) surveillance as specified by each individual study.
Reflects receipt of intense (vs. minimal) surveillance as specified by each individual study.
Refers to SEER Hawaii location (vs. Atlanta, Detroit, Seattle-Puget Sound, San Francisco-Oakland, Connecticut, Iowa, New Mexico, and Utah locations).
Refers to SEER Atlanta and Seattle Puget Sound locations (vs. Detroit, San Francisco-Oakland, Connecticut, Hawaii, Iowa, New Mexico, and Utah locations).
Refers to SEER Michigan, Georgia, California, Kentucky, Louisiana, and New Jersey locations (vs. Connecticut, Hawaii, Iowa, New Mexico, Washington, and Utah locations).
Refers to SEER Connecticut, Detroit, Hawaii, Iowa, New Mexico, Utah, Atlanta, Kentucky, Louisiana, New Jersey, and California locations (vs. Seattle).
Refers to SEER Midwest, Northeast, and South locations (vs. West location).
Refers to Alabama, Los Angeles, and North Carolina sites of care (vs. CRC survivors in managed care organizations in the Cancer Research Network).
Reflects CRC survivors from Group Health Cooperative (Seattle, WA) and Health Alliance Plan/Henry Ford Health System (Detroit, MI) sites (vs. Kaiser Permanente Colorado (Denver, CO) and Kaiser Permanente Northwest (Portland, OR)).
Reflects treatment centers in the highest (vs. lowest) tertile of all surgical volume.
Reflects treatment centers where both diagnosis and treatment occurred at reporting facility (vs. diagnosed elsewhere and treated at reporting facility) or treatment centers who are members of a university health consortium (vs. not a member).
The association of gender, marital status, education, household income, geographic location (i.e., urban versus rural), stage at diagnosis, tumor differentiation, receipt of radiation, receipt of surgical resection with reanastomosis, and length of time since diagnosis on receipt of surveillance yielded inconsistent results across studies [2, 15–18, 21–26, 28, 30–31, 33–34, 37–38, 40–43, 46]. A number of studies reported no significant association of neighborhood poverty level, concentration of specialists in the county, tumor location (i.e., proximal versus distal), receipt of adequate (≥12) lymph node evaluation, receipt of polypectomy prior to CRC diagnosis, receipt of emergency surgery, or receipt of follow-up care instructions on receipt of CRC surveillance [16, 21, 28, 34, 37, 43, 47].
Completeness of Reporting on Selected Characteristics of Internal and External Validity
Completeness of reporting was variable on 6 aspects of internal and external validity (Table 3). Twelve [2, 16, 24–27, 29, 34, 39–40, 42, 44] of the 34 studies did not provide data regarding the number of potentially eligible participants. Twenty-three studies [15–23, 30–33, 35–39, 41, 43, 45–47] explicitly reported how sample size was determined; for 11 [2, 24–29, 34, 40, 42, 44] data were inferred (e.g., authors reported that sample was determined from a given administrative database) but not explicitly reported. Only 4 studies [15, 31, 37–38] explicitly addressed the issue of confounders, while 28 [2, 16– 18, 20–30, 32–36, 39–43, 45–47] made only implicit reference to potential confounders (e.g., adjusted analyses without specific rationale or justification for doing so), and 2 studies [19, 44] did not address confounders. Twenty-nine [2, 15–22, 26–27, 29–35, 37–44, 45–47] of the 34 studies provided unadjusted estimates, and all but 7 [16, 19–20, 32, 35, 39, 44] provided both unadjusted and adjusted estimates. Five studies [23–25, 28, 36] provided only adjusted estimates. Related to generalizability, only 14 [16, 18, 20–22, 27, 34–36, 40, 42, 45–47] of the 34 studies explicitly discussed external validity, while 12 [17, 19, 24–26, 28, 32–33, 38, 41, 43–44] made only implicit reference to the issue.
Table 3.
Completeness of reporting on selected characteristics of internal and external validity utilizing STROBE guidelines
| Study
|
Study Population
|
Design and Analysis
|
Generalizability
|
|||
|---|---|---|---|---|---|---|
| First author (year) | Number of potentially eligible participants | Determination of sample size | Discussion of potential confounders | Reporting of unadjusted estimates | Reporting of adjusted estimates & precision | Discussion of external validity issues |
| Boehmer (2010) | Y | Y | Y | Y | Y | N |
| Borie (2004) | N | Y | I | Y | N | Y |
| Boulin (2005) | Y | Y | I | Y | Y | I |
| Brawarsky (2013) | I | Y | I | Y | Y | Y |
| Cardella (2008) | Y | Y | N | Y | N | I |
| Cheung (2008) | Y | Y | I | Y | N | Y |
| Cooper (1999) | Y | Y | I | Y | Y | Y |
| Cooper (2000) | Y | Y | I | Y | N | Y |
| Cooper (2006) | Y | Y | I | N | Y | N |
| Cooper (2008) | N | I | I | N | Y | I |
| Earle (2004) | Y | Y | I | Y | Y | Y |
| Ellison (2003) | N | I | I | N | Y | I |
| Elston Lafata (2001) | N | I | I | Y | Y | I |
| Elston Lafata (2005) | N | I | I | Y | Y | Y |
| Foley (2011) | I | I | I | N | Y | I |
| Fox (2013) | N | I | I | Y | Y | N |
| Haggstrom (2009) | Y | Y | I | Y | Y | N |
| Hilsden (2004) | Y | Y | Y | Y | Y | N |
| Hu (2011) | N | I | I | Y | Y | N |
| Jackson (2010) | Y | Y | I | Y | N | I |
| Knopf (2001) | Y | Y | I | Y | Y | I |
| Mahboubi (2007) | Y | Y | I | Y | Y | Y |
| Parsons (2012) | N | I | I | Y | Y | Y |
| Pollack (2009) | I | Y | I | Y | N | Y |
| Ramsey (2007) | Y | Y | I | N | Y | Y |
| Rolnick (2005) | Y | Y | Y | Y | Y | N |
| Rulyak (2004) | Y | Y | Y | Y | Y | I |
| Rulyak (2007) | N | Y | I | Y | N | N |
| Sabatino (2013) | Y | Y | I | Y | Y | Y |
| Salloum (2012) | N | I | I | Y | Y | Y |
| Salz (2010) | Y | Y | I | Y | Y | I |
| Singh (2013) | N | I | I | Y | Y | Y |
| Sisler (2012) | Y | Y | I | Y | Y | I |
| Spratlin (2008) | N | I | N | Y | N | I |
ABBREVIATIONS: Y = yes, explicitly reported by study authors; I = implied by raters but not explicitly reported by study authors; N = no, unreported by study authors; STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
4. Discussion
This systematic review of post-treatment surveillance among CRC survivors demonstrates that deviation from surveillance recommendations includes both under- and overuse. Across all study populations, receipt of colonoscopy among CRC survivors was suboptimal and was not markedly better than recent estimates of colonoscopy (47.5%) in average-risk populations [53]. Such low prevalence of adherence to surveillance recommendations among CRC survivors is concerning as it compromises the usefulness of post-treatment surveillance for early detection of recurrent or second cancers.
Our review also found overuse of recommended surveillance tests and procedures. Such findings align with recent work by Potosky and colleagues [54] which found that both U.S. oncologists and primary care providers endorse more surveillance tests and at more frequent intervals than recommended by current guidelines. Because younger age and regional stage disease have been associated with overuse of surveillance, these findings may indicate risk stratification on the part of physicians to more aggressively monitor survivors at greater risk for recurrence [24]. However, it has also been posited that physicians may feel pressured to order unnecessary surveillance tests and/or procedures in order to reassure anxious patients [14]. Regardless of the reasons, it is important to emphasize that overuse is just as problematic as suboptimal use. Surveillance is costly and has potential adverse effects (e.g., perforation, false-positive findings); thus, its use should follow established guidelines [9–13].
Studies of factors associated with adherence to surveillance have focused almost exclusively on patient-level socio-demographic and disease/treatment factors associated with receipt of surveillance. While these findings are useful for identifying groups that need to be prioritized for surveillance (e.g., those lacking reliable access to healthcare), they do not identify modifiable patient factors or other contributing factors beyond the patient (e.g., provider, healthcare system) that may impact adherence to recommended guidelines. This lack of information stands in contrast to the literature on factors associated with CRC screening in average-risk populations. A number of psychosocial variables, including preventive health orientation, knowledge of cancer risk factors, perceived benefits and barriers to screening, self-efficacy, fear or worry about CRC, physician recommendation, and intention have been consistently associated with CRC screening [55]. Results of a single study to date which examined modifiable psychosocial (i.e., health belief model) factors associated with surveillance among CRC survivors found that only greater perceived likelihood of CRC recurrence was associated with intention to have a colonoscopy; however, completion of the procedure was not examined [56]. Because of the disease experience, it may be that the factors associated with surveillance differ from those associated with screening. Additional research is needed to identify modifiable factors associated with both under- and over-utilization of surveillance that can be targeted in future evidence-based interventions with CRC survivors.
A multilevel perspective may represent one useful way of conceptualizing, and ultimately intervening, with the problem of suboptimal and overuse of recommended surveillance tests and procedures among CRC survivors. This perspective recognizes that there are a number of contextual influences (i.e., individual patient, family and social supports, provider/team, local community environment, and state and national health policy) that impact individual behavior throughout the cancer care continuum, from risk assessment through diagnosis and treatment to post-treatment survivorship [57], as highlighted in a special issue of JNCI monographs [58]. To date, only a few multilevel intervention trials have been conducted targeting health outcomes such as smoking cessation [59] and cardiovascular health [60]. To our knowledge, no interventions have targeted adherence to recommended surveillance among CRC survivors.
At the patient-level, CRC survivors may benefit from self-management strategies such as problem-solving, decision-making, resource utilization, forming partnerships with healthcare providers, and taking action [61]. Support for the utility of self-management interventions in improving cancer survivorship outcomes (e.g., distress, energy, physical activity) has recently been examined among breast cancer survivors [62–64]. At the provider-level, improved communication between oncologists and primary care providers is critical [65–66]. Survivorship care plans can help facilitate this transition by clearly identifying which provider is responsible for each aspect of follow-up care, thereby reducing the potential for role ambiguity [65]. Results from the few randomized trials to date suggest that primary care providers who received a brief survivorship care plan to inform their care of breast and CRC survivors achieved identical outcomes (e.g., time to detection of recurrence, rate of recurrence-related serious events) as cancer specialists [67–69]. Primary care providers also appear willing to assume exclusive care of CRC survivors, with appropriate supports (e.g., patient-specific letters from the specialist, printed guidelines, expedited referral sources and access to investigations for suspected recurrence) [70]. Finally, at a healthcare systems-level, a better understanding of survivors’ healthcare environments and national healthcare policies is critical, particularly given the observed trend toward greater receipt of surveillance among non-U.S. populations, such as Canada, where universal health care is the norm [19–20, 31, 43–44]. Careful examination of the referral patterns for and financing of surveillance tests and procedures is important in optimizing the CRC surveillance process.
There are several limitations of this review. Many of the studies utilized the same or overlapping populations from large publicly-available databases (e.g., SEER-Medicare). There were also significant differences in how studies operationally defined adherence to surveillance, and some definitions were easier to adhere to than others (e.g., office visits and physical exams versus colonoscopy and CEA testing). As noted by others [5, 14], this wide variation in surveillance programs has made it difficult to identify the most effective test/procedure (or combination thereof) or the optimal schedule for follow-up, a limitation that warrants additional investigation. The wide-ranging estimates of surveillance found in this review precluded the use of meta-analysis to aggregate effect sizes of adherence to surveillance. Likewise, because of the limited number of correlates examined and the inconsistent patterns of association, it was not feasible to provide summary estimates.
Despite these limitations, our review provides a comprehensive examination of surveillance practices among heterogeneous groups of CRC survivors from clinic settings and administrative databases (i.e., SEER-Medicare) which include only persons 65 years and older. With few exceptions, only the non-U.S. studies used population-based cancer registries; the use of such registries would represent a useful direction for future research in the U.S. Assessment of the completeness of reporting on selected aspects of internal and external validity revealed that relatively little attention was paid to issues of potential confounders and generalizability. This lack of attention in the conduct and reporting of these studies makes it difficult to assess potential threats to internal and external validity [71]. Moving forward, investigations into the prevalence of adherence to post-treatment surveillance among CRC survivors should more clearly examine and report aspects of both internal and external validity. Given that the studies summarized in our review focused predominantly on non-modifiable factors associated with CRC surveillance, there is a need to conduct studies that assess modifiable determinants of surveillance at the patient, provider, and healthcare system levels. Although the feasibility of conducting studies that do not rely on administrative data is admittedly much more challenging, it is only once modifiable determinants have been identified that interventions to increase adherence to recommended surveillance guidelines among CRC survivors can be developed and evaluated. In addition to targeting patient-level modifiable determinants, such interventions should incorporate a multilevel perspective in order to directly and indirectly target a range of modifiable factors that influence both short- (i.e., receipt of surveillance) and long-term (i.e., survival) outcomes of interest.
Acknowledgments
The authors would like to thank Helena Vonville, MLS for her assistance in developing the search strategy. This work was supported by the National Cancer Institute at the National Institutes of Health (K07CA140159 to M.Y.C. and R01CA112223 to S.W.V.); and the Susan G. Komen Foundation (KG111378 to S.M.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Susan G. Komen Foundation.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Reference List
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Hu C-Y, Delclos GL, Chan W, et al. Post-treatment surveillance in a large cohort of patients with colon cancer. Am J Manag Care. 2011;17:329–36. [PubMed] [Google Scholar]
- 3.Hammond K, Margolin DA. The role of postoperative surveillance in colorectal cancer. Clin Colon Rectal Surg. 2007;20:249–54. doi: 10.1055/s-2007-984869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24. doi: 10.1007/BF02055676. [DOI] [PubMed] [Google Scholar]
- 5.Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: A meta-analysis. Dis Colon Rectum. 2007;50:1783–99. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow-up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ. 2002;324:1–8. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunitake H, Zheng P, Land SR, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol LTS-01. J Clin Oncol. 2010;28:5274–9. doi: 10.1200/JCO.2010.30.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–17. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 10.American Cancer Society. Colorectal cancer detailed guide; 6–17–2011. Available at: http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/index.
- 11.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: A consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–71. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Desch CE, Benson AB, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer, Version 1.2012. 2011. pp. 8–30. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007:CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Boehmer U, Harris J, Bowen DJ, et al. Surveillance after colorectal cancer diagnosis in a safety net hospital. J Health Care Poor Underserved. 2010;21:1138–51. doi: 10.1353/hpu.2010.0918. [DOI] [PubMed] [Google Scholar]
- 16.Borie F, Daures J-P, Millat B. Cost and effectiveness of follow-up examinations in patients with colorectal cancer resected for cure in a French population-based study. J Gastrointest Surg. 2004;8:552–558. doi: 10.1016/j.gassur.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Boulin M, Lejeune C, Le Teuff G, et al. Patterns of surveillance practices after curative surgery for colorectal cancer in a French population. Dis Colon Rectum. 2005;48:1890–1899. doi: 10.1007/s10350-005-0096-7. [DOI] [PubMed] [Google Scholar]
- 18.Brawarsky P, Neville BA, Fitzmaurice GM, et al. Surveillance after resection for colorectal cancer. Cancer. 2013;119:1235–1242. doi: 10.1002/cncr.27852. [DOI] [PubMed] [Google Scholar]
- 19.Cardella J, Coburn NG, Gagliardi A. Compliance, attitudes and barriers to post-operative colorectal cancer follow-up. J Eval Clin Pract. 2008;14:407–415. doi: 10.1111/j.1365-2753.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheung WY, Pond GR, Rother M, et al. Adherence to surveillance guidelines after curative resection for stage II/III colorectal cancer. Clin Colorectal Canc. 2008;7:191–196. doi: 10.3816/CCC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 21.Cooper GS, Yuan Z, Chak A, et al. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85:2124–2131. [PubMed] [Google Scholar]
- 22.Cooper GS, Yuan Z, Chak A, et al. Patterns of endoscopic follow-up after surgery for nonmetastatic colorectal cancer. Gastrointest Endosc. 2000;52:33–38. doi: 10.1067/mge.2000.106685. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Payes JD. Temporal trends in colorectal procedure use after colorectal cancer resection. Gastrointest Endosc. 2006;64:933–40. doi: 10.1016/j.gie.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Kou TD, Reynolds HL. Receipt of guideline-recommended follow-up in older colorectal cancer survivors. Cancer. 2008;113:2029–37. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 25.Ellison GL, Warren JL, Knopf KB, et al. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38:1885–904. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elston Lafata J, Johnson CC, Ben-Menaghem T, et al. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39:361–72. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–9. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 28.Foley KL, Song E-Y, Klepin H, et al. Screening colonoscopy among colorectal cancer survivors insured by Medicaid. Am J Clin Oncol. 2011;35:205–11. doi: 10.1097/COC.0b013e318209d21e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox JP, Jeffery DD, Williams TV, et al. Quality of cancer survivorship care in the military health system (TRICARE) Cancer J. 2013;19:1–9. doi: 10.1097/PPO.0b013e3182821930. [DOI] [PubMed] [Google Scholar]
- 30.Haggstrom DA, Arora NK, Helft P, et al. Follow-up care delivery among colorectal cancer survivors most often seen by primary and subspecialty care physicians. J Gen Intern Med. 2009;24:472–479. doi: 10.1007/s11606-009-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilsden RJ, Bryant HE, Sutherland LR, et al. A retrospective study on the use of post-operative colonoscopy following potentially curative surgery for colorectal cancer in a Canadian province. BMC Cancer. 2004;4:14. doi: 10.1186/1471-2407-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson GL, Melton D, Abbot DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28:3176–81. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopf KB, Warren JL, Feuer EJ, et al. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc. 2001;54:563–71. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 34.Parsons HM, Tuttle TM, Kuntz KM, et al. Quality of care along the cancer continuum: Does receiving adequate lymph node evaluation for colon cancer lead to comprehensive postsurgical care? J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Pollack LA, Adamache W, Ryerson AB, et al. Care of long-term cancer survivors: Physicians seen by Medicare enrollees surviving longer than 5 years. Cancer. 2009;115:5284–5295. doi: 10.1002/cncr.24624. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey SD, Howlader N, Etzioni R, et al. Surveillance endoscopy does not improve survival for patients with local and regional stage colorectal cancer. Cancer. 2007;109:2222–8. doi: 10.1002/cncr.22673. [DOI] [PubMed] [Google Scholar]
- 37.Rolnick S, Alford SH, Kucera GP, et al. Racial and age differences in colon examination surveillance following a diagnosis of colorectal cancer. J Natl Cancer Inst Monogr. 2005;35:96–101. doi: 10.1093/jncimonographs/lgi045. [DOI] [PubMed] [Google Scholar]
- 38.Rulyak SJ, Madelson MT, Brentnall TA, et al. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59:239–47. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 39.Rulyak SJ, Lieberman DA, Wagner EH, et al. Outcome of follow-up colon examination among a population-based cohort of colorectal cancer patients. Clin Gastroenterol H. 2007;5:470–476. doi: 10.1016/j.cgh.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Salloum RG, Hornbrook MC, Fishman PA, et al. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer. 2012 doi: 10.1002/cncr.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256–63. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh A, Kuo Y-F, Goodwin JS. Many patients who undergo surgery for colorectal cancer receive surveillance colonoscopies earlier than recommended by guidelines. Clin Gastroenterol H. 2013;11:65–72. doi: 10.1016/j.cgh.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: A population-based analysis. J Oncol Pract. 2012;8:e69–79. doi: 10.1200/JOP.2011.000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spratlin JL, Hui D, Hanson J, et al. Community compliance with carcinoembryonic antigen: Follow-up of patients with colorectal cancer. Clin Colorectal Canc. 2008;7:118–125. doi: 10.3816/CCC.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 45.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 46.Mahboubi A, Lejeune C, Coriat R, et al. Which patients with colorectal cancer are followed up by general practitioners? A population-based study. Eur J Cancer Prev. 2007;16:535–541. doi: 10.1097/CEJ.0b013e32801023a2. [DOI] [PubMed] [Google Scholar]
- 47.Sabatino SA, Thompson TD, Smith JL, et al. Receipt of cancer treatment summaries and follow-up instructions among adult cancer survivors: Results from a national survey. J Cancer Surviv. 2013;7:32–43. doi: 10.1007/s11764-012-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treanor C, Donnelly M. An international review of the patterns and determinants of health service utilization by adult cancer survivors. BMC Health Serv Res. 2012;12:316. doi: 10.1186/1472-6963-12-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salz T, Woo H, Starr TD, et al. Ethnic disparities in colonoscopy use among colorectal cancer survivors: A systematic review. J Cancer Surviv. 2012;6:372–378. doi: 10.1007/s11764-012-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLos Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 52.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, West Sussex, England: John Wiley & Sons; 2008. [Google Scholar]
- 53.Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potosky AL, Han PKJ, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403–10. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vernon SW, McQueen A. Colorectal cancer screening. In: Holland JC, et al., editors. Psycho-oncology. 2. New York, NY: Oxford University Press; 2010. pp. 71–83. [Google Scholar]
- 56.Salz T, Brewer NT, Sandler RS, et al. Association of health beliefs and colonoscopy use among survivors of colorectal cancer. J Cancer Surviv. 2009;3:193–201. doi: 10.1007/s11764-009-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taplin SH, Price RA, Edwards HM, et al. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012:2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Cancer Institute. Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012:1–134. doi: 10.1093/jncimonographs/lgs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The COMMIT Research Group. Community intervention trial for smoking cessation (COMMIT): II. Changes in adult cigarette smoking prevalence. Am J Public Health. 1995;85:193–200. doi: 10.2105/ajph.85.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lytle LA, Stone EJ, Nichaman MZ, et al. Changes in nutrient intakes of elementary school children following a school-based intervention: Results from the CATCH study. Prev Med. 1996;25:465–77. doi: 10.1006/pmed.1996.0078. [DOI] [PubMed] [Google Scholar]
- 61.Lorig KR, Holman HR. Self-management education: History, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 62.Cimprich B, Janz NK, Northouse L, et al. Taking charge: A self-management program for women following breast cancer treatment. Psychooncology. 2005;14:704–17. doi: 10.1002/pon.891. [DOI] [PubMed] [Google Scholar]
- 63.Damush TM, Perkins A. The implementation of an oncologist referred, exercise self-management program for older breast cancer survivors. Psychooncology. 2006;15:884–90. doi: 10.1002/pon.1020. [DOI] [PubMed] [Google Scholar]
- 64.Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol. 2008;24:5132–7. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 65.Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol. 2008;26:2246–7. doi: 10.1200/JCO.2007.15.7081. [DOI] [PubMed] [Google Scholar]
- 66.Hudson MM, Landier W, Ganz PA. Impact of survivorship-based research on defining clinical care guidelines. Cancer Epidemiol Biomarkers Prev. 2011;20:2085–92. doi: 10.1158/1055-9965.EPI-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grunfeld E, Mant D, Yudkin P, et al. Routine follow up of breast cancer in primary care: Randomised trial. BMJ. 1996;313:665–9. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–55. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 69.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: Randomised controlled trial. Br J Cancer. 2006;94:1116–21. doi: 10.1038/sj.bjc.6603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Del Giudice M, Grunfeld E, Harvey BJ, et al. Primary care physicians’ views of routine follow-up care of cancer survivors. J Clin Oncol. 2009;27:3338–45. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 71.Steckler A, McLeroy KR. The importance of external validity. Am J Public Health. 2008;98:9–10. doi: 10.2105/AJPH.2007.126847. [DOI] [PMC free article] [PubMed] [Google Scholar]