Abstract
Many cognitively normal older adults have underlying neuropathologic changes of Alzheimer’s disease (AD), vascular brain injury (VBI), or Lewy body disease (LBD), which confer an increased risk of dementia. The current study focused on the association between multiple neuropathologic indices and performance on specific cognitive domains in a community sample of older adults. Of 438 participants in the Adult Changes in Thought population-based study of brain aging who were autopsied, 363 subjects had cognitive testing at their final study visit and were included. Associations were measured between performance on the Cognitive Abilities Screening Instrument prior to death and neuropathologic endpoints, including AD neuropathologic changes, LBD, cerebral amyloid angiopathy, and measures of VBI. Braak stage for neurofibrillary tangles, lower brain weight, and VBI as measured by cerebral cortical microvascular lesions (μVBI) explained a significant proportion of the variance associated with global cognitive test performance (R2=0.31, p< 0.0001) both in the entire sample and when analysis was restricted to non-demented subjects (R2= 0.23, p< 0.0001). Specific cognitive domains were differentially related to neuropathologic lesion type: memory and executive function with AD pathologic changes and cortical μVBI, executive function with subcortical μVBI, and visuospatial construction with LBD. Thus, neuropathologic lesions of LBD and μVBI are associated with poorer cognitive performance over and above AD neuropathologic changes in subjects without dementia in this cohort. These findings underscore that cognitive impairment is a complex convergent trait that has important implications for clinical investigation and medical management of older adults.
Keywords: Alzheimer’s disease, brain, cerebrovascular disorders, cognition, dementia, Lewy bodies, pathologic processes
INTRODUCTION
Many studies of brain aging and dementia have observed that neuropathologic changes of Alzheimer’s disease (AD), vascular brain injury (VBI), and/or Lewy body disease (LBD) are the major predictors of clinical dementia diagnosis in older individuals [1–3]. It is important to recognize that estimates of the relative contribution of these diseases to dementia and the extent of their co-morbidity varies significantly depending upon the cohort investigated, with discrepant results reported from research center cohorts versus population- or community-based cohorts, as well as variability due to difference sin methodology and criteria [4, 5]. Although many clinical-pathological studies primarily focus on clinical dementia diagnosis as the primary cognitive outcome, others also have associated neuropathologic changes with various measures of cognitive performance. However, many of these investigations have relied on data gathered from research center cohorts, have small sample sizes, and/or focus primarily on AD neuropathologic changes [6–9]. There have been fewer large, community-based investigations attempting to associate a range of neuropathologic changes with cognitive performance, potentially limiting generalization of results from smaller research cohorts to the community. The primary goal of our study is to address these gaps in our knowledge.
The studies mentioned above and others have observed repeatedly that the neuropathologic features of AD, VBI, and LBD are variably present and commonly co-morbid among older adults without dementia or even without evidence of cognitive impairment. While clinical laboratory and radiologic evaluations have provided new insights into the natural history of apparently pre-clinical AD [10–12], a remaining advantage of autopsy evaluations is that they currently provide the most comprehensive assessment of brain diseases. We previously reported neuropathologic correlates of clinical dementia in the Adult Changes in Thought (ACT) cohort [13]. Given the current focus on the study of early cognitive changes that may signal latent and prodromal cerebral illnesses, we extend our findings here to address the association between neuropathologic changes and specific cognitive functions in older individuals without dementia.
MATERIALS AND METHODS
Participants
This study was approved by the Human Subjects Review Committees of the University of Washington and Group Health Research Institute. The study sample included autopsied participants from the ACT population-based cohort; detailed design and methods for ACT and for neuropathologic evaluation of ACT cases have been published previously [13–16]. In brief, the ACT cohort enrolls non-demented adults aged 65 and over, recruited from a random sample of Group Health Cooperative subscribers across the greater Seattle area. Cognitive screening, physical function, medical history review, and functional status assessments are administered to ACT participants at study entry and subsequently every two years unless, or until, a diagnosis of dementia is made. Participants who score below a cutoff of 86 out of 100 on the cognitive screening test (described below) or who are referred based on family and/or ACT interviewer concerns undergo a full dementia evaluation, which includes comprehensive cognitive and medical assessments. Dementia diagnosis is made by a consensus panel of experts using Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) [17] and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [18] standard criteria.
Autopsy sample
Of 4,530 ACT participants with at least one visit, 1,774 died; of those participants, 438 were autopsied. All autopsied participants provided written informed consent prior to death. As required by Washington state law, next-of-kin also provided informed postmortem consent prior to autopsy. Those who had cognitive testing at their final study visit (within two years of death for non-demented participants) and who underwent complete postmortem neuropathologic examination were included in the final analyses (cognitive sample, n= 363). Ninety percent (n = 327) of the cognitive sample had blood drawn for APOE genotyping prior to death, as previously described [14].
Autopsy procedures
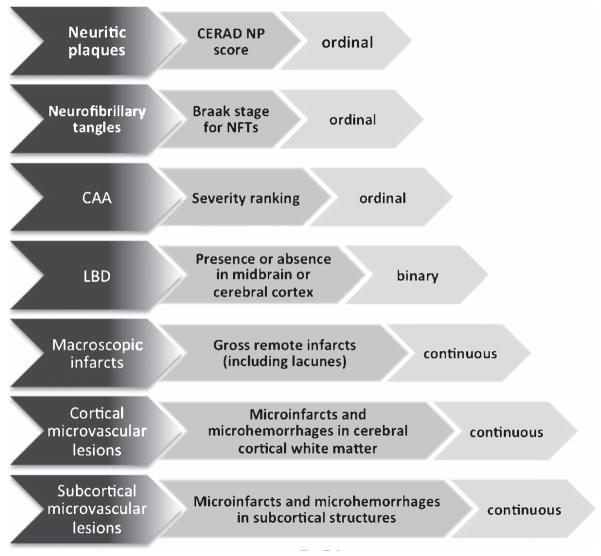
Neuropathologic evaluations were conducted exactly as described previously [13, 16]. Neuropathologic measures (Fig. 1) included total brain weight; hallmark AD neuropathologic features (Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaques (NP) score [19] and Braak staging for neurofibrillary tangles (NFTs) as visualized by silver stain [20]); LBD as detected by Lewy bodies or Lewy neurites in the midbrain or cerebral cortex [13]; ranking of severity of cerebral amyloid angiopathy (CAA) [21]; and VBI from (i)large caliber vessels in the cerebrum as measured by remote gross (macroscopic) infarcts, including lacunar infarcts, and (ii) from small caliber vessels(microvascular brain injury or μVBI) as measured by enumerating microvascular lesions (microinfarcts and microhemorrhages, although the vast majority are microinfarcts with rare bona fide microhemorrhages) in a standardized screening section of cerebral cortex and subcortical structures (cerebral white matter, basal ganglia, and thalamus) [13, 16] based on the method developed in the Honolulu Asia Aging Study [22] and recommended by a recent consensus panel appointed by the National Institute on Aging and the Alzheimer’s Association [23, 24]. Other potentially informative markers of white matter injury, such as leukoaraiosis or leukoencephalopathy, were not collected as part of ACT. Given that acute microvascular lesions would have occurred subsequent to the final cognitive testing visit in most individuals, these lesions were excluded from analyses.
Fig. 1.
Operationalization and levels of measurement of neuropathologic variables measured in the ACT cognitive sample.
Cognitive examination
A cognitive screening test, the Cognitive Abilities Screening Instrument (CASI) [25], is administered to all ACT participants at their biennial study visit. The CASI consists of items from the Mini-Mental State Examination [26], the modified Mini Mental State Examination [27], and the Hasegawa Dementia Scale [28], and screens for impairments in a range of cognitive functions including attention, concentration, orientation, memory, language, visuospatial construction, verbal fluency, and abstraction. The CASI was originally developed as a cross-cultural dementia screen [25] and has been validated as a tool for the assessment of early to severe cognitive decline across populations [25, 29, 30].
Total CASI score as well as subscale scores reflecting performance in selected cognitive domains (executive function, verbal memory, visuospatial construction, and language) were calculated. Table 1 describes the test items that comprised each domain-specific score. Subdomains were determined as described by Teng et al. [25]; however, attention, concentration, abstraction, and verbal fluency items were combined to create a composite executive function index similar to the memory and language indices. To permit calculation of sub-domain scores and comparison of scores across domains, raw scores were converted to z-scores using the mean and standard deviation obtained from the entire, nondemented cohort at baseline. These standard scores were then summed and averaged to create a composite z-score for each cognitive domain. Z-scores were also calculated for total CASI score to facilitate comparison with sub-domain scores. In addition, item response theory (IRT) scores have been generated for the ACT sample based on previously published findings that the CASI has curvilinear scaling properties (e.g., a given number of standard CASI points are associated with variability depending upon such factors as age and education) [31]. IRT scores generated from previously published parameter estimates were found to produce less-biased estimates of global ability [32]. Thus, both CASI z-scores and CASI IRT scores were analyzed for the current study when examining global cognitive performance.
Table 1.
Constituent CASI subtests by cognitive domain
| Cognitive domain | CASI subtests |
|---|---|
| Executive function | attention, concentration, verbal fluency, abstraction |
| Memory | verbal list recall, object recall |
| Visuospatial construction | cube drawing |
| Language | command, reading, writing, naming |
Analyses
T-tests and Fisher’s exact tests were performed to examine differences between diagnostic groups (dementia, no dementia) on demographic variables and neuropathologic features. To evaluate the relationship between final cognitive performance and neuropathologic changes, linear regression was performed using total CASI score or CASI IRT score as the response variable and demographic and neuropathologic indices as predictors. Separate linear regression analyses were then performed by cognitive domain (CASI subscales measuring executive function, memory, visuospatial construction, and language), with demographic and neuropathology indices entered as predictors. Age, education, and gender were entered first in all regression models. Braak staging, CERAD score, and CAA were coded as ordinal scores based on standard criteria for each, LBD was coded as a binary variable (present versus absent), and brain weight, gross infarcts, and microvascular lesions were continuous data. Variance inflation factors were examined for each variable entered into the regression model to determine the presence or absence of multi-collinearity between the predictor variables. To determine whether APOE 4 genotype was a significant predictor of cognitive performance over and above demographic and neuropathologic variables, secondary analyses were carried out on the subset of ACT participants who had undergone APOE genotyping prior to death. All statistical analyses were performed using Stata 12 (StataCorp., College Station, TX).
RESULTS
Participant characteristics
Sample characteristics are provided in Table 2. The sample included in this investigation did not differ from the total autopsy cohort with respect to demographic or neuropathologic measures. As we have observed previously [33], when the autopsy sample was compared to the entire ACT cohort who died, the autopsy group was older at death and had higher education than the group that did not undergo autopsy. Among those who underwent autopsy, the demented group was significantly older at death, had lower education level, had a lower final total CASI score, and was more likely to have neuropathologic changes across all indices than the non-demented group. As mentioned, biennial visits are discontinued once a participant is diagnosed with dementia in the ACT study; thus, there was a longer average period of time between final cognitive testing and autopsy for demented (~3.7 years) versus nondemented (~1 year) groups.
Table 2. Sample characteristics.
Percentages in the table may not equal 100 due to rounding.
| Autopsy cohort | Total included sample | No Dementia | Dementia only | sig.† | ||
|---|---|---|---|---|---|---|
| (n = 438) | (n = 363) | (n = 196) | (n = 167) | |||
| Age at visit | mean (sd) | 85.0 (6.6) | 85.1 (6.5) | 85.1 (7.2) | 85.2 (5.8) | |
| Age at death | mean (sd) | 87.4 (6.8) | 87.4 (6.7) | 86.0 (7.2) | 89.0 (5.7) | *** |
| Education | mean (sd) | 14.3 (3.1) | 14.2 (3.0) | 14.5 (3.0) | 13.9 (3.1) | * |
| Gender | % women | 55.9 | 54.0 | 52.0 | 56.3 | |
| Final CASI | mean (sd) | 86.3 (10.5) | 85.2 (10.7) | 92.0 (5.7) | 77.4 (9.9) | *** |
| Braak stage for NFTs | % 0 | 4.1 | 3.9 | 5.6 | 1.8 | |
| % I/II | 34.5 | 31.7 | 44.4 | 16.8 | *** | |
| % III/IV | 33.6 | 33.6 | 39.3 | 27.0 | ||
| % V/VI | 27.9 | 30.9 | 10.7 | 54.5 | ||
| CERAD NP score | % None | 22.8 | 22.3 | 29.1 | 14.4 | |
| % Sparse | 30.6 | 30.9 | 37.8 | 22.8 | *** | |
| % Moderate | 24.0 | 23.7 | 21.4 | 26.4 | ||
| % Frequent | 22.6 | 23.1 | 11.7 | 36.5 | ||
| Brain weight | mean (sd) | 1214.2 (138.0) | 1212.3 (137.3) | 1240.2 (126.2) | 1179.6 (142.9) | *** |
| CAA ranking | % None | 70.5 | 69.4 | 79.1 | 58.1 | |
| % Mild | 14.8 | 15.7 | 11.7 | 20.4 | *** | |
| % Moderate | 12.9 | 13.2 | 8.2 | 19.2 | ||
| % Severe | 1.8 | 1.7 | 1.0 | 2.4 | ||
| Lewy body disease | % | 13.8 | 13.8 | 11.7 | 16.2 | |
| Gross remote cerebral infarcts | % | 30.3 | 31.7 | 22.5 | 42.5 | *** |
| Cortical microvascular lesions | % | 34.6 | 35.5 | 27.0 | 45.5 | *** |
| Subcortical microvascular lesions | % | 29.6 | 31.1 | 25.0 | 38.3 | ** |
significance reported for pairwise comparisons (t-tests and Fisher’s exact tests) between dementia and no dementia groups.
Braak NFT stage comparisons: 0, I, II versus III, IV, V, VI; CERAD NP score comparisons: none/sparse versus moderate/frequent; CAA ranking comparisons: none/mild versus moderate/severe; LBD, gross cerebral infarcts, cortical and subcortical microvascular lesion comparisons: present versus absent.
p < 0.001
p < 0.01
p < 0.05.
A substantial proportion of the cognitive sample had AD neuropathologic changes: 65% had Braak NFT staging of III to VI, and 47% had moderate or frequent CERAD score for NPs. Even when those who received a clinical diagnosis of dementia were excluded, one-half of the sample had Braak NFT stage of III to VI, and one-third had a moderate or frequent CERAD score for NPs. No differences were noted for those with a Braak stage III or less as compared with Braak stage IV–VI with regard to proportion of LBD, macroscopic infarcts, or subcortical microvascular lesions. A lower proportion of participants classified as Braak stage none–III had cerebral cortical microvascular lesions(31%) and CAA (19%) as compared to those with Braak stage IV–VI (41% and 45%, respectively).
Global cognition and neuropathologic indices
Demographic factors alone accounted for 8% of the total variance in CASI score (F(3,332)= 9.46, p< 0.0001). When all neuropathologic indices were entered at once into the model, the total explained variance increased to 31% (F(11,324)= 13.04, p< 0.0001). Braak stage for NFTs, brain weight, and cerebral cortical μVBI were significant predictors of total CASI score (p values ≤0.01) (Table 3). Low variable inflation factors (VIF range = 1.01–1.75) suggest that the magnitude of multi-collinearity between the predictors did not substantially impact the results. When CASI IRT scores were substituted for CASI total z-scores, the findings were similar, with a total explained variance of37% (F(11,322)= 16.86, p< 0.0001). Again, Braak stage for NFTs, brain weight, and cerebral cortical microvascular lesions were significant predictors of CASI IRT scores. To determine if our results were largely influenced by subjects with dementia, analyses were re-run excluding those diagnosed with dementia during life. As with the total group, global cognitive function (total CASI score) was predicted by Braak stage for NFTs (p = 0.01), brain weight (p = 0.04), and cerebral cortical microvascular lesions (p = 0.02) when all variables were entered into the model (F(11,169)= 4.59, p< 0.0001).
Table 3.
Linear regression results for total CASI score, cognitive subdomains, and neuropathology variables
| Variables | CASI (total) | Executive | Memory | Construction | Language | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total group | Non- demented | Total group | Non- demented | Total group | Non- demented | Total group | Non- demented | Total group | Non- demented | |
| Demographic model | R2=0.08*** | R2=0.14*** | R2=0.05*** | R2=0.08** | R2=0.03* | R2=0.10*** | R2=0.05*** | R2=0.07** | R2=0.02‡ | R2=0.02 |
| Age | −2.90** | −3.38*** | −2.34* | −3.31*** | −2.44* | −3.05** | −4.25*** | −3.61*** | −2.51* | −2.20* |
| Gender | 0.54 | 0.97 | −1.25 | −0.70 | 0.68 | 2.88** | 0.24 | 0.09 | 0.01 | −0.68 |
| Education | 4.47*** | 4.72*** | 2.99** | 1.66‡ | 2.15* | 1.96* | −0.29 | −0.14 | 1.09 | −0.64 |
| Total model | R2=0.31*** | R2=0.23*** | R2=0.18*** | R2=0.15*** | R2=0.30*** | R2=0.21*** | R2=0.15*** | R2=0.15** | R2=0.09*** | |
| Age | −1.20 | −1.49 | −0.78 | −1.68‡ | −0.54 | −1.24 | −3.01** | −2.58** | −1.34 | −0.97 |
| Gender | 2.54** | 2.80** | 0.39 | 0.15 | 2.82** | 3.03** | 1.38 | 0.74 | 0.80 | −0.53 |
| Education | 3.95*** | 3.55*** | 2.30* | 1.05 | 1.68‡ | 1.93‡ | −0.78 | −0.35 | 0.58 | −0.97 |
| Brain weight | 3.43*** | 2.10* | 3.13** | 2.26* | 3.03** | 0.75 | 3.04** | 1.71‡ | 1.89‡ | 0.71 |
| CERAD NP score | −1.11 | 0.26 | −0.12 | −0.30 | −0.55 | 0.76 | −1.56 | −0.56 | 0.14 | −0.71 |
| Braak stage for NFTs | −5.30*** | −2.60** | −2.79** | −1.84‡ | −6.87*** | −3.65*** | −0.37 | −0.11 | −2.11* | −1.59 |
| Cerebral amyloid angiopathy | −1.17 | −0.48 | 0.28 | 1.47 | −1.24 | −1.25 | 0.36 | −0.05 | 0.76 | 0.58 |
| Lewy body disease | −0.85 | 0.35 | −0.77 | −0.30 | −1.22 | −1.43 | −4.04*** | −3.33*** | −2.37* | − 2.86** |
| Gross remote cerebral infarcts | 0.71 | 1.20 | −0.52 | 0.77 | 1.16 | 0.86 | 0.68 | 0.40 | 0.27 | 0.30 |
| Cortical microvascular lesions | −2.46** | −2.28* | −2.03* | −0.25 | −2.52** | −1.60 | −1.93* | −0.57 | −1.14 | 0.00 |
| Subcortical microvascular lesions | −1.10 | −0.61 | −3.47*** | −1.93‡ | −0.56 | −1.64 | −1.06 | 0.75 | −2.22* | −1.21 |
Results are expressed as t-scores.
p≤0.001
p≤0.01
p≤0.05
p < 0.10
CASI subscales by cognitive domain and neuropathologic indices
Demographic factors alone accounted for only between 2–5% of the total variance in cognitive subdomain scores for the total group (Table 3). When all demographic and neuropathologic predictors were entered, age was associated with visuospatial construction, education with executive and memory functioning, and gender with memory. Brain weight was positively associated with performance across all cognitive domains.
When all neuropathologic and demographic variables were entered at once, total explained variance for the memory subdomain increased to 30% (F(11,342)= 13.09, p< 0.0001). Negative neuropathologic predictors of memory performance included Braak stage for NFTs (p < 0.0001) and cerebral cortical microvascular lesions (p = 0.01). When only the non-demented group was analyzed (F(11,180)= 4.42, p< 0.0001), only Braak stage for NFTs significantly predicted memory performance (p < 0.0001).
Demographic and neuropathologic variables together accounted for 18% of the executive subdomain score (F(11,351)= 6.95, p< 0.0001). Lower performance on the executive function index was predicted strongly by higher Braak stage for NFTs(p = 0.006), and subcortical microvascular lesions (p = 0.001), and was less strongly associated with cerebral cortical microvascular lesions (p = 0.04). When the nondemented group was examined alone, the total explained variance was 15% (F(11,184)= 2.96, p=0.001) As with the total group, performance on the executive function index was negatively associated with both subcortical microvascular lesions (p = 0.06, statistical trend) and Braak stage for NFTs (p = 0.07, statistical trend), although the associations were less strong than when the entire group was included in the analyses.
Total variance explained by the model accounted for 15% of the variance of the visuospatial construction index score (F(11,339)= 5.46, p< 0.0001). Poorer visuospatial construction performance was primarily predicted by presence of LBD (p < 0.0001), with a secondary association noted for cerebral cortical microvascular lesions (p = 0.05). When the demented participants were excluded from analyses (F(11,178)= 2.67, p= 0.003), LBD continued to be a significant predictor of performance on visuospatial construction performance (p = 0.001).
Only 9% of the language subdomain score variance was explained by the demographic and neuropathologic model (F(11,338)= 3.08, p< 0.001). Language performance negatively correlated with LBD (p = 0.02) and subcortical microvascular lesions (p = 0.03), and Braak stage for NFTs (p = 0.04). When the demented group was omitted from analyses, the total model remained associated with language performance (F(11,177)= 2.07, p= 0.03). Similar results were noted for LBD (p = 0.005), but not NFTs or subcortical microvascular lesions.
Because prior studies have found an association between gross or macroscopic infarcts and cognitive function [34], we ran follow up analyses to determine whether infarcts identified grossly were associated with cognitive outcomes when microvascular lesions were omitted from the multivariate analyses. Across all cognitive domains, gross infarcts were associated with performance on the executive subdomain (p = 0.009) but not with any other cognitive test performance.
APOE sample
In the sample of subjects with completed APOE genotype, demographic factors, including APOE genotype (ε4+, ε4−), accounted for 15% of the total variance in CASI score (F(4,296)= 13.01, p< 0.0001). When all neuropathologic indices were entered at once into the model, the total explained variance increased to 37% (F(12,288)= 13.82, p< 0.0001). As with the total cognitive sample when APOE was not included, Braak stage for NFTs (p < 0.0001) and cerebral microvascular lesions (p = 0.006) were associated with total CASI performance. Significant neuropathologic predictors for the cognitive subdomains were likewise unchanged as compared to the total cognitive sample. APOE genotype was not a significant predictor of cognitive performance over and above the other neuropathologic predictors.
DISCUSSION
In the present study, we examined the relationships among neuropathologic lesions and cognitive performance prior to death in the ACT study autopsy sample. We previously found that multiple lesion types were associated with clinical dementia diagnosis [13]; in the current study, we focused on associations between neuropathologic changes and specific cognitive functions in subjects with or without dementia. Although Braak stage for NFTs was a significant predictor of global cognitive function, our results also indicated that non-AD lesions contributed. Specifically, we found that type of neuropathologic lesion differentially predicts performance across and within specific cognitive domains.
Recent efforts examining the relationship between neuropathologic changes and cognitive function have been undertaken, most notably in the combined analyses of the Religious Orders Study and Memory and Aging Project [34]. Similar to the current study, these investigators found that AD neuropathologic changes were related to memory in nondemented subjects; however, several differences exist between our findings. For example, we found that microvascular lesions were related to cognitive function independently from gross infarcts. This is consistent with findings reported by the Honolulu Asia Aging Study (which also utilizes the CASI to measure cognition) [35, 36], and underscores the importance of “silent” μVBI on everyday functioning.
Further, our study differs from others in that we separated VBI according to whether the lesions were cerebral cortical or subcortical. While total CASI score and memory performance were predicted by cerebral cortical, but not subcortical, microvascular lesions, performance on executive function tasks was associated with both cerebral cortical and subcortical microvascular lesions. We speculate that while subcortical VBI may not be associated with global cognitive functioning per se, such lesions may nonetheless result in subtle yet potentially meaningful changes in executive function among older individuals.
Visuospatial ability was primarily associated with LBD in this sample, consistent with the observation that visuospatial impairment is often one of the earliest cognitive symptoms in Parkinson’s disease [37,38] and is a primary feature of LBD [39]. A recent community-based autopsy study also found an association between visuospatial task performance and LBD [40]; results from the current study extend this research by demonstrating that this association exists even when analysis is restricted to subjects without dementia. Our unique finding that LBD was associated with a simple visuo-construction task even in nondemented individuals underscores the importance of identifying early visuospatial impairments in older adults. Although the neuropathologic substrate of impaired visuospatial abilities in LBD-related conditions is not well understood, it is hypothesized to result from pathophysiologic processes that promote Lewy body accumulation in posterior cortical regions, asymmetric dopamine loss in the right basal ganglia, disruptions in the fronto-striatal loop, or impairments in the dorsolateral prefrontal pathways [41–45]. Unlike other cognitive domains, visuospatial abilities were not specifically related to AD lesions over and above other neuropathologic changes. This is perhaps not surprising, given the general relative sparing of pure visuospatial functions in early AD [46, 47], and suggests that impairment on visuospatial function alone may be a specific indicator for non-AD related pathology.
The total variance for the language subdomain that was explained by the neuropathologic and demographic model was less than that of the other variables. Language functions are quite variable and can be disrupted by multiple pathological processes. For example, semantic processing is commonly disrupted in AD patients, while subcortical μVBI is associated with impairments in speech production, language processing, and naming [48–50]. LBD also is associated with disrupted language, but such impairments differ qualitatively from AD and may be influenced by reduced fluency, impaired sentence processing, and slowed speech [51, 52]. Unlike the other cognitive subdomains, language was not associated with cortical μVBI. Given that many language-based cognitive processes are primarily cortical in nature, this finding is somewhat surprising. However, subcortical μVBI is associated with impairments in speech production, language processing, and naming [48–50], consistent with our finding that subcortical microvascular lesions may be associated with reduced performance on the language composite.
A potential limitation of our study relates to the variability in time between cognitive testing and death, and the possibility that those who were non-demented at the time of their final visit could potentially have developed dementia during the months prior to death. In addition, we did not have information concerning the presence of mild cognitive impairment in this sample, so the nondemented group likely includes a substantial proportion of participants who had cognitive impairment but lacked sufficient functional impairment to meet criteria for a diagnosis of dementia. This is a particularly important point given the amount of AD neuropathologic changes present in the clinically non-demented group; prior research has suggested that subjects with no cognitive impairment have relatively little AD neuropathologic change [53]. Due to these limitations, we chose to analyze cognitive performance in relation to severity of neuropathologic changes but without regard to clinical diagnosis at the final visit. This approach, which has been used by others [40], provides increased statistical power and is consistent with the recent conceptual shift that distinguishes AD neuropathologic changes from clinical dementia diagnosis, in large part as a recognition of latent and prodromal stages of disease. To verify that the results were not solely related to clinical dementia diagnosis, we did secondary analyses of both global and subdomain cognitive performances in the clinically non-demented group only; the results were generally similar to findings from the combined sample.
A further limitation of the study involves the use of a single global cognitive test score and derived composites indexing different cognitive domains. Given that the CASI is designed as an enhanced screening instrument, the cognitive domain subscales produced a limited range of scores as well as ceiling effects. Unfortunately, because ceiling effects are present across all the subscales, our ability to assess the relationship between neuropathologic indices and cognition for those performing in the higher ranges was limited. Particularly given that this is a relatively highly educated sample, more sensitive neuropsychological measures of individual cognitive domains would have been preferable. However, our results with these less sensitive indices of cognition support the use of additional investigation of the relationship between specific cognitive domains and neuropathologic lesions in community-based samples. In addition to cognitive measures, the neuropathologic measures used may also have impacted our results. Our demographic and neuropathologic model accounted for 31% of the variance in total CASI score, and for varying lesser degrees on the cognitive subscales. Use of more quantitative measures of brain injury or disease burden may have increased the correlations with cognitive test scores. For example, only 65% of our sample had hippocampal sclerosis measured; of those, 80% were free from lesions. Thus, we did not have sufficient statistical power to detect the impact of these potentially important lesions. However, cognitive test performance may be affected by numerous environmental, genetic, and other biologic factors; thus it is likely that unmeasured (e.g., mood states, fatigue) or unknown factors also contributed to the variance associated with cognitive test performance. Finally, a limitation of this study, as well as most autopsy studies, is the relatively small proportion of the total sample that consents to and undergoes autopsy at death. Thus, we are constrained in our ability to generalize from these results to the population at large.
This study links cognitive subscales contained within a brief global screening measure to a variety of underlying neuropathologic indices in a non-clinically defined community sample. Although global functioning itself is related to certain (primarily AD) neuropathologic changes, directing attention to performance across specific cognitive domains permits more in-depth analysis of the potential impact of other, non-AD lesions, on cognition. In sum, our findings indicate that the type and quantity of neuropathologic lesions may be differentially related to cognitive domains affected by disease thus potentially highlighting different pathways to cognitive decline in older individuals.
Figure 2.
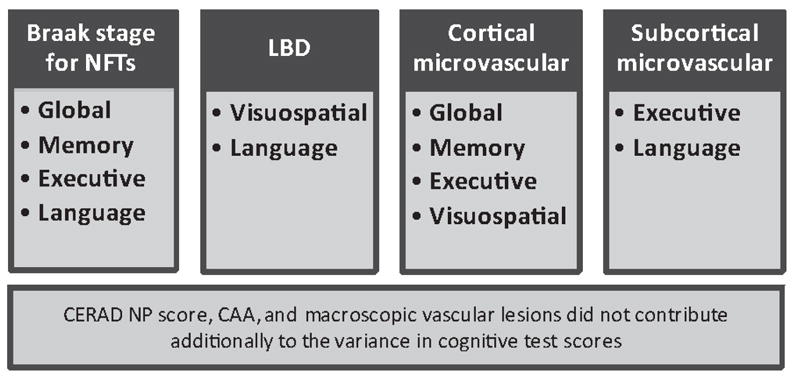
Neuropathologic variables that contribute to cognitive domains in the ACT study sample.
Acknowledgments
This research was supported by NS062684, AG006781, AG024180, the Group Health Research Institute, the Nancy and Buster Alvord Endowment, and the Department of Veterans Affairs. The funding sources did not provide scientific input for the study.
References
- 1.Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, O’Brien JT, Kalaria RN. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133:2225–2231. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massoud F, Devi G, Stern Y, Lawton A, Goldman JE, Liu Y, Chin SS, Mayeux R. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Arch Neurol. 1999;56:1368–1373. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 6.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duy-ckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79:915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 9.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewers M, Insel P, Jagust WJ, Shaw L, Trojanowski JJ, Aisen P, Petersen RC, Schuff N, Weiner MW. CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cereb Cortex. 2012;22:1993–2004. doi: 10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popp J, Lewczuk P, Frommann I, Kolsch H, Kornhuber J, Maier W, Jessen F. Cerebrospinal fluid markers for Alzheimer’s disease over the lifespan: Effects of age and the APOE epsilon4 genotype. J Alzheimers Dis. 2010;22:459–468. doi: 10.3233/JAD-2010-100561. [DOI] [PubMed] [Google Scholar]
- 12.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. AnnNeurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 14.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 15.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, Peskind ER, Raskind MA, Breitner JC, Montine TJ. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological stageing ofAlzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 21.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 22.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 23.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT National Institute on Aging, Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. Mini Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chiu HC, Schneider LS, Metzger LE. Alzheimer’s dementia: Performance on the Mini Mental State Examination. J Consult Clin Psychol. 1987;55:96–100. doi: 10.1037//0022-006x.55.1.96. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa K. The clinical assessment of dementia in the aged: A dementia screening scale for psychogeriatric patients. In: Bergener M, Lehr U, Lang E, editors. Aging in the Eighties and Beyond. Springer; New York: 1983. pp. 207–218. [Google Scholar]
- 29.Liu HC, Teng EL, Lin KN, Chuang YY, Wang PN, Fuh JL, Liu CY. Performance on the cognitive abilities screening instrument at different stages of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;13:244–248. doi: 10.1159/000057703. [DOI] [PubMed] [Google Scholar]
- 30.Graves AB, Mortimer JA, Larson EB, Wenzlow A, Bowen JD, McCormick WC. Head circumference as a measure of cognitive reserve. Association with severity of impairment in Alzheimer’s disease. Br J Psychiatry. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- 31.Crane PK, Narasimhalu K, Gibbons LE, Mungas DM, Haneuse S, Larson EB, Kuller L, Hall K, van Belle G. Item response theory facilitated cocalibrating cognitive testsand reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018–1027. e1019. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LY, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, Montine TJ, Li G. Blood pressure and brain injury in older adults: Findings from a community-based autopsy study. J Am Geriatr Soc. 2009;57:1975–1981. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72:599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: The Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70:774–780. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, Nelson J, Hardman J, Masaki K, Vogt MR, Launer L, White LR. AD lesions and infarcts in demented and non-demented Japanese-American men. AnnNeurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 37.Song IU, Kim JS, Yoo JY, Song HJ, Lee KS. Cognitive dysfunctions in mild Parkinson’s disease dementia: Comparison with patients having mild Alzheimer’s disease and normal controls. Eur Neurol. 2008;59:49–54. doi: 10.1159/000109261. [DOI] [PubMed] [Google Scholar]
- 38.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson’s disease: Subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16:177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, CarlosMachado J, O’Brien J, Playfer J, Reid W. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 40.Dowling NM, Tomaszewski Farias S, Reed BR, Sonnen JA, Strauss ME, Schneider JA, Bennett DA, Mungas D. Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. J Int Neuropsychol Soc. 2010:1–13. doi: 10.1017/S1355617710001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schendan HE, Amick MM, Cronin-Golomb A. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: Evidence from Parkinson’s disease. BehavNeurosci. 2009;123:125–136. doi: 10.1037/a0013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cronin-Golomb A, Braun AE. Visuospatial dysfunction and problem solving in Parkinson’s disease. Neuropsychology. 1997;11:44–52. doi: 10.1037//0894-4105.11.1.44. [DOI] [PubMed] [Google Scholar]
- 43.Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O’Brien JT. Regional cerebral blood flow in Parkinson’s disease with and without dementia. Neuroimage. 2003;20:1309–1319. doi: 10.1016/S1053-8119(03)00364-1. [DOI] [PubMed] [Google Scholar]
- 44.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: Mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44:339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. MovDisord. 2009;24:1193–1199. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- 46.Carter SF, Caine D, Burns A, Herholz K, Lambon Ralph MA. Staging of the cognitive decline in Alzheimer’s disease: Insights from a detailed neuropsychological investigation of mild cognitive impairment and mild Alzheimer’s disease. Int J Geriatr Psychiatry. 2012;27:423–432. doi: 10.1002/gps.2738. [DOI] [PubMed] [Google Scholar]
- 47.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomic G, Stojanovic M, Pavlovic A, Stankovic P, Zidverc-Trajkovic J, Pavlovic D, Markovic-Jovanovic Z, Covickovic-Sternic N. Speech and language disorders secondary to diffuse subcortical vascular lesions: Neurolinguistic and acoustic analysis. A case report. J Neurol Sci. 2009;283:163–169. doi: 10.1016/j.jns.2009.02.361. [DOI] [PubMed] [Google Scholar]
- 49.Gainotti G, Ferraccioli M, Vita MG, Marra C. Patternsof neuropsychological impairment in MCI patients with small subcortical infarcts or hippocampal atrophy. J Int Neuropsychol Soc. 2008;14:611–619. doi: 10.1017/S1355617708080831. [DOI] [PubMed] [Google Scholar]
- 50.Nadeau SE, Crosson B. Subcortical aphasia. BrainLang. 1997;58:355–402. doi: 10.1006/brln.1997.1707. discussion 418–323. [DOI] [PubMed] [Google Scholar]
- 51.Gross RG, McMillan CT, Chandrasekaran K, Dreyfuss M, Ash S, Avants B, Cook P, Moore P, Libon DJ, Siderowf A, Grossman M. Sentence processing in Lewy body spectrum disorder: The role of working memory. Brain Cogn. 2012;78:85–93. doi: 10.1016/j.bandc.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ash S, McMillan C, Gross RG, Cook P, Gunawardena D, Morgan B, Boller A, Siderowf A, Grossman M. Impairments of speech fluency in Lewy body spectrum disorder. Brain Lang. 2012;120:290–302. doi: 10.1016/j.bandl.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]