Abstract
Objective
To evaluate subclinical macular findings in premature patients at risk of retinopathy of prematurity (ROP) with the use of handheld spectral domain-optical coherence tomography (SD-OCT).
Design
Prospective, observational case series.
Participants
Forty-nine prematurely born neonates.
Methods
Forty-nine infants were imaged using a handheld SD-OCT. Images were acquired in non-sedated infants in the neonatal intensive care unit. Some patients were followed and re-imaged over the course of several weeks. Two hundred ninety-eight total images were acquired, and evaluated for cystoid macular edema (CME) and persistence of inner retinal layers.
Main Outcome Measures
In vivo determination of foveal retinal lamination, image analysis and clinical observation.
Results
Two hundred forty (81%) of the images from 45 patients were usable (defined as having scans passing through the fovea with clearly identifiable retinal layers). Persistence of one or more inner retinal layers was seen in 42 patients (93%). Patients with at least one persistent layer, 16, 5, 7, 13 and 1 had a maximum ROP stage of 0, 1, 2, 3, and 4A respectively. CME was seen in 25 of the 45 patients (56%) during one or more imaging sessions. CME was present in 9, 1, 5, 9, and 1 patient with maximum ROP stage of 0, 1, 2, 3, and 4A respectively.
Conclusions
Our data suggests there is persistence of inner retinal layers in premature infants, regardless of maximal ROP stage. Subclinical CME is seen in premature infants; however, CME does not appear to be correlated with ROP stage. This suggests that there maybe other etiologies for the CME seen in this patient population. Hand-held SD-OCT imaging is a viable technique for evaluating subclinical macular findings in premature infants, though larger datasets are needed from multiple centers to further evaluate the generalizability of these findings.
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative retinal disorder seen in infants who are premature and have low birth weight. Patients with a history of ROP can have variable visual acuity despite a normal appearing macula on clinical exam.1 It is unclear to what degree prematurity of the visual system, other systemic factors and/ or structural changes in the macula itself account for these differences. Optical coherence tomography (OCT) has proven a useful modality to assess anatomic changes in the macula, which may help explain these outcomes. For example, studies have shown that patients with a history of ROP have many foveal anomalies, including abnormal foveal contour, preservation of multiple inner retinal layers within the fovea, and increased central retinal thickness but these have not always been correlated with decreased vision.2–6
OCT is widely used in adults to help diagnose and manage various retinal diseases. The use of OCT is limited in young children and infants due to issues with positioning and fixation. Handheld OCT has made it possible to image infants with and without sedation in a noninvasive and noncontact manner. 7–14 This advancement has facilitated the imaging of premature infants with acute ROP. Previous reports using this technology have shown the presence of cystoid macular edema (CME) in patients with ROP.7–10,13 OCT images of patients with advanced ROP have shown retinoschisis, preretinal structures, and retinal detachments not seen on clinical exam.11,15 Chavala et al.11 described a patient who appeared to have stage 4A disease based on indirect ophthalmoscopy but was found to have stage 4B disease on SD-OCT imaging. These findings have important implications for management and treatment of these patients. Nevertheless the use of OCT in this patient population is not widespread, so the generalization of previous findings to different pediatric populations remains unclear. Here we sought to examine a series of patients undergoing ROP screenings using OCT imaging and compared our findings with those previously reported.
Methods
This study was approved by the Institutional Review Boards at the Medical College of Wisconsin and Children’s Hospital of Wisconsin, conformed to the requirements of the United States Health Insurance Portability and Privacy Act, and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the parent or legal guardian of all subjects. Forty-nine infants in the neonatal intensive care unit at Children’s Hospital of Wisconsin were enrolled. Children’s Hospital of Wisconsin is a tertiary unit with both inborn infants and infants transferred from multiple outside hospitals. All parents of infants undergoing ROP screening were asked to participate in imaging and those whose parents consented were enrolled. There were patients whose parents did not agree to participate until after initial screening exams were performed. There were 27 males and 18 females from age 30 weeks postmenstrual age (PMA = gestational age + chronological age in weeks) to 57 6/7 weeks PMA. Gestational age was determined by the neonatologist based on obstetric records when available, or best estimate at birth for those without prenatal records. We collected birth weight, gestational age at birth, age at each exam and imaging session, race, and gender. We also reviewed patient charts to record major medical problems that could possibly be associated with CME, such as heart defects, intraventricular hemorrhage, sepsis, and necrotizing enterocolitis.
Eyes were dilated using Cyclomydril (Alcon, Inc., Fort Worth, TX, USA). Clinical examinations were performed by a pediatric ophthalmologist (DMC), who documented ROP zone (1 to 3) ROP stage (0 representing avascular retina to 4), presence of plus disease according to the International classification of Retinopathy of Prematurity (ICROP), and presence and severity of CME at any time. All cases of stage 3 or 4 with plus disease were treated with either bevacizumab or laser. Postoperatively eyes treated with laser were given tobramycin 0.3% / dexamethasone 0.1% ophthalmic ointment four times daily and cyclopentalate hydrochloride 0.2% / phenylephrine hydrochloride 1% one drop each eye three times daily for 14 days. Eyes injected with bevacizumab were given moxifloxacin hydrochloride 0.5% four times daily for one week.
OCT images of the macular region were acquired using the Bioptigen Hand Held Probe SD-OCT (HHP-SDOCT) (Bioptigen, Research Triangle Park, NC, USA). SD-OCT imaging parameters provided by Maldonado et al.16 were used to optimize image quality. The scan size was 8×8mm and scan density was 1000 A scans/100 B scans. Images were acquired between April 2010 and June 2012. Imaging was always performed after the clinical exam. After instilling proparacaine for topical anesthesia, a lid speculum was used to keep the eye open during imaging. Artificial tears were used to lubricate the cornea (Saline drops or Systane Ultra; Alcon, Inc., Fort Worth, TX, USA). The subjects were not sedated during imaging and a pacifier dipped in “sweet-ease” sucrose solution was used to help calm the infants.
Individual B-scans in which the fovea was visible were manually selected from each macular volume scan, with a total of 298 images obtained. Images were included in the study only if the entire foveal region was visible, and image quality was adequate to assess presence and severity of CME and inner retinal layers (determined by consensus of 4 graders). Severity of CME was graded on a scale of 0 for no CME, 1 for a single cyst at the fovea, 2 for a few parafoveal cysts, 3 for multiple cysts at the fovea with preservation of the foveal pit, and 4 for multiple cysts at the fovea with loss of foveal contour. The number of imaging sessions per subject ranged from one to eight. Longitudinal exams were done after consent was obtained in conjunction with follow up ROP exams. Longitudinal exams were not obtained for patients who were transferred at a late gestational age and discharged prior to a follow up exam or when consent was obtained at a late gestational age and the patient was subsequently discharged or the retinal was fully vascularized and no further screening for ROP was indicated. In all but two cases follow up ended when patients were transferred to another hospital or discharged. In these two subjects, images were acquired during follow up examination.
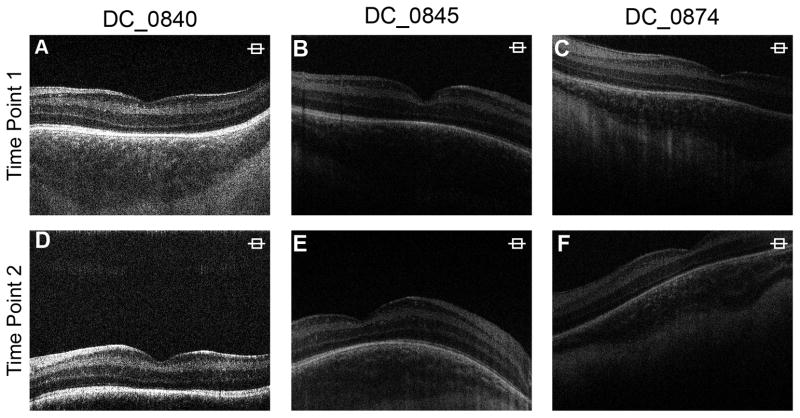
To ensure accurate tracking of development and disease progression, repeatability was established despite challenges in the neonatal population, such as the subjects’ inability to fixate and poor cooperation. We were able to image the same retinal area in our subjects serially throughout the follow-up period (Figure 1).
Figure 1.
Repeatability of imaging in infants. Images from two different time points in patient DC_0840 (A–D), DC_0845 (B–E), and DC_0874 (C–F) demonstrate the ability to image the same retinal area during follow up. Retinal and choroidal vasculatures serve as landmarks to determine corresponding retinal locations.
Results
There were 240 (81%) usable scans obtained from 45 of the 49 subjects. Images were evaluated for presence of inner retinal layers at the foveal center. Only three subjects showed complete excavation of the fovea with no residual inner retinal layers presents. Subject specific data for residual inner retinal layers can be found in Table 1 (available at http://aaojournal.org). Persistence of one or more retinal layers was present in forty-two infants (93%). In three subjects only the outer plexiform layer was present. In 26 subjects both the outer and inner plexiform layers were present. Both plexiform layers and the ganglion cell layer remained in 13 subjects. (Figure 2). There were 16 infants with stage 0 ROP, 5 infants with Stage 1 ROP, 7 infants with stage 2 ROP, 13 infants with stage 3 ROP, and 1 infant with stage 4A ROP infant with at least one persistent layer. Thus, persistence of inner retinal layers occurs in all stages of ROP. This finding was present on the initial exam and did not seem to change over longitudinal scans.
Figure 2.
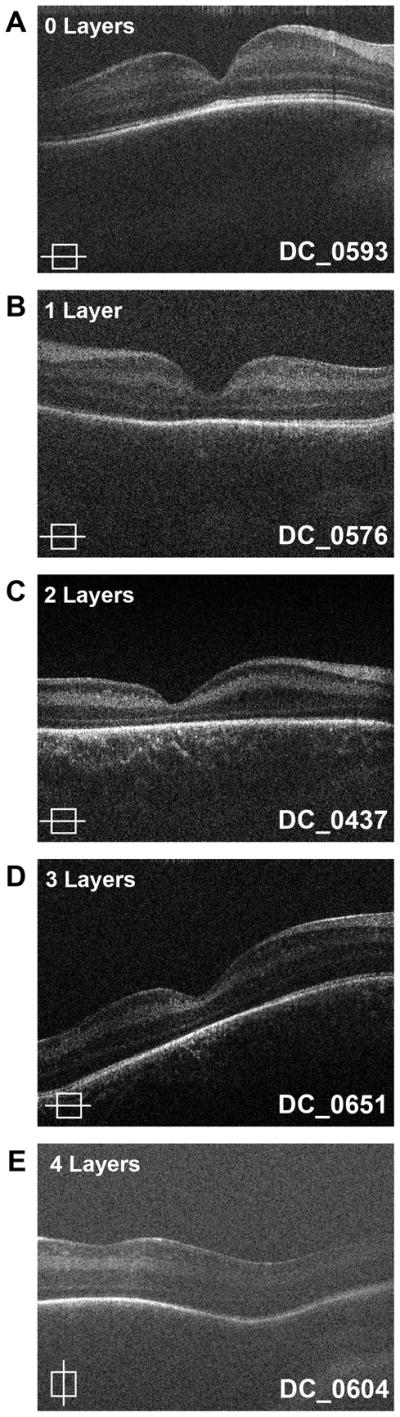
Persistence of inner retinal layers at the foveal center. Some individuals exhibited complete excavation with no residual retinal layers present (A). Three patients had only the inner plexiform layer (1 layer) present (inner nuclear and ganglion cell and nerve fiber layers are absent) (B), while other subjects also had the inner plexiform and nuclear layer (2 layers) (C). Less developed retinas had residual ganglion cell, inner nuclear and inner plexiform layers (3 layers) (D). One subject had residual nerve fiber layer (additionally ganglion cell, inner nuclear and plexiform layers) over the macula (E).
Cystoid Macular Edema
Of the 45 subjects deemed to have usable image, twenty-five (56%) had CME during one or more imaging sessions. CME developed at different times over longitudinal scans but was bilateral in all cases. There was a wide variation in the severity of CME (Figure 3). The most severe CME in a particular patient was graded. While twenty patients did not have CME (grade 0), four had maximum CME grade 1 (16%), ten had maximum CME grade 2 (40%), five had maximum CME grade 3 (20%), and six had maximum CME grade 4 (24%). Only one patient had CME that was severe enough to be detected clinically.
Figure 3.
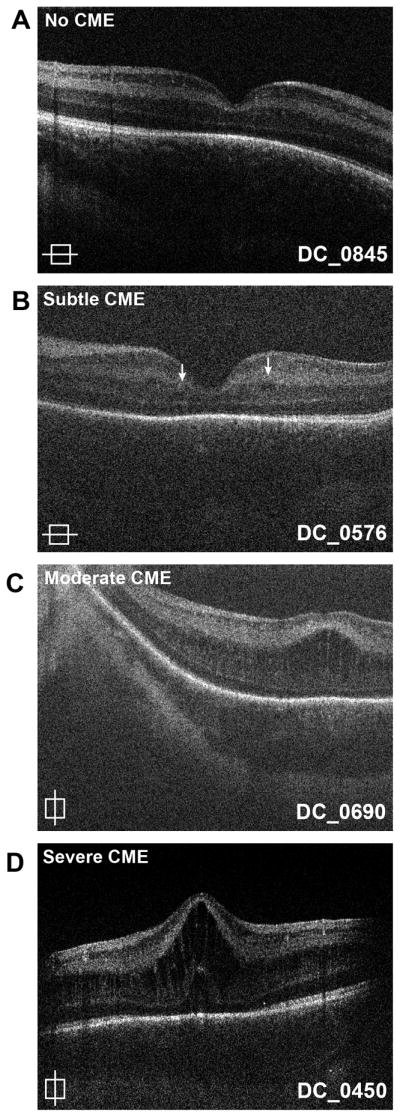
Variability in cystoid macular edema (CME). Shown here are examples of optical coherence tomography (OCT) images with no CME (A), one single cyst at the fovea (B), a few parafoveal cysts (C), multiple cysts at the fovea with preserved foveal pit (D), and multiple cysts at the fovea with loss of foveal depression (E).
CME was seen in 72% of females and 44% of males, this difference was not statistically significant (p=0.077, Fisher’s exact test, with Bonferroni correction). The group with CME at any exam and the group without CME had similar birth weights (p=0.1163, Fisher’s exact test, with Bonferroni correction). The CME group was younger at birth compared to the group without CME (p=0.0167, Fisher’s exact test, with Bonferroni correction). CME was equally present across all races (p=0.82, Kruskal-Wallis test).
CME was seen in 9 (50%) of subjects with maximum ROP stage 0, 1 (20%) subject with stage 1 ROP, 5 (71%) subjects with stage 2 ROP and 9 (64%) subjects with stage 3 ROP. Similar to presence of inner retinal layers, there was not an association between most severe ROP stage and presence of CME (p=0.31, Chi-squared test). Of the patients with plus disease, 7 (78%) had CME. However, the majority of patients with CME did not have plus disease. In six subjects the age of CME onset could not be determined, as it was observed in the first exam. In the remaining subjects the average age on CME onset was 36.08 ± 2.29 weeks (average ± standard deviation. range 33.14–41.43 weeks) (Table 1, available at http://aaojournal.org). There were eight subjects who required laser treatment during follow up, and four of these went on to develop CME after laser was performed. Three of four of these were imaged prior to laser treatment during which time CME was not present. In these cases CME was noted between 1–2 weeks post laser. In the fourth case an image prior to laser treatment was not obtained.
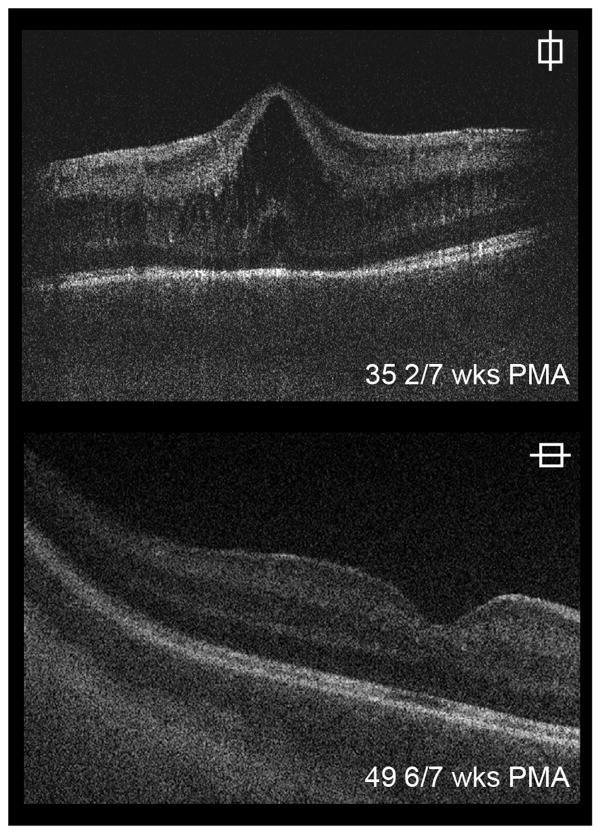
Four infants were treated for ROP with bevacizumab. Three patients had CME prior to receiving bevacizumab, which did not resolve by last imaging (Table 1, available at http://aaojournal.org). CME developed after bevacizumab injection in one subject. The patient received bevacizumab at 33 6/7 weeks PMA and CME was first seen on imaging at 34 6/7 weeks PMA (Figure 4). The time between treatment and onset of CME was similar for laser and bevacizumab.
Figure 4.
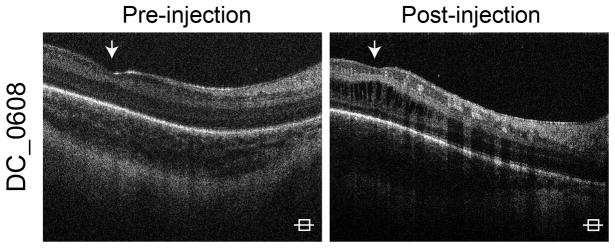
Cystoid macular edema (CME) in patients treated with bevacizumab. Shown here is imaging from a patient who had no CME on imaging prior to receiving bevacizumab (A). The patient received bevacizumab at 33 6/7 weeks postmenstrual age (PMA) and then developed CME by 34 6/7 weeks PMA (B).
Resolution of CME was seen in only 3 patients. In the first subject CME was first detected at 35 2/7 weeks PMA and it resolved by 52 2/7 weeks PMA (Figure 5). CME was observed and resolved in two other subjects (Table 1, available at http://aaojournal.org).
Figure 5.
Resolution of cystoid macular edema (CME) Three patients with CME had resolution of edema during the follow up period. Shown here is imaging from the right eye of patient DC_0450. CME was present on imaging at 35 2/7 weeks (wks) postmenstrual age (PMA) (A) but had resolved by 49 6/7weeks PMA (B).
Of the subjects with intraventricular hemorrhage, 9 (75%) developed CME. Eleven (85%) of the patients with necrotizing enterocoloitis had CME. Six (75%) of the patients with sepsis developed CME. Twenty-six patients had a heart defect, such as patent ductus arteriosus, patent foramen ovale, or tetralogy of fallot. Eighteen (69%) of these patients were in the CME group. Due to the small sample size, it was not possible to evaluate for a statistically significant association between these factors and CME.
Discussion
Foveal development involves the centrifugal displacement of inner retinal cells and centripetal displacement of photoreceptors.17 Previous studies have shown that there is persistence of inner retinal layers in children and adults with a history of ROP.5–6 In these patients, the presence of inner retinal layers does not appear to affect visual acuity.5 Studies including ours, have also shown persistence of inner retinal layers in infants undergoing screening for acute ROP.10 Our data adds that the persistence of inner retinal layers is not associated with ROP severity.
Consistent with previous reports,7–10,13 we confirmed that subclinical CME is seen in a significant number of premature infants.
Similar to our study, Maldonado et al.7 found the presence of CME was not associated with the stage of ROP or presence of plus disease however, Maldonado et al concluded that severity of CME is associated with higher ROP stage. As in our study, the majority of patients with CME had multiple cysts present but Maldonado had a higher percentage of patients with a bulging fovea (equivalent to grade 4 in our study) (62%), while only 24% of our patients with CME had a maximum severity of grade 4. Vinekar et al.8 concluded that CME was related to ROP stage. However, the weighted birth weight mean was 1255g and the weighted gestational age mean was 30.4 weeks PMA, while the mean birth weight in our study was 917g and in Maldonado et al. study was 825g and the mean gestational age was 27.4 weeks PMA in our study and 26 weeks PMA in Maldonado et al. study. The lower incidence of CME seen in the study by Vinekar et al.8 may be related to the larger, more mature patient population. A comparison of similar studies can been seen in Table 2.
With the exception of Vinekar’s data, CME does not appear to be correlated with ROP stage. This suggests that there may be other etiologies for the CME seen in this patient population. Other studies have hypothesized that the CME may be due to neurohumoral factors such as elevated VEGF levels, or mechanical traction on the macula. 7–10 However one patient that received bevacizumab developed CME one week after the anti-VEGF treatment was given. Since recurrence of ROP after anti-VEGF treatment is 16+/− 4.6 weeks, VEGF levels may not directly coincide with the development of CME.18 Direct correlation with VEGF levels is also not supported by the lack of association of the stage of ROP and presence of CME. This does not necessarily exclude a role of VEGF in the development of CME. VEGF may be involved in triggering a cascade of effects leading to the development of CME a few or several weeks before the actual development of CME. If treatment with anti-VEFG medications is given after such a cascade is initiated, treatment may not effectively prevent the development of CME.
We believe it is possible that concurrent systemic diseases may account for CME in some patients, although our study size was too small to address this effectively. Systemic factors such as hyperoxia, hypoxia, hypotension, acidosis, sepsis, patent ductus arteriosus, blood transfusions, intraventricular hemorrhage, and apnea are associated with ROP.19–20 Maldonado et al.13 described a term infant with neonatal hemochromatosis who had bilateral CME on SD-OCT which resolved after liver transplantation. More recently, Maldonado et al.7 tried to correlate certain systemic factors (Apgar score at 1 and 5 minutes, surgery for patent ductus arteriosus, culture-proven sepsis, surgery for necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia, and hydrocephalus) with CME and did not find an association.
It is unclear whether CME is related to laser in our patients. Certainly there is precedence for CME after panretinal photocoagulation (PRP) in adults. McDonald and Schatz21 showed that 43% of patient’s treated with PRP for proliferative diabetic retinopathy developed increased macular edema seen on fluorescein angiography 6–10 weeks after laser treatment. In 63% of these eyes, the increase in macular edema did not resolve to preoperative levels by the time of the last follow-up (followed an average of 15mo). More than 50% of the eyes had at least mild edema before surgery. While PRP of vascularized retina in diabetic adults does not equate to laser treatment of avascular retina in infants, this is some evidence that laser may predispose to development of CME. However, of the eight patients in our study who underwent laser four did not develop CME. Of note, four of our subjects had CME on imaging after receiving laser, however only three subjects had imaging prior to the procedure showing that CME was not yet present. Given that four of our infants did not develop CME despite needing laser it is not clear if laser could directly cause CME in this patient population. A larger sample size is needed to further evaluate any possible association.
Primary bevacizumab has been shown to be effective to treat macular edema in adults with retinal vascular disease, such as central retinal vein occlusion23 and diabetes.24 In our small sample size bevacizumab did not prevent the development of CME in 4 patients treated for aggressive posterior type-1 ROP. Interestingly, three patients had CME prior to injection, and the other developed CME after the injection. The CME did not resolve by the last imaging session in any of these patients. A larger sample size and longer follow up is needed to determine if bevacizumab affects the time to resolution of CME. It is also important to note that further studies must be done to determine the long-term safety of the use of bevacizumab in this population.
The natural history of CME resolution is unclear. CME resolved in 9 of 17 patients imaged after 37 weeks PMA in Maldonado’s study. We only had 3 cases resolve at 39 5/7 and 52 2/7 weeks PMA but expect more would resolve if followed for a longer period of time. Vinekar et al.8 documented resolution of CME in all patients by imaging at 52 weeks PMA, although 2 of the 10 patients with CME did not undergo repeat imaging at this time point. Further investigation of duration and clinical implications of CME need to be evaluated.
There are several limitations of our study. First, the study was conducted in a tertiary care center including inborn and transferred infants form outside NICUs which may not be applicable to other NICU environments. Second, the majority of subjects were male and Caucasian. In addition, none of the 45 infants analyzed in this study developed ROP more advanced than stage 4A. Also, imaging could only be performed at time of ROP screening examinations, so it was not possible to precisely determine when CME first appeared and when it resolved. There may also be a selection bias introduced in that we were not able to image all patients. Parents whose child developed more advanced ROP were more likely to consent to imaging at some point during the child’s hospital stay. Other infants transferred to the hospital for systemic surgery may be sicker than those in an outlying NICU and those transferred for treatment of ROP results in a higher percentage of stage 3 ROP infants.
In conclusion, hand-held SD-OCT imaging is a viable technique for evaluating subclinical macular findings in premature infants. Larger datasets are needed from multiple centers to develop a better understanding of what is normal development what represents subclinical pathology and how the development of CME affects vision. As these questions are answered the information may be used to guide treatment decisions in this patient population.
Supplementary Material
Table 1.
CME Reported with ROP
| Subclinical CME | ROP Stages | |
|---|---|---|
| This Study | 56% (25/45) | 0–4 |
| Lee et al.9 | 61% (23/38) | 0–4 |
| Maldonado et al.10 | 58% (18/31) | 0–2 |
| Maldonado et al.7 | 50% (21/42) | 0–3 |
| Vinekar et al. 8 | 16% (23/146) | 0–2* |
CME only seen in Stage 2 ROP
[CME – cystoid macular edema, ROP – retinopathy of prematurity]
Acknowledgments
Support: This study was supported by NIH Grants P30EY001931, T32EY014537, R01EY017607, The E. Matilda Ziegler Foundation for the Blind, RD and Linda Peters Foundation, and an unrestricted departmental grant from Research to Prevent Blindness. Part of this investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR016511 from the National Center for Research Resources, National Institutes of Health. Pooja Godara is supported by a research award from the VitreoRetinal Surgery Foundation.
Footnotes
Financial Disclosures: Adam M. Dubis, None; C. Devika Subramaniam, None; Pooja Godara, None; Joseph Carroll, None; Deborah Costakos, None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Visual acuity at 10 years in Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study eyes: effect of retinal residua of retinopathy of prematurity. Arch Ophthalmol. 2006;124:199–202. doi: 10.1001/archopht.124.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Akerblom H, Larsson E, Eriksson U, Holmström G. Central macular thickness is correlated with gestational age at birth in prematurely born children. Br J Ophthalmol. 2011;95:799–803. doi: 10.1136/bjo.2010.184747. [DOI] [PubMed] [Google Scholar]
- 3.Joshi MM, Trese MT, Capone A., Jr Optical coherence tomography findings in stage 4A retinopathy of prematurity: a theory for visual variability. Ophthalmology. 2006;113:657–60. doi: 10.1016/j.ophtha.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Ecsedy M, Szamosi A, Karko C, et al. A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci. 2007;48:5207–11. doi: 10.1167/iovs.06-1199. [DOI] [PubMed] [Google Scholar]
- 5.Recchia FM, Recchia CC. Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina. 2007;27:1221–6. doi: 10.1097/IAE.0b013e318068de2e. [DOI] [PubMed] [Google Scholar]
- 6.Hammer DX, Iftimia NV, Ferguson RD, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008;49:2061–70. doi: 10.1167/iovs.07-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012;130:569–78. doi: 10.1001/archopthalmol.2011.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5183–8. doi: 10.1167/iovs.10-7155. [DOI] [PubMed] [Google Scholar]
- 9.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31:1470–82. doi: 10.1097/IAE.0b013e31821dfa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118:2315–25. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–56. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott AW, Farsiu S, Enyedi LB, et al. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147:364–73. doi: 10.1016/j.ajo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado RS, Freedman SF, Cotten CM, et al. Reversible retinal edema in an infant with neonatal hemochromatosis and liver failure. J AAPOS. 2011;15:91–3. doi: 10.1016/j.jaapos.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koozekanani DD, Weinberg DV, Dubis AM, et al. Hemorrhagic retinoschisis in shaken baby syndrome imaged with spectral domain optical coherence tomography [report online] [Accessed December 14, 2012];Ophthalmic Surg Lasers Imaging. 2010 doi: 10.3928/15428877-20100215-87. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3182288/ [DOI] [PMC free article] [PubMed]
- 15.Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010;128:57–62. doi: 10.1001/archophthalmol.2009.361. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants and children. Invest Ophthalmol Vis Sci. 2010;51:2678–85. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54:549–81. doi: 10.1016/s0301-0082(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 18.Mintz-Hittner HA, Kennedy KA, Chuang KA BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla D, Agarwal R, Deorari AK, Paul VK. Retinopathy of prematurity. Indian J Pediatr. 2008;75:73–6. doi: 10.1007/s12098-008-0011-z. [DOI] [PubMed] [Google Scholar]
- 20.Rivera JC, Sapieha P, Joyal J, et al. Understanding retinopathy of prematurity: update on pathogenesis. Neonatology. 2011;100:343–53. doi: 10.1159/000330174. [DOI] [PubMed] [Google Scholar]
- 21.McDonald RH, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985;5:5–10. doi: 10.1097/00006982-198500510-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lee SB, Yun YJ, Kim SH, Kim JY. Changes in macular thickness after panretinal photocoagulation in patients with severe diabetic retinopathy and no macular edema. Retina. 2010;30:756–60. doi: 10.1097/IAE.0b013e3181c701e0. [DOI] [PubMed] [Google Scholar]
- 23.Epstein DL, Algvere PV, von Wendt G, et al. Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology. 2012;119:1184–9. doi: 10.1016/j.ophtha.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Arevalo JF, Sanchez JG, Wu L, et al. Pan-American Collaborative Retina Study Group (PACORES) Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488–97. doi: 10.1016/j.ophtha.2009.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.