Abstract
In the 12-month phase 3 TRANSFORMS study, fingolimod showed greater efficacy than intramuscular interferon beta (IFNβ)-1a in patients with relapsing–remitting multiple sclerosis (RRMS). This study analyzed fingolimod efficacy compared with IFNβ-1a in patient subgroups from TRANSFORMS. Patients were randomized to receive fingolimod or weekly IM IFNβ-1a for 12 months. Analyses of efficacy included annualized relapse rate (ARR), and magnetic resonance imaging (MRI) measures [gadolinium (Gd)-enhancing T1 lesions, new/newly enlarged (active) T2 lesions, brain volume change]. Subgroups were defined based on demographics, disease characteristics (baseline EDSS score, relapse rate, and MRI parameters), and response to previous therapy. Fingolimod 0.5 mg reduced ARR over 12 months by 32–59 % relative to IFNβ-1a in all subgroups defined by demographic factors or baseline disease characteristics. Fingolimod also reduced the number of new Gd-enhancing lesions, active T2 lesions, and the rate of brain volume loss, versus IFNβ-1a in most (95 %) subgroups. In patients with high disease activity despite IFNβ treatment in the year before study, fingolimod 0.5 mg reduced ARR by 61 % relative to IFNβ-1a. Reductions in lesion counts and brain volume loss also favored fingolimod in these patients. In conclusion, consistently better efficacy was observed for fingolimod compared with IFNβ-1a across different subgroups of patients with RRMS.
Keywords: Multiple sclerosis, Randomized clinical trial, Fingolimod, Interferon-beta, MRI, Subgroup analysis
Introduction
Fingolimod (FTY720; Gilenya™, Novartis Pharma AG, Basel, Switzerland) 0.5 mg once daily is the first oral disease-modifying therapy (DMT) approved for relapsing multiple sclerosis (MS) in the USA and several other countries. In the EU, fingolimod is approved for relapsing–remitting MS (RRMS) in patients with high disease activity despite previous treatment with interferon beta, and for patients with rapidly evolving severe RRMS. Compared with the first-line therapy, intramuscular (IM) interferon beta-1a (Avonex®), and with placebo, fingolimod showed superior efficacy in improving clinical and magnetic resonance imaging (MRI) outcomes in patients with RRMS [1, 2]. In the 1-year TRANSFORMS (Trial Assessing Injectable Interferon Versus FTY720 Oral in RRMS) core phase, fingolimod 0.5 mg once daily demonstrated significant benefits over interferon beta-1a on an annualized relapse rate (ARR; 0.16 and 0.33, respectively; 52 % reduction), brain volume loss, gadolinium (Gd)-enhancing T1 lesion count, and new/newly enlarged T2 lesion count [1]. In the TRANSFORMS extension, switching from interferon beta-1a to fingolimod 0.5 mg at month 12, was associated with significant improvements in ARR and MRI outcomes by month 24 [3]. In addition, patients who received continuous fingolimod for 2 years had improved ARR and MRI outcomes at month 24 compared with the switch group.
Here, we report the results of subgroup analyses from TRANSFORMS, with a focus on the approved 0.5 mg dose. We evaluated efficacy in patient subgroups defined by demographic factors, baseline disease characteristics, and responsiveness to previous treatment, some of which have been reported as potential predictors of disease progression in early MS [4–8]. Also analyzed were patient subgroups with highly active disease, defined in collaboration with the European Medicines Agency (EMA) as part of the submission and approval process.
Methods
Study design and patient population
TRANSFORMS was a randomized, double-blind, active-controlled, phase 3 trial with an optional extension (ClinicalTrials.gov number: NCT00340834). The study methodology has been published previously in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines [1]. The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. Overall, 1,292 patients with RRMS were randomly assigned with equal probability to receive fingolimod 0.5 mg or 1.25 mg once daily, or IM interferon beta-1a 30 μg weekly, for 12 months. All patients who completed the core phase on assigned study drug were eligible to enter the open-label extension phase. Individuals who received interferon beta-1a during the core phase were reassigned, in a 1:1 ratio, to receive fingolimod 0.5 mg or 1.25 mg once daily; patients on fingolimod during the core phase continued on the same assigned dose. Please see Supplementary Appendix for further details.
Analysis groups
These analyses focus on the fingolimod 0.5 mg group. The results with fingolimod 1.25 mg (Supplementary Figures 1 to 5) were consistent with the data presented here for fingolimod 0.5 mg.
Subgroups defined by demographic factors and baseline disease characteristics included sex (male or female), age (≤40 years or >40 years), treatment history (treatment-naïve or previously treated with any MS medication at any time before study entry), number of relapses in the year before study entry (≤1 or >1), number of relapses in the 2 years before study entry (1, 2, >2), baseline disability [expanded disability status scale (EDSS) score 0–3.5 or >3.5), number of Gd-enhancing T1 lesions (0 or ≥1), and T2 lesion volume (≤3,300 or >3,300 mm3). Aside from T2 lesion volume, these analysis subgroups were predefined; seven of these predefined subgroups were modified after database lock either to pool subgroups with few patients or to adjust the cut-off value. These modifications were the same as those made to the same set of predefined subgroups in the FREEDOMS (FTY720 Research Evaluating Effects of Daily Oral therapy in MS) study, and the reasons for these modifications are explained in detail in the associated publication [9]. The changes did not affect the overall conclusions.
Subgroups with highly active disease were defined after study completion and analysis during discussions surrounding licensing approval with the EMA, and reflect the populations of patients for whom fingolimod therapy is approved in the EU. These subgroups included group 1a: patients who received interferon beta during the year before study enrollment but who had as many or more relapses in the year before study entry than in the year 2 years before study entry; group 1b: patients who received any DMT during the year before study enrollment but who had as many or more relapses in the year before study entry than in the year 2 years before study entry; group 2a: patients who received interferon beta during the year before study enrollment and had ≥1 relapse in the previous year plus ≥1 Gd-enhancing T1 lesion or ≥9 T2 lesions at baseline; group 2b: patients who received any DMT during the year before study enrollment and had ≥1 relapse in the previous year plus ≥1 Gd-enhancing T1 lesion or ≥9 T2 lesions at baseline; group 3: treatment-naïve patients with rapidly evolving severe RRMS, defined as ≥2 relapses within the year before baseline and ≥1 Gd-enhancing T1 lesion at baseline.
Additional analyses were performed in a subgroup of previously treated patients defined as patients who had received any DMT in the year before study entry (group 4).
Efficacy outcomes
Analyzed endpoints included ARR (primary endpoint in TRANSFORMS), Gd-enhancing T1 lesion counts, new/newly enlarged T2 lesion counts, and normalized brain volume loss. Owing to the short duration of the study, sustained worsening of disability was infrequent in all three treatment groups of the overall population (6–8 %) [1] and the treatment effect, although numerically in favor of fingolimod 0.5 mg compared with interferon beta-1a, was not significant. Therefore, no subgroup analyses for this endpoint are reported.
The proportion of patients with active disease, defined as ≥1 relapse in the previous year and ≥1 Gd-enhancing T1 lesion at the time of assessment, was evaluated in group 4 at baseline and month 12 in the TRANSFORMS core phase and at month 24 in the extension phase. All patients in group 4 were included in each analysis if they were not missing data for the relevant time point.
Statistical analysis
Annualized relapse rates, ARR ratios, confidence intervals, and p values were estimated using a negative binomial regression model with log-link. Adjustments were used for treatment for the overall population, and for treatment, subgroup variable, and treatment by subgroup variable interaction for the subgroup analyses; log time on study was used as an offset variable to account for the varying lengths of patient time in the study.
The Gd-enhancing T1 and new/newly enlarged T2 lesion counts, lesion count ratios, confidence intervals, and corresponding p values were estimated using a negative binomial regression model with log-link. Adjustments for treatment for the overall population, and for treatment, subgroup, and treatment by subgroup variable interaction were used for the subgroup analyses. For Gd-enhancing T1 lesion analyses, scans obtained <30 days after steroid treatment for MS were excluded.
Percentage change from baseline to month 12 in normalized brain volume was investigated in an analysis of variance (ANOVA) model with adjustments for treatment for the overall population and for treatment, subgroup, and treatment by subgroup interaction for subgroup analyses.
Due to the limited size of some of the subgroups and to avoid convergence and correlative problems between baseline adjustments and the subgroup variable, the subgroup analyses presented here used simpler models (i.e., with fewer adjustments) than those employed in the previously reported analysis of the overall population [1]. The overall population was re-analyzed using these less complex models to allow comparisons between the subgroups and the overall population.
Heterogeneity of treatment effect across different levels of each subgroup was evaluated and p values for the treatment by subgroup interaction are presented. The interaction term tests whether the treatment effect is significantly different across different levels of the subgroup, between males and females, for example. If a subgroup variable had more than two levels (e.g., number of relapses in the last 2 years) then the least severe disease level was used as the reference subgroup, and the test assessed whether the treatment effect in the more severe levels differed from that in the reference group. For the highly active disease subgroups defined during discussions with the EMA (groups 1a, 1b, 2a, 2b, and 3), each subgroup was tested against the rest of the population (all those patients not in the subgroup of interest).
Results
Efficacy in subgroups defined by demographic factors and baseline disease characteristics
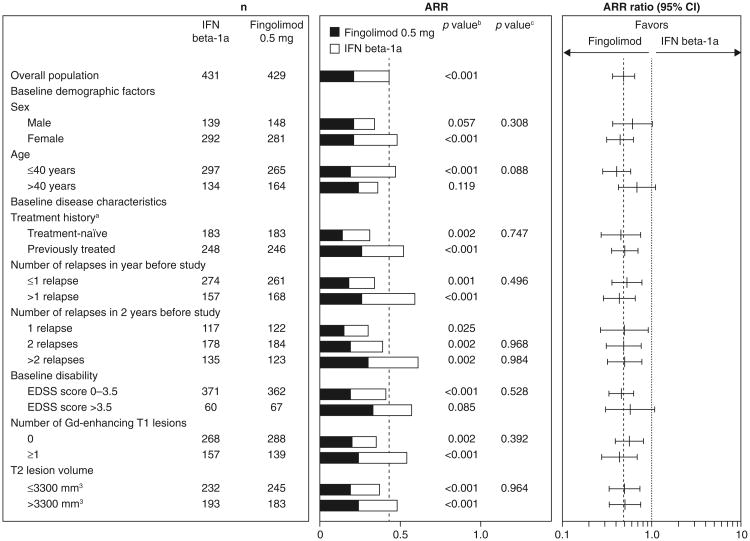
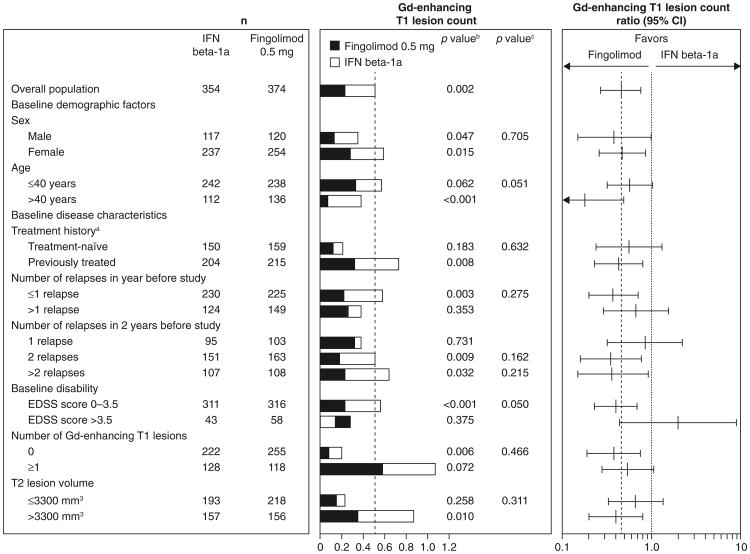
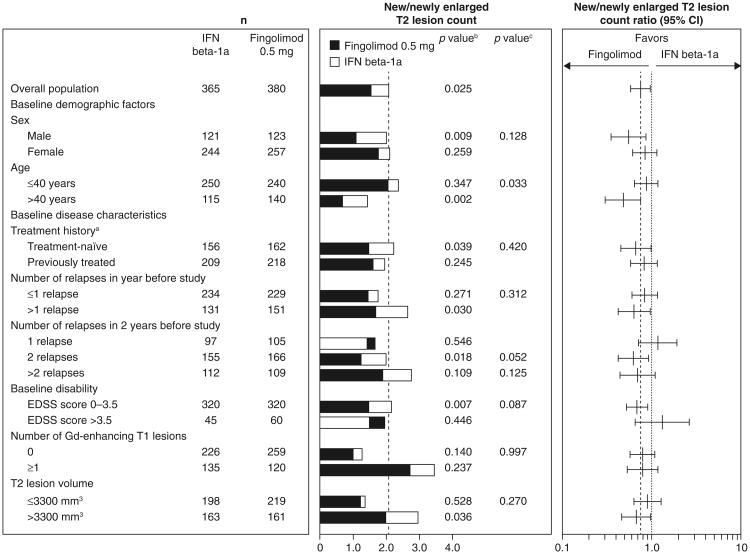
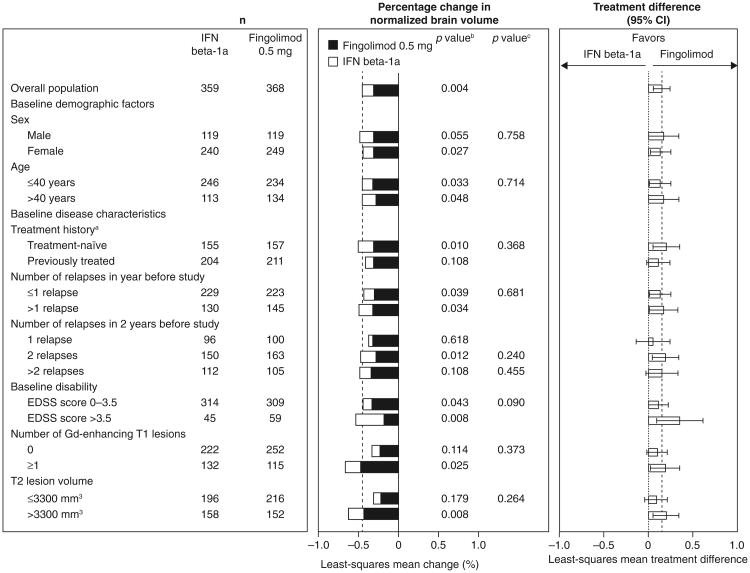
In all subgroups, fingolimod was associated with better ARR outcomes compared with patients receiving interferon beta-1a, with reductions in ARRs ranging from 32 to 59 % for fingolimod versus interferon beta-1a (Fig. 1). None of the tests for heterogeneity of the treatment effect was significant. The MRI analyses favored fingolimod over interferon beta-1a in the majority of subgroups (48/51), in many cases significantly. Fingolimod was associated with reductions in counts of Gd-enhancing T1 lesions (15–82 %) and new/newly enlarged T2 lesions (11–52 %) versus interferon beta-1a in the overall population and most subgroups (Figs. 2, 3). Reductions in brain volume loss were mainly of the order of 30–40 %, ranging from 14 % in patients with only one relapse in the 2 years before study entry to 66 % in patients with an EDSS score >3.5 at baseline (Fig. 4). The three exceptions where point estimates did not favor fingolimod were Gd-enhancing T1 and new/newly enlarging T2 lesion counts in the subgroup with a baseline disability EDSS score >3.5, and new/newly enlarging T2 lesions in the subgroup of patients who experienced only one relapse in the 2 years before study entry. In none of the subgroups analyses was interferon beta-1a statistically superior to fingolimod. Across all MRI outcomes, tests for heterogeneity were non-significant, with only one exception, indicating that the treatment effect in the overall study population applies to the subgroups. The exception was the analysis of new/newly enlarging T2 lesions in the age subgroup, in which the results indicated that the treatment effect in favor of fingolimod was stronger than average in the sub-population of patients aged >40 years and comparable to that of interferon in the subgroup of patients aged ≤40 years.
Fig. 1.
ARRs over 12 months in patient subgroups defined by demographic factors and baseline disease characteristics (intent-to-treat population). The ARRs and ARR ratios estimated using a negative binomial regression model adjusted for treatment, and (for subgroup analyses only) subgroup variable and treatment by subgroup variable interaction; log time on study was used as an offset variable. aPatients were categorized according to whether they were treatment-naïve (had received no form of medication for MS before the study) or had previously received treatment for MS with any medication at any time before study enrollment. bp value for the treatment contrast within the subgroup. cp value for the treatment by subgroup interaction, which evaluates heterogeneity of the treatment effect (see “Methods”). ARR annualized relapse rate, EDSS expanded disability status scale, Gd gadolinium, IFN interferon, MS multiple sclerosis
Fig. 2.
Gd-enhancing T1 lesion counts and ratios over 12 months in patient subgroups defined by demographic factors and baseline disease characteristics (intent-to-treat population). Gd-enhancing T1 lesion counts were estimated using a negative binomial regression model, log-link, adjusted for treatment for the overall result, and adjusted for treatment subgroup and treatment by subgroup variable interaction for the subgroup analyses. n number of observations included in the analysis (patients with non-missing Gd-enhancing T1 lesion count assessments at month 12; scans obtained <30 days after the last use of steroids to treat MS were excluded from this analysis). aPatients were categorized according to whether they were treatment-naïve (had received no form of medication for MS before the study) or had previously received treatment for MS with any medication at any time before study enrollment. bp value for the treatment contrast within the subgroup. cp value for the treatment by subgroup interaction, which evaluates heterogeneity of the treatment effect (see “Methods”). EDSS expanded disability status scale, Gd gadolinium, IF interferon, MS multiple sclerosis
Fig. 3.
New/newly enlarging T2 lesion counts and ratios over 12 months in patient subgroups defined by demographic factors and baseline disease characteristics (intent-to-treat population). New/newly enlarged T2 lesion counts were estimated using a negative binomial regression model, log-link, adjusted for treatment for the overall result, and adjusted for treatment subgroup and treatment by subgroup variable interaction for the subgroup analyses. n number of observations included in the analysis (patients with non-missing new/newly enlarged T2 lesion count assessments at month 12). aPatients were categorized according to whether they were treatment-naïve (had received no form of medication for MS before the study) or had previously received treatment for MS with any medication at any time before study enrollment. bp value for the treatment contrast within the subgroup. cp value for the treatment by subgroup interaction, which evaluates heterogeneity of the treatment effect (see “Methods”). EDSS expanded disability status scale, Gd gadolinium, IFN interferon, MS multiple sclerosis
Fig. 4.
Percentage change in normalized brain volume over 12 months in patient subgroups defined by demographic factors and baseline disease characteristics (intent-to-treat population). The percentage change in brain volume was analyzed using an ANOVA model adjusted for treatment for the overall result and for treatment, subgroup, and treatment by subgroup interaction for subgroup analyses. The p value for the ANOVA analysis refers to a t test for the treatment contrast. n number of patients in each group with a non-missing assessment of percentage change from baseline in normalized brain volume at month 12. aPatients were categorized according to whether they were treatment-naïve (had received no form of medication for MS before the study) or had previously received treatment for MS with any medication at any time before study enrollment. bp value for the treatment contrast within the subgroup. cp value for the treatment by subgroup interaction, which evaluates heterogeneity of the treatment effect (see “Methods”). ANOVA analysis of variance, EDSS expanded disability status scale, Gd gadolinium, IFN interferon, MS multiple sclerosis
Efficacy in previously treated patients or treatment-naïve patients with highly active disease
Relapse rates
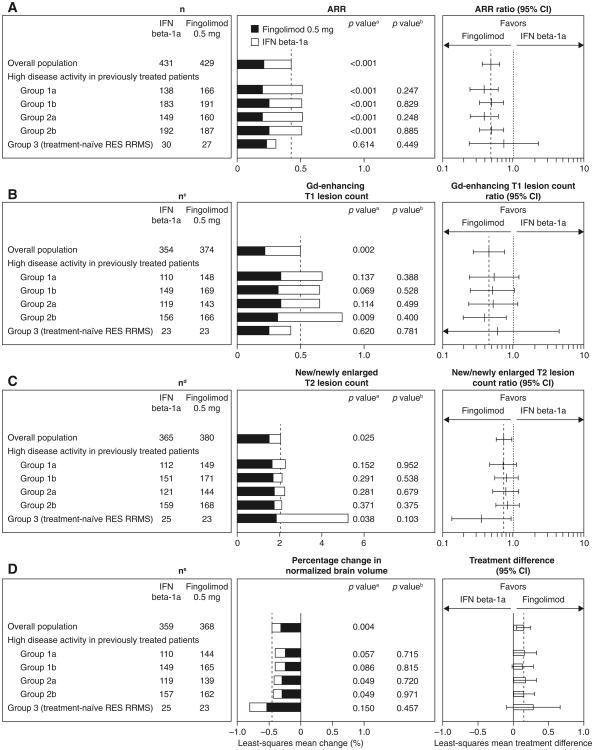
In patients with high disease activity despite receiving interferon beta in the year before study entry (groups 1a and 2a), fingolimod reduced ARR by 61 % versus interferon beta-1a over 12 months (Fig. 5a). In patients with high disease activity despite any DMT in the year before study entry (groups 1b and 2b), fingolimod reduced ARR by 50 % versus interferon beta-1a over 12 months. In the small subgroup of treatment-naïve patients with rapidly evolving severe RRMS (group 3; n = 57), the ARR reduction with fingolimod versus interferon beta-1a was 25 %.
Fig. 5.
a Annualized relapse rates, b Gd-enhancing T1 lesion count, c estimated new/newly enlarged T2 lesion count and d brain volume loss for fingolimod versus interferon beta-1a over 12 months in previously treated or treatment-naïve patients with highly active disease (intent-to-treat population). For definition of the patient groups, see “Methods”. ARRs and ARR ratios were estimated using a negative binomial regression model adjusted for treatment, and (for subgroup analyses only) subgroup variable and treatment by subgroup variable interaction; log time on study was used as an offset variable. Gd-enhancing T1 and new/newly enlarged T2 lesion counts were estimated using a negative binomial regression model, log-link, adjusted for treatment for the overall result, and adjusted for treatment subgroup and treatment by subgroup variable interaction for the subgroup analyses. Least-squares mean change in brain volume calculated using an ANOVA model adjusted for treatment for the overall result and for treatment, subgroup, and treatment by subgroup interaction for subgroup analyses. ap value for the treatment contrast within the subgroup. bp value for the treatment by subgroup interaction, which evaluates heterogeneity of the treatment effect (see “Methods”). cn number of observations included in this analysis (patients with non-missing Gd-enhancing T1 lesion count assessments at month 12; scans obtained <30 days after the last use of steroids to treat MS were excluded from this analysis). dn number of observations included in this analysis (patients with non-missing new/newly enlarged T2 lesion count assessments at month 12). en number of patients in each group with a non-missing assessment of percentage change from baseline in normalized brain volume at month 12. ANOVA analysis of variance, ARR annualized relapse rate, DMT disease-modifying therapy, Gd gadolinium, IFN interferon, RES rapidly evolving severe, RRMS relapsing–remitting multiple sclerosis
MRI lesion counts
In patients who had high disease activity despite receiving interferon beta in the year before study entry (groups 1a and 2a), fingolimod reduced Gd-enhancing T1 lesion counts by 46–48 % relative to interferon beta-1a at month 12 (Fig. 5b). In the same subgroups, fingolimod reduced new/newly enlarged T2 lesion counts relative to interferon beta-1a at month 12 by 21–27 % (Fig. 5c). Reductions in lesion counts favoring fingolimod over interferon beta-1a also were observed in patients who had high disease activity despite any DMT in the year before study entry (groups 1b and 2b). In group 3, fingolimod reduced Gd-enhancing T1 lesion counts by 40 % and reduced new/newly enlarged T2 lesion counts by 64 % relative to interferon beta-1a at month 12.
Brain volume loss
Treatment with fingolimod was associated with a reduction in brain volume loss from baseline to month 12 of 31–40 % relative to interferon beta-1a, in patients who had high disease activity despite receiving interferon beta (groups 1a and 2a) or any DMT (groups 1b and 2b) in the year before study entry (Fig. 5d). The results also favored fingolimod in group 3.
Proportion of patients with active disease despite DMT
Overall, 745/1,292 (57.7 %) patients enrolled in TRANSFORMS had previously received DMT for MS, 635/745 (85.2 %) had previously received interferon beta. Among the patients who received DMT in the year before entering TRANSFORMS (group 4; n = 641), the proportion with active disease at month 12 (defined as ≥1 relapses in year 1 and ≥1 Gd-enhancing T1 lesions at month 12) was substantially lower with fingolimod (1.6 %; 3/191) than with interferon beta-1a (12.1 %; 21/173).
For patients who switched from interferon beta-1a to fingolimod in the extension (n = 79), the proportion of patients with active disease at month 12 prior to switch was 13.7 % (10/73) and fell to 3.0 % (2/66) at month 24. Among patients who received continuous fingolimod for 2 years (n = 177), few had active disease at either month 12 (1.2 %; 2/169) or month 24 (2.0 %; 3/152).
Discussion
Fingolimod demonstrated consistently greater efficacy versus interferon beta-1a on all outcomes in the majority (∼95 %) of patient subgroups analyzed, including those defined by demographic factors and baseline disease activity, and subgroups of patients who had high disease activity despite previous treatment with approved MS therapies (which reflect the populations for whom fingolimod therapy is approved in the EU). In none of the subgroups were the results statistically in favor of interferon beta-1a. These results are consistent with findings from the overall intent-to-treat population in the TRANSFORMS study and extension [1, 3].
Subgroup analyses have intrinsic limitations and present methodological challenges. TRANSFORMS was not prospectively designed or powered to test for treatment differences within subgroups, or to test formally for heterogeneity among subgroups. In addition, statistical power for MRI outcomes is probably higher than for clinical outcomes. The p values may not represent nominal values and should be interpreted with care. A sizable number of subgroups was analyzed here without formal adjustment for multiple comparisons, in accordance with a previous recommendation [10], which may have led to false-positive results. Conversely, some of the subgroups were small, potentially making some clinically relevant differences not statistically significant. Tests of heterogeneity were not significant, with only one exception, indicating that the treatment effect in favor of fingolimod observed in the overall population also applies to the subgroups.
Possible heterogeneity of the treatment effect was found in subgroups defined by age. The treatment benefit of fingolimod over interferon beta-1a on ARR was greater in patients aged ≤40 years than in those >40 years, although the test for heterogeneity between the two subgroups was not significant. A similar effect of age on ARR was observed in the FREEDOMS study [9], and the heterogeneity of effect was statistically significant. With respect to MRI activity in TRANSFORMS, the treatment effect favored fingolimod over interferon beta-1a particularly strongly in the subgroup of patients aged >40 years, in terms of both Gd-enhancing T1 and new/newly enlarging T2 lesions, while in patients aged ≤40 years the treatment effect of fingolimod was comparable to that of interferon beta-1a.
In subgroups with highly active disease, fingolimod was more beneficial than interferon beta-1a in improving ARRs and MRI outcomes. Significant ARR reductions were observed in patients who had high disease activity despite interferon beta therapy in the year before study entry (relative ARR reduction: 61 %), and were accompanied by reductions in Gd-enhancing T1 and new/newly enlarged T2 lesion counts, and brain volume loss, relative to interferon beta-1a. Analysis of the efficacy of fingolimod in such patients is clinically relevant given that a large proportion of patients (up to 36 %) who receive first-line MS treatments discontinue due to perceived lack of efficacy [11–13]. In these subgroups and in the overall population, the relative treatment effect of fingolimod versus interferon beta-1a was greater for ARRs than for new/newly enlarged T2 lesion counts. Given that treatment with interferon beta has been associated with an ARR reduction of approximately 30 % versus placebo but with larger relative reductions in MRI outcomes [14–17], the lesser effect on MRI outcomes compared with ARR in TRANSFORMS may reflect a floor effect on the ability to reduce lesion counts further compared with active therapy. In treatment-naïve patients with highly active RRMS, the benefit of fingolimod over interferon beta-1a in preventing new T2 lesions and additional brain volume loss was particularly pronounced compared with the overall population, which is likely to be of clinical relevance.
The analysis of continued active disease in patients who had received DMT in the previous year provides further evidence that fingolimod improves outcomes in patients who had active disease despite standard treatment. Within the subgroup of previously treated patients, fingolimod markedly reduced the proportion of patients with active disease relative to interferon beta-1a, over 12 months in the core phase of the study. After patients treated with interferon beta-1a during the first year switched to fingolimod in the second year (extension phase) there was a substantial reduction in the proportion of patients with active disease by month 24, to a level similar to that observed in the continuous fingolimod group. These results indicate that fingolimod can provide added benefit in patients who still have active disease following a year of interferon treatment. The data are also in agreement with published clinical and MRI data from the overall extension population, showing improved outcomes following switching from interferon beta-1a to fingolimod [3].
The overall consistency of the results favoring fingolimod, and similarities in the relative treatment effect across many subgroups, provide evidence of a clinically relevant benefit for fingolimod over interferon beta-1a across a range of subgroups of patients with RRMS. The results presented here are particularly relevant to the populations for whom approval of fingolimod exists in the EU, including those with highly active RRMS, given the need for effective therapy for such patients. The data regarding continued active disease while on interferon therapy and the reduction after switching to fingolimod support the prospectively collected data in the TRANSFORMS core and extension phases, providing direct evidence of the potential benefit of switching from interferon beta-1a to fingolimod.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the study, the study site personnel, and C. Brechin PhD (Oxford PharmaGenesis™ Ltd) and H. Salloukh (Novartis Pharma AG) for editorial assistance. All authors had full access to all data in the study, participated in writing the manuscript, and approved the full manuscript before the corresponding author took final responsibility for the decision to submit for publication. This study was funded by Novartis Pharma AG, Basel, Switzerland.
Footnotes
On behalf of the TRANSFORMS Study Group
Electronic supplementary material The online version of this article (doi:10.1007/s00415-013-6932-0) contains supplementary material, which is available to authorized users.
Conflicts of interest Prof. Cohen has received reimbursement for travel or consulting from Biogen Idec, Elan, Novartis, Teva, and Vaccinex. He has also received research support paid to his institution from Biogen Idec, Novartis, Receptos, Teva, Synthon, and Vaccinex. Dr. Barkhof has received consultancy fees from Bayer Schering Pharma, Sanofi-Aventis, Biogen Idec, GE Medical Systems, Genzyme, Synthon, Novartis and Roche. He has served as an editorial board member for Brain, Journal of Neurology, European Radiology and Neuroradiology. He has received payment for speaker services from Novartis and Serono. Barkhof has also received research support from the Dutch Foundation for MS Research. Prof. Comi has received consulting fees for speaker services from Novartis, Teva Pharmaceutical Industries, Sanofi-Aventis, Merck Serono, Bayer and Actelion. He has also received lecture fees from Novartis, Teva Pharmaceutical Industries, Sanofi-Aventis, Merck Serono, Biogen Dompè, Bayer and Serono Symposia International Foundation. Prof. Izquierdo has received payment for board membership of Biogen, Novartis, Sanofi, Serono and Teva. He has also received consultancy fees or honorarium from Biogen Idec, Merck, Bayern, Sanofi, Teva and Novartis. Dr. Khatri has received consultancy fees from Teva, Biogen Idec, Bayer, Pfizer, Genzyme, Questcor, Avanit, Novartis and Teurimo BCT. He has received research support from Biogen Idec and Novartis. Dr. Montalban has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceutical Industries and Almirall. Prof. Pelletier has received payment for scientific advisory boards for Allergan, Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi and Teva. He has received non-profit foundation support from ARSEP, academic research support from PHRC, and unconditional research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Sanofi and Teva. Dr. Eckert is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Dr. Häring is an employee of Novartis Pharma AG, Basel, Switzerland. Dr. Francis is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Contributor Information
Jeffrey A. Cohen, Email: cohenj@ccf.org, Mellen Center U-10, Neurological Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Frederik Barkhof, Image Analysis Center, VU University Medical Center, Amsterdam, the Netherlands.
Giancarlo Comi, Department of Neurology, Vita-Salute San Raffaele University, Milan, Italy.
Guillermo Izquierdo, Department of Neurology, Virgen Macarena University Hospital, Seville, Spain.
Bhupendra Khatri, St Luke's Medical Center, Milwaukee, WI, USA.
Xavier Montalban, Department of Neurology-Neuroimmunology and Cemcat, Vall d'Hebron University Hospital, Barcelona, Spain.
Jean Pelletier, Department of Neurology and CRMBM CNRS7339, CHU La Timone, Marseille, France.
Benjamin Eckert, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Dieter A. Häring, Novartis Pharma AG, Basel, Switzerland
Gordon Francis, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
References
- 1.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 3.Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, Pelletier J, Stites T, Wu S, Holdbrook F, Zhang-Auberson L, Francis G, Cohen JA. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–529. doi: 10.1016/S1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- 4.Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346(3):158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 5.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 6.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 7.Losseff NA, Miller DH, Kidd D, Thompson AJ. The predictive value of gadolinium enhancement for long term disability in relapsing-remitting multiple sclerosis—preliminary results. Mult Scler. 2001;7(1):23–25. doi: 10.1177/135245850100700105. [DOI] [PubMed] [Google Scholar]
- 8.Scott TF, Schramke CJ, Novero J, Chieffe C. Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology. 2000;55(5):689–693. doi: 10.1212/wnl.55.5.689. [DOI] [PubMed] [Google Scholar]
- 9.Devonshire V, Havrdova E, Radue EW, O'Connor P, Zhang-Auberson L, Agoropoulou C, Haring DA, Francis G, Kappos L. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420–428. doi: 10.1016/S1474-4422(12)70056-X. [DOI] [PubMed] [Google Scholar]
- 10.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 11.Tremlett H, Zhao Y, Joseph J, Devonshire V. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–1374. doi: 10.1136/jnnp.2008.145805. [DOI] [PubMed] [Google Scholar]
- 12.Portaccio E, Zipoli V, Siracusa G, Sorbi S, Amato MP. Long-term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. Eur Neurol. 2008;59(3–4):131–135. doi: 10.1159/000111875. [DOI] [PubMed] [Google Scholar]
- 13.Zwibel HL. Glatiramer acetate in treatment-naive and prior interferon-beta-1b-treated multiple sclerosis patients. Acta Neurol Scand. 2006;113(6):378–386. doi: 10.1111/j.1600-0404.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343(13):898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer FE, 3rd, Priore RL, Pullicino PM, Scherokman BJ, Whitham RH, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996;39(3):285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 16.Li DK, Paty DW. Magnetic resonance imaging results of the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-beta1a in relapsing-remitting multiple sclerosis. Prevention of relapses and disability by interferon-beta1a subcutaneously in multiple sclerosis. Ann Neurol. 1999;46(2):197–206. doi: 10.1002/1531-8249(199908)46:2<197::aid-ana9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.PRISMS study group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (prevention of relapses and disability by interferon beta-1a subcutaneously in multiple sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.