Abstract
Liposarcomas are tumors arising in white adipose tissue (WAT) with avidity for local recurrence. Aggressive dedifferentiated liposarcomas (DDLS) may arise from well-differentiated subtypes (WDLS) upon disease progression, however, this key issue is unresolved due in large part to knowledge gaps about liposarcoma cellular composition. Here, we wished to improve insights into liposarcoma cellular hierarchy. Tumor section analysis indicated that the populations, distinguishable based on expression of CD34 (a marker of adipocyte progenitors) and CD36 (a marker of adipocyte differentiation), occupy distinct intra-tumoral locations in both WDLS and DDLS. Taking advantage of these markers, we separated cells from a panel of fresh human surgical specimens by fluorescence-activated cell sorting (FACS). Based on chromosome analysis and the culture phenotypes of the composing populations, we demonstrate that malignant cells comprise four mesenchymal populations distinguished by expression of CD34 and CD36, while vascular (CD31+) and hematopoietic (CD45+) components are non-neoplastic. Finally, we show that mouse xenografts are derivable from both CD36-negative and CD36-positive DDLS cells, and that each population recreates the heterogeneity of CD36 expression in vivo. Combined, our results show that malignant cells in WDLS and DDLS can be classified according to distinct stages of adipogenesis and indicate immonophenotypic plasticity of malignant liposarcoma cells.
Keywords: Liposarcoma, adipose, mesenchymal, stromal, progenitor, cancer
Introduction
Sarcomas, cancers of connective tissues, are diagnosed in approximately 10,000 U.S. patients annually with a five-year survival rate of only 50% (Anaya et al., 2009). Liposarcomas, including well-differentiated (WDLS), dedifferentiated (DDLS), pleomorphic, and myxoid variants, arise in white adipose tissue (WAT) and are among the deadliest of these tumors (Kooby et al., 2004; Lahat et al., 2008). WDLS are more common and characterized by repetitive local recurrence with minimal risk of metastasis. In contrast, DDLS are aggressive malignancies with the capacity for distant spread and lethal outcome even after initially successful treatment (Kooby et al., 2004; Gilbert et al., 2009). Malignant cells within WDLS and DDLS typically demonstrate amplification of chromosome 12 (12q13∼15) the locus of MDM2, CDK4 and SAS genes (Weaver et al., 2009). The liposarcoma cell type in which genetic changes first occur is unknown. It is also unclear if WDLS is the predecessor of DDLS or whether these two subtypes, often found within the same tumor, arise independently. To date, characterization of liposarcoma cells has only been performed following expansion in culture (Peng et al., 2011). Lack of information on the in vivo cellular liposarcoma hierarchy has hampered understanding of the mechanisms underlying the disease progression.
Investigation of many solid cancers has been facilitated by classifying constituent malignant cells into distinct populations corresponding to the differentiation stages of benign tissue counterparts (Matsui et al., 2004; Tang, 2012). In response to metabolic imbalance, WAT has a capacity to quickly grow in mass, resulting in obesity (Daquinag et al., 2011a; Sun et al., 2011). WAT expansion is as a result of proliferation and differentiation of a progenitor population that is similar to mesenchymal stromal/stem cells (MSC) initially described in the bone marrow (Prockop, 1997; Pittenger et al., 1999; Bianco et al., 2008; Caplan and Correa, 2011). These adipose MSC, termed adipose stromal cells (ASC), serve as progenitors of preadipocytes (Rodeheffer et al., 2008; Tang et al., 2008), ultimately differentiating into white adipocytes, which are large cells accumulating triglycerides in lipid droplets and the main cellular component of WAT (Cinti, 2011; Daquinag et al., 2011b;). In addition to ASC, WAT contains endothelial cells and infiltrating leukocytes, which may also contribute to the adipocyte pool in pathological conditions (Daquinag et al., 2011b; Kolonin et al., 2012). Gene expression profiles (Matushansky et al., 2008) and adipogenenic potential of liposarcoma cells (Peng et al., 2011) have indicated the mesenchymal origin of liposarcomas, however the possibility of hematopoietic or endothelial cells also undergoing malignant transformation has not been ruled out.
We hypothesized that, by analogy with benign cells of adipocyte lineage (Fig. 1A), malignant cells in WDLS and DDLS could be classified as per distinct stages of adipogenesis. Our studies identify four distinct mesenchymal populations of malignant cells in both WDLS and DDLS and establish a protocol by which they can be separated from non-malignant (hematopoietic and endothelial) cells of tumor microenvironment. We show that a population of malignant cells in both WDLS and DDLS has features of ASC, whereas other cell populations have immunophenotypes corresponding to variable degrees of adipocyte differentiation. Our experiments in DDLS xenograft mouse models show that cell populations separated based on distinct immunophenotypes have comparable tumor-initiation capacities and can re-generate the distinct immunophenotypic populations in vivo.
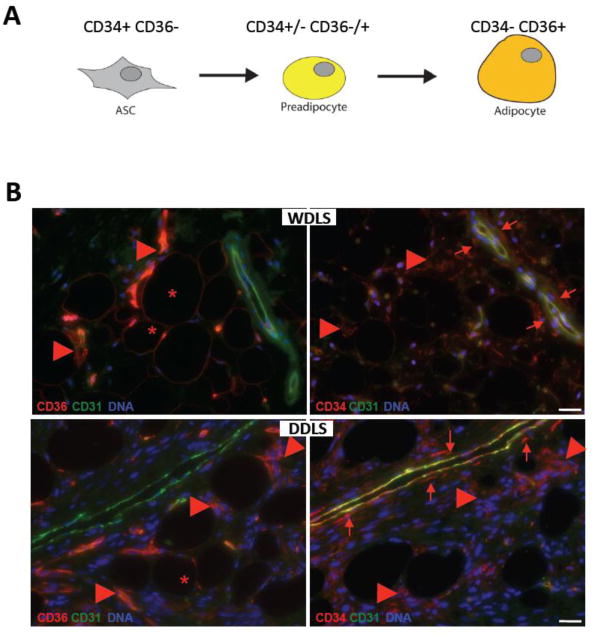
Figure 1.
WDLS and DDLS cells at distinct differentiation stages. (A) A schematic depicting expression of CD34 and CD36, cell surface markers used for liposarcoma cell classification, during differentiation of mesenchymal adipocyte progenitors. (B) Immunolocalization of distinct liposarcoma populations in serial paraffin sections of representative WDLS and DDLS samples subjected to immunofluorescence with antibodies against CD36 or CD34 (red). CD36+CD34- cells with adipocyte morphology (*), adjacent stromal CD36+CD34+ cells (arrowheads) and mainly perivascular CD36-CD34+ cells (arrows) are indicated relative to non-malignant vasculature expressing CD31 (green). Nuclei are blue. Scale bar: 50 μm.
Results
Heterogeneity of WDLS and DDLS cells revealed in tumor sections
As markers for the analysis of liposarcoma cell heterogeneity, we chose CD34, a single-pass type 1 transmembrane sialomucin expressed in adipose and some other progenitor cells (Daquinag et al., 2011b), and CD36, a scavenger receptor that functions as a fatty acid transporter in adipocytes (Kampf et al., 2007). Expression of both CD34 and CD36 has been previously reported for liposarcomas (Mechtersheimer, 1991). It has been shown that ASC express high levels of CD34 but low levels of CD36 (Festy et al., 2005). During adipocyte differentiation, the expression of CD34 decreases whereas the expression of CD36 increases (Kampf et al., 2007), resulting in preadipocytes with intermediate expression levels of these molecules (Fig. 1A). In differentiated adipocytes, CD34 is not expressed, whereas the expression of CD36 becomes prominent, rendering them as CD34-CD36+.
To investigate the niches that distinct populations occupy within the tumor, we immunolocalized cells expressing CD36+ and CD34+ relative to endothelial cells expressing CD31/PECAM-1 in human liposarcomas. Immunofluorescence analysis of tumor sections revealed several distinct cell types. Cells with the adipocyte morphology positive for CD36 and negative for CD34 were observed in both WDLS and DDLS (Fig. 1B), and were predictably more abundant in WDLS. Clusters of cells expressing both CD34 and CD36 were frequently observed in the vicinity of differentiated adipocytes and were equally common in both WDLS and DDLS (Fig. 1B). This cell population likely represents partially differentiated liposarcoma cells. Finally, a population of CD34+ CD36- cells was observed surrounding the vasculature in both WDLS and DDLS (Fig. 1B). This immunophenotype and also this localization is characteristic of ASC, which are known to serve as pericytes and adventitial cells in WAT (Traktuev et al., 2008; Corselli et al., 2011).
Malignant cells comprise the mesenchymal liposarcoma population
For systematic analysis of human liposarcomas, we isolated tumor cell suspensions from multiple WDLS and DDLS using enzymatic tissue digestion. To initiate characterization of the cellular hierarchy composing WDLS and DDLS, we subjected freshly isolated tumor cells to flow cytometry. First, we deemed it important to confirm that the malignant liposarcoma cells are restricted to the mesenchymal lineage. In analyzing several WDLS and DDLS cases, we observed a large proportion of tumor cells as CD36+ or CD34+ (Fig. 2A). We subjected cytospins of the FACS-sorted cells to fluorescence in situ hybridization (FISH), detecting 12q15 chromosomal segment amplification. This hallmark of liposarcoma was observed for the majority of CD36+ (66%) and CD34+ (74%) cells, indicating that cells at various stages of differentiation are malignantly transformed (Fig. 2B). The minor fraction of CD34+ cells lacking this amplification apparently correspond to benign progenitors, previously reported to infiltrate liposarcomas (Morozov et al., 2010). We also performed FACS with antibodies to CD31 (not shown) and pan-leukocyte marker CD45 (Fig. 2A). Consistent with previous reports (Tseng et al., 2012), a significant fraction of liposarcoma cells corresponded to vascular endothelial cells and infiltrating leukocytes, some of which express variable levels of CD34 and CD36. Importantly, we could not detect 12q15 amplification in these populations (Fig. 2B).
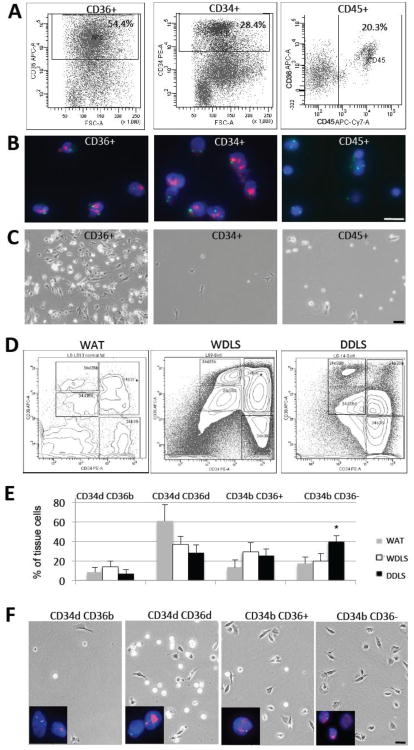
Figure 2.
Characterization of WDLS and DDLS populations. (A) FACS separation of cells based on the expression of CD36, CD34, and CD45 in a representative WDLS. Percentages of cells positive for a marker among viable cells are indicated. (B) Identification of malignant cells through the detection of the 12q15 amplification (red) by FISH in CD36+ and CD34+ cells. (C) Morphology of cells sorted based on the expression of CD36, CD34, or CD45 from the WDLS tumor upon adherence. In (B-C), WDLS cells isolated in (A) were used. (D) FACS 3D contour graphs for representative samples of benign WAT, as well as for WDLS and DDLS samples analyzed in Fig. 1 (B). Gates for CD34dCD36d, CD34dCD36b, CD34bCD36-, and CD34bCD36+ cells are shown. (E) Percentages among viable cells analyzed in multiple samples were calculated for normal WAT, WDLS and DDLS (N=5 for each). Mean frequencies of each population +/- SD among viable tumor cells in are shown. * P<0.05. (F) Morphology of cells sorted in (D) from a representative DDLS sample upon plastic adherence in culture. FISH detecting the 12q15 amplicon (red) confirms cells in the four populations as malignant. Green: centromeric probe; blue: DAPI. Scale bar: 50 μm.
Consistent with our previous observations (Peng et al., 2011), adherent cells were isolated from both WDLS and DDLS cases based on conditions used to culture ASC (Zhang et al., 2009; Daquinag et al., 2011a; Klopp et al., 2012; Zhang et al., 2012). The majority of cells sorted as CD36+ had morphologies typical of preadipocytes and contained phase-bright lipid droplets, indicating their partial differentiation (Fig. 2C). In contrast, cells sorted as CD34+ did not contain lipid droplets and had the appearance of mesenchymal fibroblasts with defined nuclei and nucleoli (Fig. 2C), which is typical ASC/MSC morphology as reported previously (Zhang et al., 2009; Daquinag et al., 2011a; Klopp et al., 2012). Both CD36+ and CD34+ cells had a high plating efficiency (>1/20). In contrast, plating efficiency of the non-malignant CD45+ population was remarkably lower (1/340). The adherent CD45+ cells had characteristic myelomonocytic morphologies (Fig. 2C). Both CD34+ and CD36+ cells were highly proliferative and could be indefinitely passaged in standard MSC maintenance conditions, as expected of malignant cells, while CD45+ and CD31+ cells failed to proliferate. Combined, these data indicate that liposarcoma-infiltrating hematopoietic and endothelial cells are benign whereas counterpart malignant cells are comprised by mesenchymal populations at different stages of adipogenesis.
Classification of malignant cell populations in WDLS and DDLS
Next, we established a flow cytometric methodology to separate distinct malignant liposarcoma populations. We analyzed cells from a panel of human WDLS (N=5) and DDLS (N=5) after double labeling with CD34 and CD36 antibodies. Four distinct populations were observed: CD34dim/CD36dim (CD34dCD36d), CD34dim/CD36bright (CD34dCD36b), CD34bright/CD36- (CD34bCD36-) and CD34bright/CD36+ (CD34bCD36+). According to the adipogenesis model (Fig. 1A), the CD34dCD36b population contains adipocytes, the CD34bCD36- population contains ASC, while the CD34bCD36+ and CD34dCD36d populations contain partially differentiated cells. As expected, a variably abundant sub-population of cells negative for both CD34 and CD36 was observed, which accounted for infiltrating non-malignant and possibly a fraction of malignant cells (Fig. 2D). The four CD34/CD36 populations were clearly defined in all analyzed WDLS and DDLS samples, although absolute marker expression levels for a respective population varied. Cell separation into the corresponding four populations was also observed for benign WAT resected from the patients (N=5) during surgery (Fig. 2D).
Although the levels of CD34 and CD36 expression in the respective populations of normal WAT and tumors were variable, these data show that cells in liposarcomas maintain the phenotypic diversity of parental WAT tissue. Quantification of these sub-populations in different samples of WAT, WDLS and DDLS (N=5 each), revealed that the CD34bCD36- cells are significantly more abundant in DDLS than in WDLS and benign WAT (Fig. 2E). Importantly, we observed the same trend when comparing the WDLS and DDLS components of the same tumor (data not shown). These observations suggest that malignant cells with the phenotype of adipose mesenchymal progenitors are enriched during liposarcoma progression. It should be noted that in both, WAT and tumors, the differentiated CD34dCD36b fraction was underrepresented because adipocytes mainly do not register in flow cytometry due to their buoyancy, large size, and fragility.
For each population (CD34dCD36d, CD34dCD36b, CD34bCD36- and CD34bCD36+), FISH analysis of cells sorted from tumors revealed a high frequency of 12q15 amplification, indicating them as malignant (Fig. 2F). Liposarcoma cells negative for 12q15 amplification were observed as a variable minor fraction of each population, accounting for the contributing benign mesenchymal, hematopoietic, and endothelial cells (Morozov et al., 2010). As revealed by flow cytometric analysis (Supplemental Fig. 1), virtually all cells in CD34bCD36+ and CD34bCD36- populations are negative for CD45 and CD31, consistent with them being mesenchymal malignant cells. About 5% of CD34bCD36+ cells express CD31, indicating this sub-fraction being endothelial cells. In CD34dCD36d and CD34dCD36b populations, a significant sub-fraction of CD45+CD31- cells was observed, which accounts for infiltrating leukocytes. These data are in agreement with CD36 expression reported for sub-populations of leukocytes and endothelial cells also observed in tissue sections (Fig. 1B).
We also analyzed the morphology of individual cell populations upon plastic attachment in culture. Only scant adherent cells could be isolated from the CD34dCD36b sub-population (Fig. 2F), consistent with it containing endothelial cells (some of which express CD36) and occasional viable adipocytes, which are both poorly adherent. The CD34dCD36d and CD34bCD36+ cells were mostly adherent and contained lipid droplets, consistent with these sub-populations corresponding to malignant preadipocyte-like cells (Fig. 2F). Finally, the CD34bCD36- cells were lipid droplet-free, consistent with this sub-population corresponding to malignant ASC-like cells (Fig. 2F). Combined, these observations reinforce our tumor section analysis data, indicating that in both WDLS and DDLS malignant cells comprise a heterogeneous hierarchy composed of cellular populations undergoing varying degrees of adipogenic differentiation.
Analysis of freshly isolated CD34/CD36 populations revealed variable expression of CD73, CD90, and CD150, the markers expressed by MSC and ASC (Bianco et al., 2008; Bellows et al., 2011a). In WDLS, all four populations contained a significant component of CD73+ and CD90+ cells, while cells with significant CD105 expression were not observed (Supplemental Fig. 2A). Cells lacking MSC marker expression, particularly prominent in the two CD34d populations, likely correspond to differentiating adipocytes, as well as the endothelial and leukocyte populations detected through the use of CD31 and CD45 (Supplemental Fig. 1) In DDLS, the frequency of CD73+ and CD90+ cells in each of the four populations was increased compared to WDLS, while CD105 expression was still insignificant (Supplemental Fig. 2B). These data are consistent with the decreased extent of adipocyte differentiation in progressed disease and with enrichment for cells with the ASC properties detected though the use of CD34 as a marker (Fig. 2D-E).
Plasticity of liposarcoma populations in culture and in mouse xenografts
Next we performed tumorigenicity studies in mouse xenograft models. In our previous reports, we have successfully established xenografts in immunodeficient mice via injection of DDLS (but not WDLS) cells cultured for several passages (Peng et al., 2011). While for many human cancers tumors can grow in mice upon injection of only few malignant cells (Matsui et al., 2004; Tang, 2012), for unclear reasons liposarcoma cells are poorly tumorigenic in the xenograft setting. Injection of as many as 8×106 unsorted freshly isolated liposarcoma cells have not generated xenografts in all immunodeficient host strains (Nude, SCID and NSG) that we have tested. We have also injected up to 2×106 uncultured cells separated by FACS from two different DDLS tumors. Neither bulk tumor cells nor freshly sorted CD34dCD36d, CD34dCD36b, CD34bCD36-, or CD34bCD36+ populations generated xenografts, indicating that ex vivo cell passaging is a requisite for liposarcoma xenograft take. We, therefore, chose cells from a DDLS sample (termed Lipo863), shown to express adipogenesis genes, accumulate lipid droplets upon differentiation induction, and grow tumors in immunodeficient mice (Peng et al., 2011) to perform the remainder of our studies. Analysis of Lipo863 cells by flow cytometry indicated that CD34 expression was lost in these cells in culture (Fig. 3A), which is also typical of benign ASC (Gimble et al., 2007). Expression of CD36 was also reduced compared to the parental tumor; however, the cells were clearly separated into two distinct populations (CD36- and CD36+) based on the level of CD36 expression (Fig. 3A). Upon FACS, each population, in culture, presented as adherent cells morphologically similar to normal ASC (Fig. 3A). Both CD36- and CD36+ populations of cultured Lipo863 cells were found to unanimously express CD73, CD90, as well as CD105 (Supplemental Fig. 3), confirming their similarity to ASC/MSC.
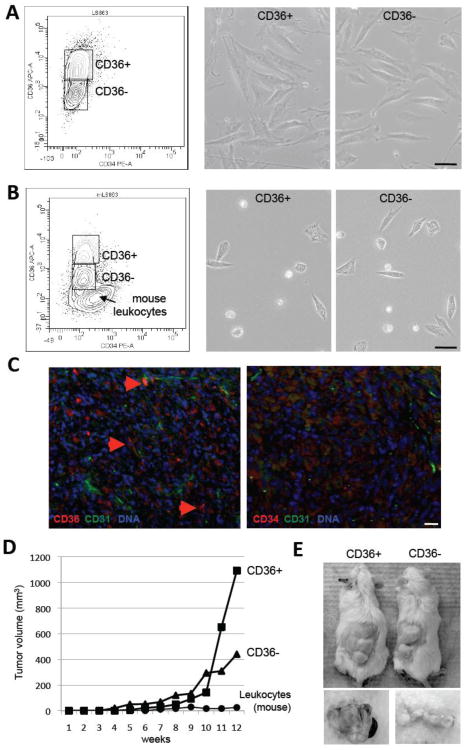
Figure 3.
Culture plasticity and tumorogenicity of liposarcoma cells. (A) Separation of early passage Lipo863 cells derived from a representative DDLS sample (Fig. 2D) based on CD36 and CD34 expression (left) and morphology of sorted CD36+ and CD36- Lipo863 cells upon adherence (right). (B) Separation of cells derived from a Lipo863 mouse xenograft based on CD36 and CD34 expression (left) and morphology of CD36+ and CD36- cells sorted from the mouse Lipo863 tumor upon adherence (right). (C) Paraffin sections of xenografts grown from Lipo863 cells subjected to immunofluorescence with antibodies against CD36 or CD34 (red) and against CD31 (green). Arrows indicate CD36 expression. Nuclei are blue. Scale bar: 50 μm. (D) Tumor growth in mice xenografted with 106 cells of the indicated Lipo863 (B) populations or with 106 tumor-infiltrating mouse leukocytes. (E) Photographs of indicated tumor-bearing mice and resected tumors at week 12 post-grafting.
To test whether expression of CD34 could be re-created in vivo, we injected 106 Lipo863 cells into immunodeficient mice. Cell suspensions were prepared from resultant tumor xenografts and analyzed by flow cytometry. Our data show that both CD36- and CD36+ populations were expanded in tumors; however, the CD34-positive populations could not be regenerated (Fig. 3B). A population with weak level of CD34 signal observed in Lipo863 xenografts (Fig. 3B) has been identified as mouse leukocytes infiltrating the tumor (data not shown). Analysis of tumor sections by immunofluorescence confirmed heterogeneous expression of CD36 and the general lack of CD34 expression in vivo (Fig. 3C). These observations indicate that CD34 expression is not a requisite for the tumorigenicity of liposarcoma cells.
Examination of mouse Lipo863 tumor xenograft cells upon adherence in culture did not reveal noticeable morphological differences between populations sorted as CD36- and CD36+. Both CD36- and CD36+ cells displayed comparable plating efficiency and a phenotype typical of freshly isolated ASC (Fig. 3B). To compare the tumor-initiating capacities of these populations, we performed in vivo limiting dilution experiments (Supplemental Fig. 4A). Both CD36- and C36+ populations produced tumors by 12 weeks upon subcutaneous injection of as few as 104 cells into host immunodeficient mice, while neither population produced tumors upon injection of 103 cells.
We also separately injected matched numbers of CD36- and CD36+ cells freshly sorted from primary Lipo863 xenografts (Fig. 3B) into secondary recipients; mouse leukocyte populations sorted from the primary xenografts were injected as controls. After four weeks, we observed growth of tumors derived from both CD36- and CD36+ cells (Fig. 3D). Tumors reached maximal tolerable size at week 12 and were resected then (Fig. 3E). Analysis of the secondary Lipo863 xenografts by flow cytometry demonstrated that in vivo both CD36- and CD36+ cells regenerated the original heterogeneity of Lipo863 cells. The two distinct populations (CD36- and CD36+) were present in secondary transplants initiated by either CD36- or CD36+ cells, as assessed by flow cytometry (Fig. 4A). Analysis of tumor sections by immunofluorescence also indicated comparable frequencies and distribution patterns for CD36-expressing cells and comparable levels of CD36 expression and morphology of these cells (Fig. 4B). These observations indicate that tumor formation capacity is not related to the expression of CD36 and reveal the immunophenotypic plasticity of populations identified within liposarcomas.
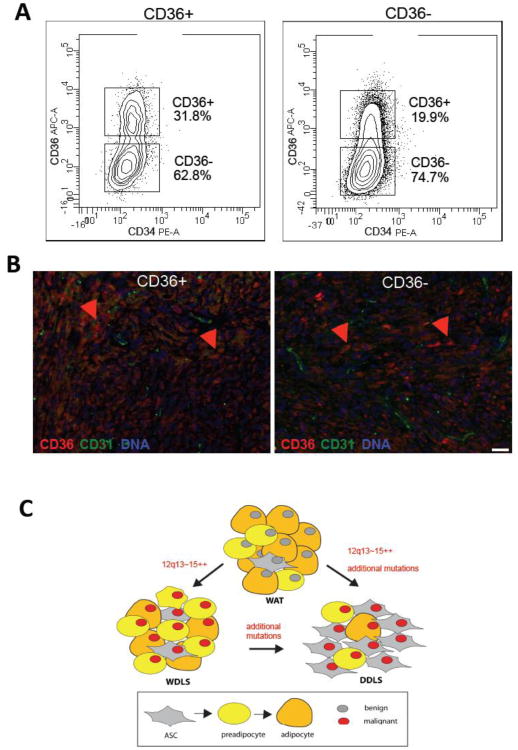
Figure 4.
Plasticity of liposarcoma populations in mouse xenografts. (A) Separation of cells from secondary xenografts of CD36+ (left) and CD36- (right) cells (Figure 3D-E) into CD36+ and CD36- populations. (B) Paraffin sections of xenografts grown from CD36+ cells (left) and CD36- cells (right) subjected to immunofluorescence with antibodies against CD36 (red) indicating comparable distribution of CD36-expressing cells relative to vasculature expressing CD31 (green). Nuclei are blue. Scale bar: 50 μm. (C) A model of liposarcomagenesis. Liposarcoma arises in benign WAT as a result of 12q13-15 amplification and MDM2 overexpression (red nucleus), which occurs at one of the stages of adipogenesis (box). DDLS arise either from WDLS though gradual dedifferentiation of progressive percentage of tumor cells to the ASC-like phenotype or independently of WDLS by separate mutations in WAT leading to a comparatively more aggressive domination of malignant ASC-like cells.
Discussion
The relative rarity of liposarcomas has posed a challenge for investigations of these lethal soft tissue cancers (Kooby et al., 2004; Lahat et al., 2008; Anaya et al., 2009; Gilbert et al., 2009). To begin characterization of their cellular organization, we systematically analyzed multiple WDLS and DDLS surgical specimens. Analysis of freshly isolated human cells based on flow cytometry, FISH, and immunofluorescence microscopy of tissue sections has indicated that malignant cells in both WDLS and DDLS comprise exclusively mesenchymal populations at several distinct stages of adipogenesis. Building on previous reports addressing CD34 and CD36 expression in liposarcomas (Mechtersheimer, 1991), our work provides new information on the distribution of individual cell types distinguished through these markers in WDLS and DDLS. Experiments with Lipo863, the adipogenic DDLS cells with ASC morphology (Peng et al., 2011), demonstrate CD34 and CD36 expression changes in liposarcoma cells upon ex vivo propagation. The capacity of each distinct Lipo863 population identified based on CD36 expression to re-create the initial Lipo863 complexity in the mouse xenograft model indicates immunophenotypic plasticity of malignant liposarcoma cells. This phenomenon may reflect the recently revealed plasticity of benign white adipocytes, which are capable of de-differentiation and trans-differentiation (Cinti, 2011).
Based on our combined data and the frequencies and phenotypes of the individual populations isolated from primary human tumors, we propose a hierarchical model of liposarcoma progression (Fig. 4C). According to this model, malignant transformation of one of the cell populations in WAT, which involves 12q13-15 amplification and concomitant molecular changes, leads to the formation of WDLS or DDLS. We hypothesize that expansion of the malignant liposarcoma populations result in equilibrium of cell phenotypes that differ by the status of differentiation, in which distinct cell populations serve mutually beneficial roles. The observed enrichment of malignant cells with the ASC phenotype in DDLS compared to WDLS could be explained by WDLS progressing to DDLS through progressive transformation of malignant ASC-like cells that eventually over proliferate and dominate. However, it is equally possible that critical additional mutations take place de novo, thus leading to a more aggressive clone forming DDLS with decreased capacity to differentiate. These non-exclusive scenarios may both occur in the clinical context.
In hematological malignancies, cancer-initiating cells have been identified as a population potentially representing a key clinical target (Matsui et al., 2004; Dick and Lapidot, 2005; Gupta et al., 2009). This paradigm, now extended to some solid cancers, is based on the concept that a single immunophenotypic cell population can re-create the diversity of cells in the original tumor upon xenotransplantation (Reya et al., 2001; Tan et al., 2006; Tang, 2012). Previously, ASC have been proposed as the origin of liposarcomas (Gimble et al., 2007). A direct test of this hypothesis is currently not technically feasible due to inability of cells sorted from freshly isolated tumors to survive in immunodeficient mice for reasons that are not clear. While additional studies will be necessary to establish the etiology of liposarcomas, at this juncture our data indicate that malignant populations similar to ASC are indeed present in both WDLS and DDLS. Expression of MSC markers by both tumorigenic Lipo836 populations confirms that aggressive liposarcoma cells are ASC/MSC-like and indicates that CD105 expression is induced in culture. Compared to adenocarcinoma cells, both primary and cultured liposarcoma cells showed low capacity to migrate, invade matrix, and form soft agar colonies (Supplemental Fig. 4) in assays performed as described (Peng et al., 2011). Interestingly, primary cells with the ASC phenotype (CD34bCD36-) sorted from DDLS displayed higher motility than the other three populations (Supplemental Fig. 4C). This migratory capacity, also observed for tumorigenic Lipo863 cells, is consistent with the properties of ASC (Zhang et al., 2009; Zhang et al., 2012). Our data indicating the expression of ΔDCN, the recently described adipose progenitors marker (Daquinag et al., 2011a), by Lipo863 cells and their sensitivity to an experimental ΔDCN-targeting compound (data not shown) support the resemblance between tumorigenic liposarcoma cells and benign adipose progenitors. However, because in our experiments both less and more differentiated cells formed tumors and recreated immunophenotypic heterogeneity, our data does not indicate ASC being the exclusive origin of ‘liposarcoma stem cells’.
Regardless of the tumor cell origin, similarity of the aggressive liposarcoma fraction to ASC has potentially important implications. It has been previously shown that ASC are recruited by malignant lesions and become an important component of the trophic microenvironment in adenocarcinomas (Zhang et al., 2009; Kolonin et al., 2012; Zhang et al., 2012). These supportive cells integrating into tumor stromal and perivascular niches have paracrine mitogenic and cytoprotective tumor-stimulating growth effects. In addition, mesenchymal progenitors, suppressing inflammation and muting the T cell-mediated response, may be at least partially responsible for immune system evasion by the tumor (Kolonin et al., 2012). Finally, MSC in general (and ASC specifically) are resistant to hypoxia, chemotherapy, and radiotherapy, and have been shown to have protective effects on adjacent malignant cells (Bianco et al., 2008; Caplan and Correa, 2011). This opens a possibility that the ASC-like fraction of liposarcoma may not only serve as a cancer-initiating population, but could also confer treatment resistance to other cell populations within WDLS and DDLS.
Conclusions
In summary, our study provides new insights into the cell hierarchy of WDLS and DDLS and helps elucidate the remarkably complex liposarcoma internal milieu. The results of this work may improve our understanding of sarcomagenesis, leading to new approaches to diagnosis, prognosis and treatment. The uncovered plasticity of liposarcoma-initiating cells, previously observed for other cancer types (Tang, 2012), is likely to explain the challenges of pharmacological liposarcoma treatment (Kooby et al., 2004; Lahat et al., 2008; Anaya et al., 2009; Gilbert et al., 2009). Future studies will further dissect the individual roles of the distinct cell types maintained in soft tissue tumors and help generate improved reagents targeting clinically important cell populations.
Materials and methods
Matched pathologically graded tumor samples were used for immunohistological and flow cytometric analysis.
Tissue Section Analysis
Analysis of formalin-fixed paraffin-embedded tissue sections was performed as described (Klopp et al., 2012) after antigen retrieval, washing with 0.2% Triton X-100, blocking in Serum-Free Protein Block (DAKO), followed by incubation with primary antibodies (4°C, 12 h) and secondary antibodies (room temperature, 1 h) in PBS containing 0.05% Tween 20. Primary antibodies: PA1-16813 rabbit anti-CD36 (Pierce), SC-9095 rabbit anti-CD34 (Santa Cruz), and SC-1506 goat anti-CD31 (Santa Cruz). Secondary donkey IgG anti-goat Alexa 488-conjugated was from Invitrogen; anti-rabbit Cy3-conjugated was from Jackson ImmunoResearch. Manufacturer-recommended antibody concentrations were used. Nuclei were stained with Hoechst 33258 (Invitrogen). Images were acquired with Olympus IX70 inverted fluorescence microscope/MagnaFire software.
Cell Phenotyping
Cell suspensions of surgically resected tissues were prepared through collagenase/dispase digestion as described (Zhang et al., 2009; Klopp et al., 2012). Fluorescence-activated cell sorting (FACS) was performed with a FACSAria flow cytometer and the FACSDiva software (BD Bioscience) as described (Bellows et al., 2011a; Bellows et al., 2011b). Cells were gated to exclude cell clumps, contaminating polymorphonuclear cells, red blood cells, platelets, endothelial microparticles, debris, and dead cells based on 7-AAD staining. Viable cells (>200,000 per sample) were then used to enumerate individual populations. Antibodies used along with appropriate isotype control IgGs: fluorescein isothiocyanate-conjugated anti-CD31 (clone WM59, BD Bioscience), phycoerythrin-conjugated anti-CD34 (clone 8G12, BD Bioscience), allophycocyanin-Cy7-conjugated anti-CD45 (clone HI30, BD Bioscience) and eFluor® 660-conjugated anti-CD36 (eBioNL07, Ebioscience). Cells from the sorted populations were immobilized by cytospins and FISH analysis for 12q15 amplification was performed as described (Peng et al., 2011).
Cell Culture
Cells were cultured in DMEM containing 10% fetal bovine serum, L-glutamine and penicillin/streptomycin. After 1 day, non-adherent cells were removed by washing with phosphate-buffered saline (PBS), and adherent cells were quantified and analyzed. For expansion, cells were passaged using 0.25% trypsin/0.1% EDTA at densities not exceeding 5,000 cells/cm2.
In vivo Studies
As described previously (Peng et al., 2011), 6-8 week-old NOD scid gamma (NSG) mice (Jackson Laboratory, USA) were injected subcutaneously with indicated numbers of cells in 150 μl of PBS. Tumor size was measured with a caliper and volume was calculated as length × width2 × 0.52. Once tumors surpassed 1 cm3 in size, mice were euthanized and tumors were resected.
Statistical Analysis was performed with unpaired Student's t test.
Supplementary Material
Highlights.
Malignant cells in liposarcomas can be classified into distinct populations
Populations are conserved in well and poorly differentiated liposarcoma
Populations of liposarcoma cells correspond to stages of adipogenesis
Liposarcoma cells are tumorigenic regardless of their immunophenotype
Xenografted liposarcoma cells display immunophenotypic plasticity
Acknowledgments
Grant support for D. Lev was provided in part from NIH/NCI RO1CA138345, an NCI Cancer Center Support Grant CA#16672, a Liddy Shriver Foundation Seed Grant and the Amschwand foundation. Support for M. Kolonin was provided in part from the American Cancer Society Research Scholar Grant CNE-119003. We thank Theresa Nguyen for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anaya DA, Lahat G, Wang X, Xiao L, Tuvin D, Pisters PW, Lev DC, Pollock RE. Establishing prognosis in retroperitoneal sarcoma: a new histology-based paradigm. Ann Surg Oncol. 2009;16:667–675. doi: 10.1245/s10434-008-0250-2. [DOI] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol Biomarkers Prev. 2011a;20:2461–2468. doi: 10.1158/1055-9965.EPI-11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG. Influence of BMI on level of circulating progenitor cells. Obesity. 2011b;19:1722–1726. doi: 10.1038/oby.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Ann Med. 2011;43:104–115. doi: 10.3109/07853890.2010.535557. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The Tunica Adventitia of Human Arteries and Veins as a Source of Mesenchymal Stem Cells. Stem Cells Dev. 2011;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011a;9:74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci. 2011b;32:300–307. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Dick JE, Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int J Hematol. 2005;82:389–396. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- Festy F, Hoareau L, Bes-Houtmann S, Pequin AM, Gonthier MP, Munstun A, Hoarau JJ, Cesari M, Roche R. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol. 2005;124:113–121. doi: 10.1007/s00418-005-0014-z. [DOI] [PubMed] [Google Scholar]
- Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40–47. doi: 10.5435/00124635-200901000-00006. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf JP, Parmley D, Kleinfeld AM. Free fatty acid transport across adipocytes is mediated by an unknown membrane protein pump. Am J Physiol Endocrinol Metab. 2007;293:1207–1214. doi: 10.1152/ajpendo.00259.2007. [DOI] [PubMed] [Google Scholar]
- Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, Woodward W, Schmandt R, Broaddus R, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin MG, Evans KW, Mani SA, Gomer RH. Alternative origins of stroma in normal organs and disease. Stem Cell Res. 2012;8:312–323. doi: 10.1016/j.scr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooby DA, Antonescu CR, Brennan MF, Singer S. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2004;11:78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15:1585–1593. doi: 10.1245/s10434-007-9805-x. [DOI] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushansky I, Hernando E, Socci ND, Matos T, Mills J, Edgar MA, Schwartz GK, Singer S, Cordon-Cardo C, Maki RG. A developmental model of sarcomagenesis defines a differentiation-based classification for liposarcomas. Am J Pathol. 2008;172:1069–1080. doi: 10.2353/ajpath.2008.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer G. Towards the phenotyping of soft tissue tumours by cell surface molecules. Virchows Arch A Pathol Anat Histopathol. 1991;419:7–28. doi: 10.1007/BF01600148. [DOI] [PubMed] [Google Scholar]
- Morozov A, Downey RJ, Healey J, Moreira AL, Lou E, Franceschino A, Dogan Y, Leung R, Edgar M, LaQuaglia M, et al. Benign mesenchymal stromal cells in human sarcomas. Clin Cancer Res. 2010;16:5630–5640. doi: 10.1158/1078-0432.CCR-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Zhang P, Liu J, Nguyen T, Bolshakov S, Belousov R, Young ED, Wang X, Brewer K, Lopez-Terrada DH, et al. An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors. Lab Invest. 2011;91:392–403. doi: 10.1038/labinvest.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BT, Park CY, Ailles LE, Weissman IL. The cancer stem cell hypothesis: a work in progress. Lab Invest. 2006;86:1203–1207. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev D, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A Population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Tseng WW, Demicco EG, Lazar AJ, Lev DC, Pollock RE. Lymphocyte Composition and Distribution in Inflammatory, Well-differentiated Retroperitonea Liposarcoma: Clues to a Potential Adaptive Immune Response and Therapeutic Implications. Am J Surg Pathol. 2012;36:941–944. doi: 10.1097/PAS.0b013e31824f2594. [DOI] [PubMed] [Google Scholar]
- Weaver J, Goldblum JR, Turner S, Tubbs RR, Wang WL, Lazar AJ, Rubin BP. Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol. 2009;22:66–70. doi: 10.1038/modpathol.2008.153. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Daquinag A, Traktuev DO, Amaya F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin, MG White adipose tissue cells are recruited by experimenta tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.