Abstract
Objective
Anti-vascular endothelial growth factor therapies have revolutionized the treatment of clinically significant diabetic macular (CSDME); yet these agents are expensive, and whether they are cost-effective is unclear. The purpose of this study is to determine the most cost-effective treatment option for patients with newly diagnosed CSDME: focal laser photocoagulation alone (L), focal laser plus intravitreal ranibizumab (L+R), focal laser plus intravitreal bevacizumab (L+B), or focal laser plus intravitreal triamcinolone (L+T) injections.
Design
Cost effectiveness analysis
Participants
Hypothetical cohort of 57 year old patients with newly-diagnosed CSDME.
Methods
Using a Markov model with a 25-year time horizon, we compared the incremental cost-effectiveness of treating patients with newly-diagnosed CSDME using L, L+R, L+B, or L+T. Data came from the DRCRnet randomized controlled trial, the Medicare Fee Schedule, and the medical literature.
Main Outcome Measures
Costs, quality-adjusted life years (QALYs), and incremental costs per QALY gained.
Results
Compared with L, the incremental cost-effectiveness of L+R and L+B were $89,903/QALY and $11,138/QALY, respectively. L+T was dominated by L. A probabilistic sensitivity analysis demonstrated, at a willingness-to-pay (WTP) of $50,000/QALY, that L was approximately 70% likely to be the preferred therapy over L+R and L+T. However, at a WTP of $100,000/QALY, more than 90% of the time, L+R therapy was the preferred therapy, compared with L and L+T. In the probabilistic sensitivity analysis, L+B was found to be the preferred therapy over L and L+T for any WTP value above $10,000/QALY. Sensitivity analyses revealed that the annual risk of cerebrovascular accident would have to be at least 1.5% higher with L+B than with L+R for L+R to be the preferred treatment. In another sensitivity analysis, if patients require < 8 injections per year over the remainder of the 25-year time horizon, L+B would cost less than $100,000/QALY, whereas L+R would be cost-effective at a WTP of $100,000/QALY if patients require fewer than 0.45 injections per year after year 2.
Conclusion
With bevacizumab and ranibizumab assumed to have equivalent effectiveness and similar safety profiles when used in the management of CSDME, bevacizumab therapy confers the greatest value among the different treatment options for CSDME.
Diabetes mellitus is a major public health problem, affecting 8% of the United States (U.S.) population. An estimated 300 million persons will have this condition by 2025.1 Clinically significant diabetic macular edema (CSDME) is a common microvascular complication of diabetes, affecting 18% of patients with diabetes mellitus for more than 10 years.2 CSDME is also a major cause of visual impairment, with a 25-year mortality-adjusted cumulative incidence of blindness of 9.5%.3 Given the impact of CSDME on visual acuity, it is unsurprising that this ocular condition can profoundly affect patients’ health-related quality of life (HRQL).4–7
For many years, the conventional first-line treatment for CSDME has been focal argon laser photocoagulation (FALP). FALP works by selectively coagulating leaky retinal blood vessels. In 1985, the landmark Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that patients who underwent FALP were 50% less likely than untreated patients to experience moderate vision loss.8, 9 In recent years, new treatment options have become available for CSDME. Anti-vascular endothelial growth factor (anti-VEGF) agents, including ranibizumab (Lucentis, Genentech/Roche) and bevacizumab (Avastin, Genentech/Roche), are antibodies or antibody fragments that bind and block VEGF. These medications can decrease foveal thickness caused by CSDME and improve best-corrected visual acuity (BCVA). For example, in the Ranibizumab for Edema of the Macula in Diabetes-2 trial, which compared 126 eyes randomly assigned to ranibizumab alone, FALP alone, or both interventions, BCVA showed improvement at more than 6 months’ follow-up in approximately one-quarter of those receiving ranibizumab, compared with no eyes in the FALP-only group.10, 11 In another trial, involving 854 eyes with CSDME, 28–30% of eyes receiving bevacizumab had significantly improved BCVA after 1 year of follow-up, compared with only 15% of those randomized to FALP.12 Although these findings suggest that anti-VEGF agents may be a better alternative to conventional FALP, successfully resolving CSDME or preventing recurrence often requires multiple anti-VEGF injections. Such repeated injections can be costly and carry a small, albeit real risk of sight-threatening complications (e.g., endophthalmitis).
Another relatively new CSDME treatment is intravitreal corticosteroid therapy. Corticosteroids are theorized to reduce CSDME by inhibiting VEGF-induced fluid leakage from retinal vessels. Studies have demonstrated CSDME resolution and significant BCVA improvement among eyes receiving intravitreal corticosteroids.13, 14 Potential downsides to intravitreal corticosteroid use include the need for repeated injections and the risk for complications, such as cataract or glaucoma development.
In 2000 Sharma and colleagues found FALP to be highly cost-effective for CSDME, at $3,101 per quality-adjusted life-year (QALY).15 We know of only one cost-effectiveness analysis comparing the newer CSDME treatment modalities—a study sponsored by Genentech/Roche, the manufacturer of ranibizumab and bevacizumab.16 Considering the high prevalence of CSDME , the questionable improvements in BCVA with relatively high costs associated with certain interventions, the risks of side effects, and many patients’ need for multiple interventions, a well-designed cost-effectiveness analysis would substantially aid clinicians managing patients with CSDME and health policymakers looking to identify treatments that confer the greatest societal value. In July 2011 the National Institute for Clinical Excellence (NICE) in the United Kingdom decided not to endorse ranibizumab as a reimbursable treatment for CSDME in the National Health Service, bringing this issue front and center.17 Given that more than $1.6 billion is spent annually on ranibizumab therapy for retinal diseases18 and that the cost per injection of ranibizumab is 7 times greater than that of bevacizumab, a rigorous cost-effectiveness analysis would be important to policymakers seeking cost savings to the U.S. health care system.
In this study, we compared the cost-effectiveness of several different treatment options for patients with newly diagnosed diabetic macular edema.
Methods
Study Design
We developed a Markov model to capture the total costs and HRQL for patients with newly diagnosed CSDME under four treatments: focal laser photocoagulation alone (L), focal laser plus intravitreal triamcinolone injections (L+T), and intravitreal ranibizumab injections with immediate (L+R) or delayed (DL+R) focal laser photocoagulation. In a sensitivity analysis, we also explored two additional interventions: intravitreal bevacizumab with immediate (L+B) or delayed (DL+B) focal laser photocoagulation. The model followed a hypothetical cohort of patients aged 57 years (the mean age for CSDME onset)19 with CSDME over a 25-year time horizon (the approximate life expectancy for 57-year-old patients with diabetes mellitus).20 Markov modeling is a standard method used in general health technology assessments21–23 and also has been used in prior cost-effectiveness analyses for CSDME.16, 24, 25
Health States
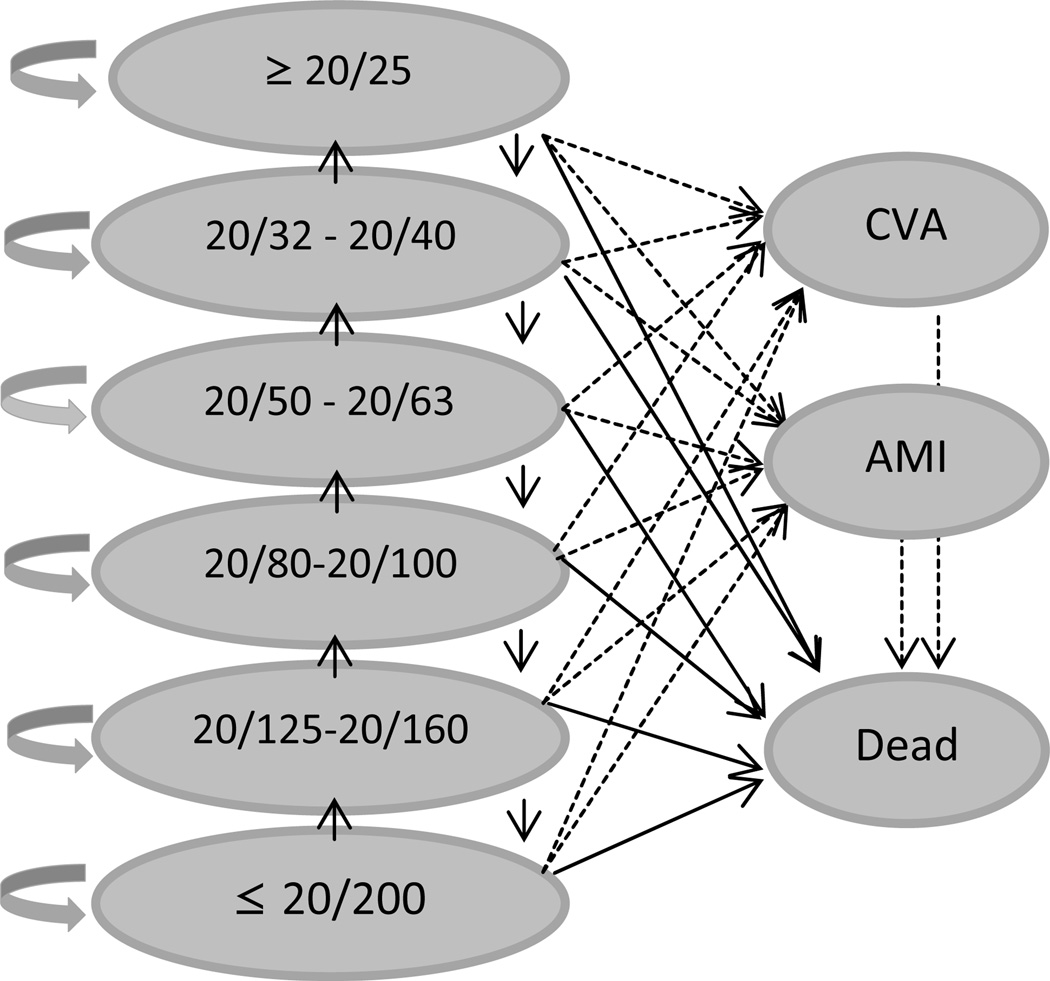
We followed patients through health states based on BCVA levels (Figure 1). In the sensitivity analysis, we also included health states associated with rare but serious systemic side effects from some of these interventions, including cerebrovascular accident (CVA), acute myocardial infarction(AMI), and death.
Figure 1.
Markov states of visual acuity and health
Circles represent levels of visual acuity and arrows represent possible annual changes in vision. Dotted lines represent secondary analysis including CVA and AMI outcomes.
AMI = acute myocardial infarction; CVA = cerebrovascular accident
Progression Rates
Vision in each intervention group followed the observed BCVAs from the DRCRnet trial at years 1 and 2 (Table 1, available at http://aaojournal.org).26, 27 Since, to our knowledge, no study to date has reported the natural history of treated or untreated CSDME beyond 2–3 years, we evaluated BCVAs in the longer term using several different scenarios. In our baseline model, we assumed that the distribution of BCVA from the DRCRnet trial did not change after year 2 for all treatment groups. In sensitivity analyses, we allowed the BCVA of patients in each treatment group to decline each year. In analyses with bevacizumab, we assumed the efficacy was equivalent to ranibizumab (except in selected sensitivity analyses where we simultaneously varied the efficacy of each agent). In sensitivity analysis, we also tracked CVA and AMI. Data on the proportions of patients experiencing CVA and AMI under each intervention were obtained from the DRCRnet trial. Once a patient experienced CVA or AMI, they experienced increased costs, lower health-related quality of life, and higher mortality for the remainder of their lifetimes28 (Figure 1). In addition, we incorporated age-adjusted mortality from U.S. life tables using the methods of Javitt and Aiello to capture the increased mortality for persons with diabetic retinopathy.25
Costs
Direct medical costs of managing CSDME were based on office-based CMS allowables29 for 2011 in Michigan and included costs of eye-care provider visits, ancillary testing (optical coherence tomography (OCT) and intravenous fluorescein angiography (IVFA)) to evaluate for and quantify the amount of CSDME present), costs of each intervention, costs of treating side effects caused by the interventions, and costs associated with blindness when BCVA remained ≤20/200 (Table 2). For pharmaceuticals administered in the office, such as the triamcinolone, bevacizumab, and ranibizumab, the cost included the drug cost, professional fee, and facility fee reimbursed by Medicare in 2011. The cost of all drugs paid for outside of the office setting was calculated based on Red Book costs from 2005 and adjusted for inflation to meet 2011 expenses.30 The number of office visits, injections, and laser treatments for each therapeutic regimen came directly from the DRCRNet trial. More details on the costs of the interventions and side effects can be found in Appendix 1 (available at http://aaojournal.org).
Table 2.
Costs and utilities Included in Markov Model
| Parameter Value | Value (2011 USD) | Reference |
|---|---|---|
| Costs (2011 USD) | ||
| Visits and diagnostic testing | ||
| Initial office visit | 236 | CPT 99204 |
| Subsequent office visits | 181 | CPT 99214 |
| Optical coherence tomography | 73 | CPT 92134 |
| Fluorescein angiography | 254 | CPT 99235 |
| Interventions | ||
| Laser photocoagulation | 1093 | CPT 67220 |
| Intravitreal ranibizumab | 2337 | CPT 67028 |
| Intravitreal bevacizumab | 348 | CPT 67028 |
| Intravitreal triamcinolone | 479 | CPT 67028 |
| Costs of Managing Sequelae | ||
| Cataract surgery* | 2763 | CPT 66984 |
| Glaucoma Drainage Device with SPG | 6532 | CPT 66180 |
| Medical glaucoma therapy† | 40 | |
| Retinal detachment repair‡ | 4996 | CPT 67040 |
| Endophthalmitis‡ | 4179 | CPT 67015/67028 |
| Vitreous hemorrhage‡ | 4868 | CPT67036 |
| Blindness | 2784 | Frick32 |
| Utilities | ||
| Health States | Brown31 | |
| ≥20/25 | 0.92 | Brown31 |
| 20/32–20/40 | 0.82 | Brown31 |
| 20/50–20/63 | 0.77 | Brown31 |
| 20/80–20/100 | 0.67 | Brown31 |
| 20/125–20/160 | 0.66 | Brown31 |
| ≤20/200 | 0.60 | Brown31 |
| Short-term Side Effects (QALYs lost)^ | ||
| Cataract surgery | −0.00 | |
| Endophthalmitis | −0.1 | Aaberg33 |
| Glaucoma surgery | −0.05 | Stein34 |
| PPV | −0.05 | Zou35 |
| Retinal detachment | −0.05 | Zou35 |
| Vitreous hemorrhage | −0.05 | Okamoto36 |
| Long-term Side Effects (annual utility)♦ | ||
| CVA | 0.39 | Freeman28 |
| AMI | 0.84 | Freeman28 |
| Glaucoma (medical) | −0.05 | Stein34 |
AMI = myocardial infarction; CVA = cerebrovascular accident; CPT = Current Procedural Terminology; QALY = quality-adjusted life years; PPV = pars plana vitrectomy; SPG = scleral patch graft; USD = United States dollars
includes cost of topical antibiotics and corticosteroids;
monthly cost;
includes cost of topical antibiotics, corticosteroids and cycloplegics;
Short term side effects affected patients only during the first year after receipt of the intervention;
Long term side effects affected patients for the remainder of time they cycled through the model.
Utilities
The main value of treating CSDME comes from the quality-of-life gained by improving or maintaining BCVA. We measured this quality of life using a QALY so that these results could be comparable with interventions for other diseases. Health-related quality of life or “utility” is quantified as a value from 1.00 (perfect health) to 0.00 (death). We incorporated utility scores for each level of BCVA as captured by Brown and colleagues. These scores range from 0.97 for 20/20 BCVA to 0.60 for 20/200 BCVA.31 Since CSDME affects the macula and often spares the peripheral retina, it is uncommon for patients to experience BCVA < 20/200 from CSDME alone. Table 2 shows the utility scores obtained from the literature for complications of the various interventions and utility scores for AMI, CVA, and death.28, 31–36 These parameters were also varied in sensitivity analyses.
All costs were in 2011 United States dollars (USD). Costs and health utilities were discounted at 3% per year and interventions a and b were compared to each other by using an incremental cost-effectiveness ratio (ICER) or Net Monetary Benefit (NMB) defined as:
ICER = (TCa - TCb) / (Ea – Eb)
NMBa = WTP * Ea - TCa
where TC is the total cost, E is effectiveness measured in QALY, WTP is willingness to pay for a QALY, and intervention a is the intervention of interest and intervention b is a lower-cost undominated alternative intervention.37 We used TreeAge Pro 2011 Health Care (TreeAge Software, Williamstown, MA) to calculate and compare costs and health effects of each of the interventions.
Sensitivity Analyses
We performed sensitivity analyses on the estimates of costs, utilities, and health state transitions. One-way sensitivity analyses were performed on all parameters to determine which parameters had the largest impact on results. We also conducted several two-way sensitivity analyses and examined a scenario using bevacizumab instead of ranibizumab as the anti-VEGF therapy. Finally, we conducted a probabilistic sensitivity analysis using Monte Carlo simulation of all input assumptions simultaneously and created cost-effectiveness acceptability curves to determine how robust the results were to changes in all parameters and how likely each therapy was to be the most cost-effective option.38
Results
Base Model (with ranibizumab)
Over 25 years, the expected costs for a single patient with newly diagnosed CSDME receiving L, L+R, DL+R, and L+T were $20,013, $58,257, $61,424, and $23,877, respectively, and the QALYs for a patient receiving these treatments were 10.41, 10.83, 10.99, and 9.54, respectively. Laser only was the least expensive option, but it also had lower health outcomes than ranibizumab therapy. The ICER of DL+R over L was $71,271/QALY, and L dominated L+T, meaning L+T was more costly and less effective. In this base-case analysis, the ICER of L+R over L was $89 ,903/QALY, and L+R provided fewer QALYs than DL+R at a higher cost per QALY (Table 3).
Table 3.
Incremental Cost Effectiveness of the Different Therapies for Diabetic Macular Edema
| Base model (using ranibizumab) | |||
|---|---|---|---|
| Therapy | Cost (USD) | QALYs | ICER |
| Laser alone | 20013 | 10.41 | Lowest cost* |
| L+T | 23877 | 9.54 | *** |
| L+R | 58257 | 10.83 | 89903** |
| DL+R | 61424 | 10.99 | 71271 |
| Base model (using bevacizumab) | |||
| Therapy | Cost (USD) | QALYs | ICER |
| Laser alone | 20013 | 10.41 | Lowest cost* |
| L+T | 23877 | 9.54 | *** |
| L+B | 27200 | 10.83 | *** |
| DL+B | 26485 | 10.99 | 11138 |
| Including CVA and AMI outcomes | |||
| Therapy | Cost (USD) | QALYs | ICER |
| Laser alone | 65603 | 10.15 | 39306** |
| L+T | 39829 | 9.49 | Lowest cost* |
| L+R | 73257 | 10.73 | 26912** |
| DL+R | 76387 | 10.88 | 26251 |
| Including CVA and AMI outcomes | |||
| Therapy | Cost (USD) | QALYs | ICER |
| Laser alone | 65603 | 10.15 | *** |
| L+T | 39829 | 9.49 | Lowest Cost* |
| L+B | 42391 | 10.73 | *** |
| DL+B | 41663 | 10.88 | 1317 |
intervention had the lowest costs so other interventions are measured compared to it. The lowest-cost intervention will not have an ICER
Dominated by extended dominance, meaning that the delayed laser strategy offers more health benefits at a lower cost per QALY
Dominated by strict dominance, meaning that another strategy has both more health benefits and a lower cost
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; L+T = laser + intravitreal triamcinolone group; L+R = laser + ranibizumab group; L+B= laser + bevacizumab group; DL+R = delayed laser + ranibizumab group; DL+B = delayed laser + bevacizumab group; CVA = cerebrovascular disease; AMI = acute myocardial infraction; USD = United States dollars
Base Model (with bevacizumab)
The 25-year costs for a patient with newly diagnosed CSDME receiving L, L+B, DL+B, and L+T were $20,013, $27,200, $26,485, and $23,877, respectively, and a patient receiving each of these therapy options would accrue 10.41, 10.83, 10.99, and 9.54 QALYs, respectively. The ICER of DL+B over L was $11,138/QALY, and L dominated L+T. L+B provided fewer QALYs at a higher cost per QALY than DL+B (Table 3).
Sensitivity Analyses
We performed several sensitivity analyses to examine the impact of changes to model assumptions.
Including side effects of CVA and AMI (with ranibizumab)
Including side effects substantially increased overall costs and lowered overall health outcomes. Since CVA rates in the laser-only arm were high in the DRCRnet (6% versus 2% in the other groups), the laser-only therapy looked more expensive with poorer HRQL. In this scenario L+T had the lowest cost and the ICER of DL+R versus L+T looked more favorable, at $26,251/QALY (Table 3).
Including side effects of CVA and AMI (with bevacizumab)
L+T still had the lowest cost and effectiveness and DL+B still had the highest cost and effectiveness, but the ICER of DL+B versus L+T was only $1,317/ QALY (Table 3). Since the DRCRnet study was not adequately powered to detect differences in CVA among the groups and the actual difference in CVA risk between bevacizumab and ranibizumab is unknown, we performed an additional sensitivity analysis to determine the difference in proportions of CVAs that would alter the preferred treatment option among these interventions. Figure 2 (available at http://aaojournal.org) shows which therapy would be preferred (maximizes health outcomes minus costs) under different assumptions of CVA risk if the decision maker values health outcomes at $50,000/QALY. At $348 per bevacizumab injection, if greater than 4% of patients developed a CVA from bevacizumab during each of the first two years, L+B would not be cost-effective at a WTP of $50,000/QALY. Likewise, at $2,337 per injection of ranibizumab, if more than 2% of patients developed a CVA from the injection, then L+R loses its status as the preferred treatment alternative, at a WTP of $50,000/QALY. The annual risk of cerebrovascular accident would have to be at least 1.5% higher with L+B than with L+R for L+R to be the preferred treatment. Figure 3 (available at http://aaojournal.org) shows qualitatively similar results for a WTP of $100,000.
Treatment of Chronic or Recurrent CSDME
In a sensitivity analysis, we explored the need for continued injections of ranibizumab or bevacizumab after year 2 for those patients who may require persistent treatment or retreatment for chronic or recurrent CSDME. If patients require fewer than 8 injections per year over the remainder of the 25-year time horizon, then L+B would cost less than $100,000/QALY, whereas if fewer than 3.5 injections were required each year, then L+B would cost less than $50,000/QALY. L+R would be cost-effective (at a WTP of $100,000/QALY) if patients require less than 0.45 injections per year after year 2.
Varying cost of anti-VEGF injections / number of injections
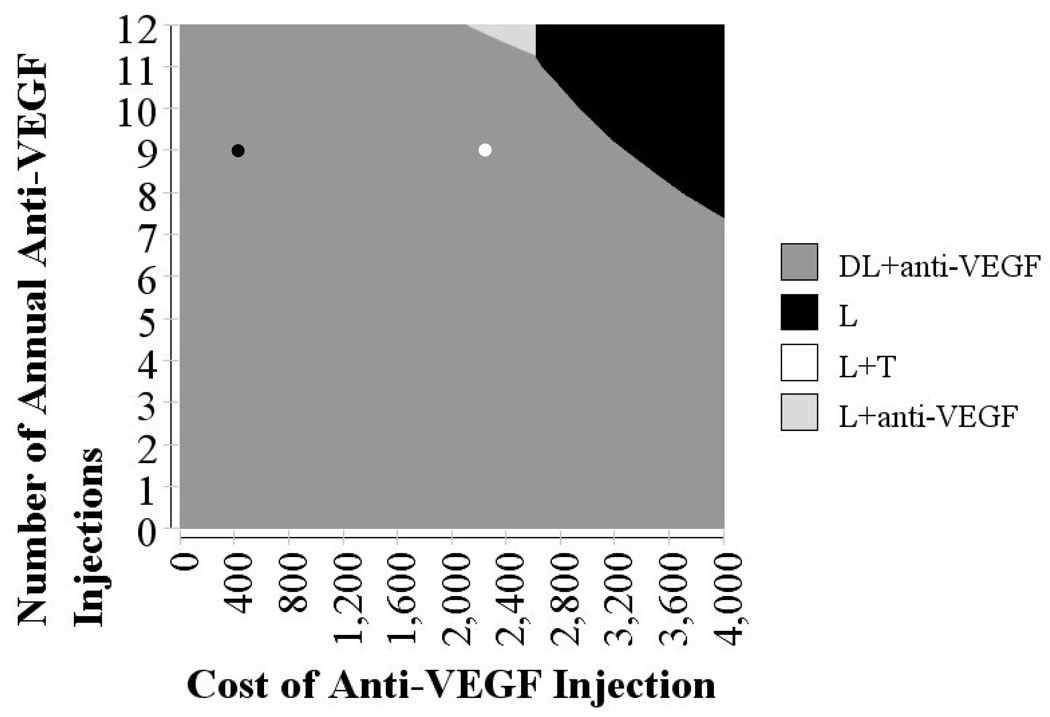
In a two-way sensitivity analysis, we simultaneously varied the cost per injection of ranibizumab and bevacizumab and the number of injections per year (during the first 2 years of the treatment period) to determine the net benefit, assuming WTP amounts of $50,000/QALY and $100,000/QALY, respectively (Figure 4, available at http://aaojournal.org, and Figure 5, respectively). At a WTP of $50,000/QALY, using ranibizumab ($2,337 per injection), a patient would need to have fewer than 7 total injections for this to be the preferable treatment option. However, using bevacizumab ($348), even if a patient has 12 injections per year during each of the first 2 years, this would be the preferred treatment option over ranibizumab. At a WTP of $100,000/QALY, using ranibizumab with 12 injections per year during the first 2 years would still be cost-effective.
Figure 5.
Sensitivity analysis varying the number of anti-VEGF injections per year and cost of each injection using a willingness-to-pay of $100,000
Base cost of ranibizumab and number of injections shown in white dot. Base cost of bevacizumab and number of injections shown in black dot.
L = laser photocoagulation only; L+T = laser photocoagulation plus intravitreal triamcinolone; L + anti-VEGF = laser photocoagulation along with an anti-VEGF agent; DL + anti-VEGF = delayed laser photocoagulation along with an anti-VEGF agent; VEGF = vascular endothelial factor
This figure shows shaded regions that represent which therapy choice is the most cost-effective for different assumptions of number of injections and costs of injections with anti-VEGF therapies. Health benefits are valued at $100,000.
Varying effectiveness of bevacizumab / ranibizumab
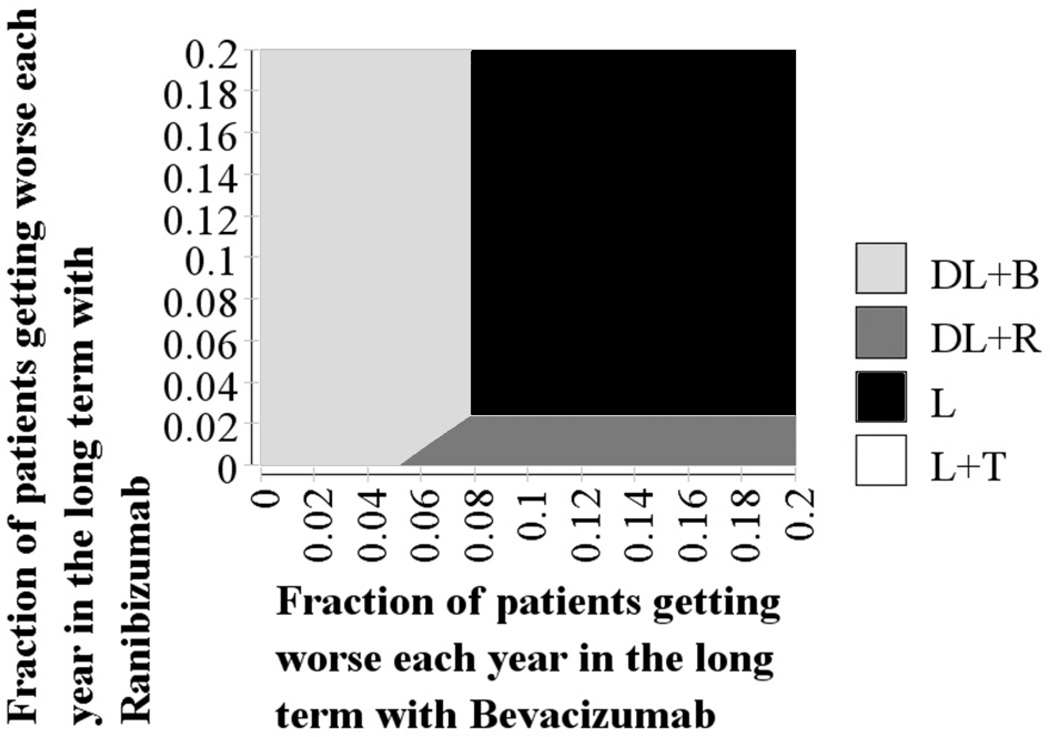
In a two-way sensitivity analysis, we simultaneously varied the effectiveness of bevacizumab and ranibizumab to determine the extent by which differences in effectiveness affect the cost-effectiveness of these interventions relative to one another. To capture the effectiveness of these interventions, we allowed for different proportions of patients treated with each anti-VEGF to experience worsening of BCVA over time. As Figure 6 demonstrates, if there is no loss of effectiveness with either intervention over time, bevacizumab would be the preferred therapy. If 6–8% or more of the patients treated with bevacizumab had worsening of BCVA and less than 2% of those treated with ranibizumab experienced a decline in BCVA over the long term, then ranibizumab would become the preferred treatment.
Figure 6.
Two-way sensitivity analysis varying the proportion of patients experiencing worsening of CSDME with ranibizumab and bevacizumab using a willingness-to-pay of $100,000
The base case assumes that patients continue with the same vision after two years. In this sensitivity analysis, we allow for patients to experience worsening vision over time. With no worsening of vision, bevacizumab would be preferred (light gray region). If 8% or more of patients treated with bevacizumab had worsening vision each year in the long term (such that they would drop down a vision “category”), then it would no longer be preferred. Ranibizumab was the preferred therapy if 2% or fewer of patients had worsening vision each year and if 6–8% of bevacizumab patients had worsening vision each year (dark gray region). In this graph, it assumes that laser therapy has no loss in vision in the long term.
L = laser photocoagulation only; L+T = laser + intravitreal triamcinolone group; DL+R = delayed laser + ranibizumab group; DL+B = delayed laser + bevacizumab group; CSDME = clinically significant diabetic macular edema
We performed sensitivity analyses varying several other model parameters. These sensitivity analyses explored the impact of varying life expectancy (Figures 7 and 8, available at http://aaojournal.org), age at CSDME onset (Figures 9 and 10, available at http://aaojournal.org), and stability of BCVA during follow-up (Figures 11 and 12, available at http://aaojournal.org). Table 4, available at http://aaojournal.org, shows results of another sensitivity analysis in which we assumed that all patients entered the model as already pseudophakic.
Probabilistic Sensitivity Analysis
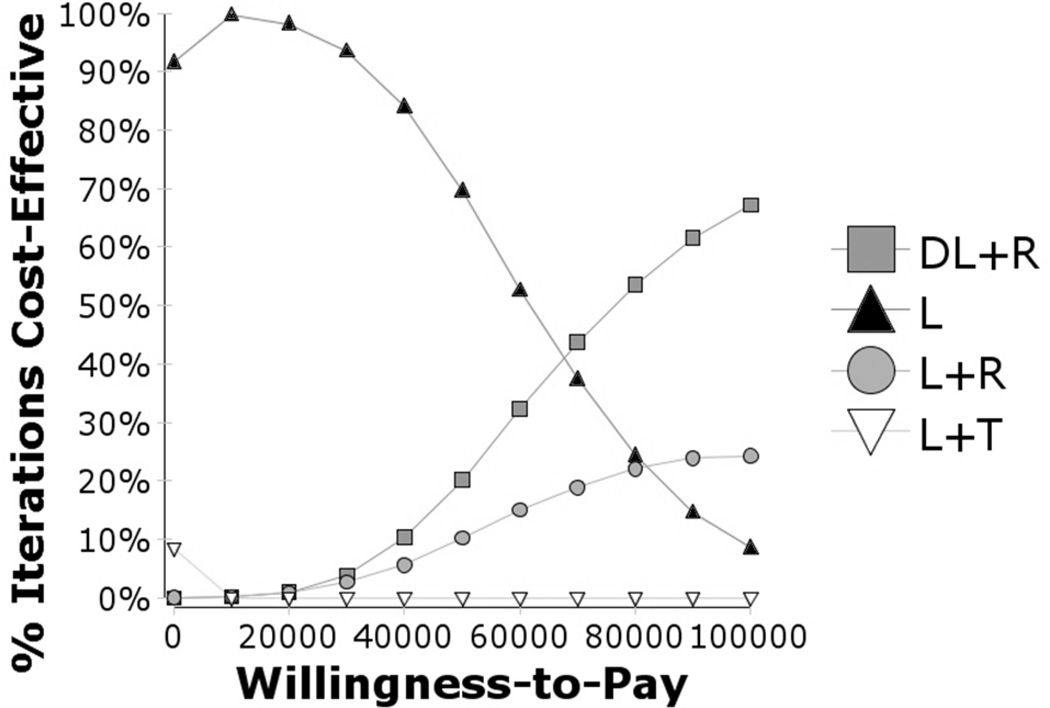
In the first probabilistic sensitivity analysis, using ranibizumab (Figure 13), we found that L would be the preferred therapy for lower WTP amounts and ranibizumab with laser (L+R or DL+R) would be preferred with higher WTP levels. Here, L+T was unlikely to be the preferred therapy irrespective of the WTP level. At a WTP of $50,000/QALY, L was almost 70% likely to be the preferred therapy, and at $100,000/QALY ranibizumab with laser (L+R or DL+R) was preferred more than 90% of the time. At higher WTP amounts, there is still substantial uncertainty about whether having immediate or delayed laser therapy with ranibizumab would be better, because the DRCRNet trial results on which this analysis is based were inconclusive.
Figure 13.
Cost effectiveness acceptability curves, ranibizumab vs. other treatments for CSDME
Figure 13 shows the cost-effectiveness acceptability curves for ranibizumab therapy. Ranibizumab therapy is about 30% likely to be cost-effective at a willingness-to-pay of $50,000/QALY (20% DL+R, plus 10% L+R) and about 90% likely to be cost-effective at a willingness-to-pay of $100,000 (67% DL+R, plus 24% L+R). Although it appears delayed laser therapy is best with the anti-VEGF therapy, there still is a reasonable chance that it is best to immediately have laser therapy with the anti-VEGF therapy. Triamcinolone is very unlikely to be cost-effective regardless of the willingness-to-pay.
L = laser photocoagulation only; L+T = laser + intravitreal triamcinolone group; DL+R = delayed laser + ranibizumab group; DL+B = delayed laser + bevicizumab group; L+R = laser + ranibizumab group; CSDME = clinically significant diabetic macular edema
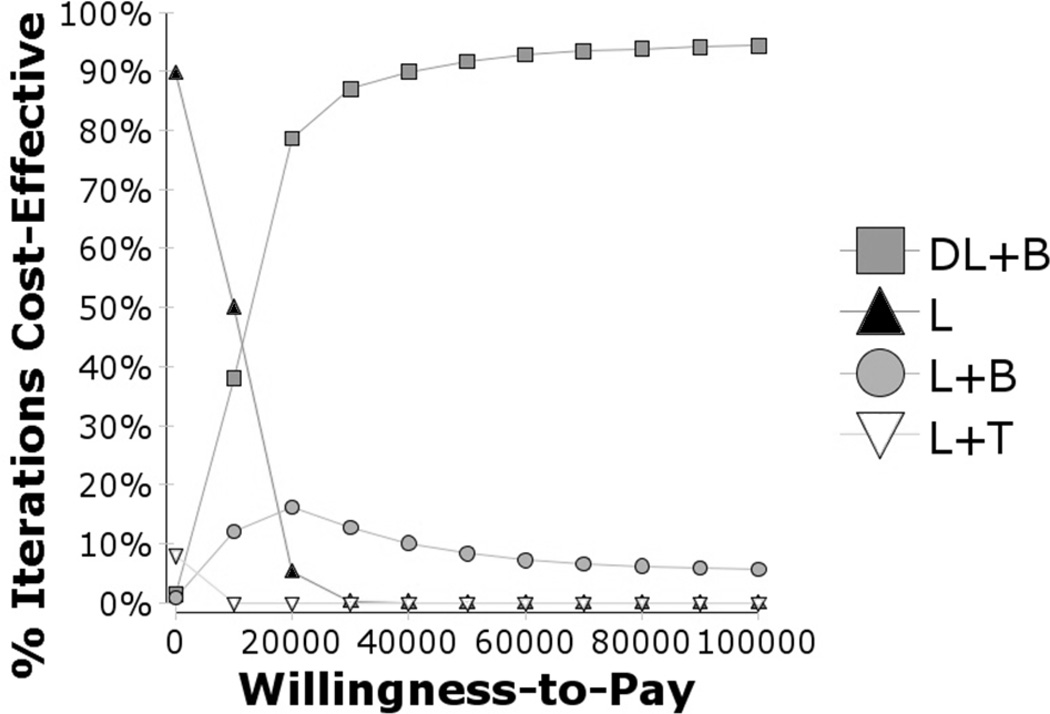
The results from the second probabilistic sensitivity analysis using bevacizumab are similar to the first, but bevacizumab is very likely to be the preferred therapy for any WTP value higher than $10,000/QALY (Figure 14).
Figure 14.
Cost effectiveness acceptability curves, bevacizumab vs. other treatments for CSDME
Figure 14 shows the cost-effectiveness acceptability curves for bevacizumab. Bevacizumab therapy is highly likely to be cost-effective at a willingness-to-pay of above $20,000/QALY. Bevacizumab therapy is about 99% likely to be cost-effective at a willingness-to-pay of $50,000/QALY (91% DL+B, plus 8% L+B). Although it appears delayed laser therapy is best with the anti-VEGF therapy, there still is a reasonable chance (10–20%) that it is best to immediately have laser therapy or with the anti-VEGF therapy regardless of the willingness-to-pay. Triamcinolone is very unlikely to be costeffective regardless of the willingness-to-pay.
L = laser photocoagulation only; L+T = laser + intravitreal triamcinolone group; DL+R = delayed laser + bevacizumab group; DL+B = delayed laser + bevacizumab group; L+B = laser + bevacizumab group
Comment
As health policymakers look to curtail rising health care costs, treatments that confer the greatest relative value need to be identified. Among the various treatment options for CSDME, we find that relative to FALP alone, assuming that ranibizumab and bevacizumab are equally effective in treating CSDME and have equivalent safety profiles, intravitreal ranibizumab is only cost-effective for those who are willing to pay at least $71,271/QALY for this intervention. By comparison, bevacizumab is a cost-effective treatment option at $11,138/QALY. Intravitreal corticosteroids were more costly and less effective than FALP alone. Sensitivity analyses highlight the impact of varying model parameters, including need to treat recurrent or chronic CSDME, number of injections administered, systemic side effects, and patient’s life expectancy on the ICER of the treatment alternatives. Finally, when each parameter was simultaneously varied in a probabilistic sensitivity analysis, ranibizumab is considered cost-effective only at relatively high WTP levels (>$100,000/QALY), whereas bevacizumab confers the greatest value at almost all WTP levels.
To our knowledge, only one other cost-effectiveness analysis has evaluated these newer treatments for CSDME. In an industry-sponsored study comparing the cost-effectiveness of ranibizumab with that of intravitreal corticosteroids using data from the DRCRnet trial,16 Dewan and colleagues found that ranibizumab met acceptable cost-effectiveness standards relative to intravitreal corticosteroids for phakic patients (those without previous cataract surgery), and intravitreal corticosteroids were the most cost-effective treatment option for pseudophakic patients (those who had undergone cataract surgery). Bevacizumab was not considered in any of their analyses. Although that study and ours used similar data sources, direct comparison of the two studies is challenging. Our study uses QALYs to compare the different interventions, whereas theirs uses cost per letter of vision gained. The analysis by Dewan and colleagues assumed that the group treated with ranibizumab maintains their level of BCVA without requiring additional ranibizumab injections beyond year 2. In our analysis, we consider the need for additional treatment beyond year 2 for a subset of patients who develop chronic or recurrent CSDME. Given that ranibizumab injections are costly and that some patients require multiple injections per year, the need for long-term treatment can dramatically affect the incremental cost-effectiveness of this intervention. Unfortunately, little has been documented on the treatment of recurrent or persistent CSDME with anti-VEGF agents beyond 2–3 years.
An interesting finding from our analysis is the impact of using bevacizumab instead of ranibizumab in the model. Bevacizumab, which has not been submitted to the FDA for approval consideration, is used off label by providers to treat CSDME because it is considerably cheaper than ranibizumab ($348 vs. $2,337 per injection) and is assumed to have similar efficacy, although no trial has directly compared these interventions. Given similar effectiveness, the price differential between these two anti-VEGF agents can dramatically affect the incremental cost-effectiveness, as observed in our analysis. Genentech (South San Francisco, CA), the manufacturer of both agents, contends that providers should use ranibizumab instead of bevacizumab because of concerns about an increased risk for serious side effects with bevacizumab. The evidence for an elevated risk of side effects comes from comparisons of systemic use, not intravitreal injection, of these agents to treat patients with colon and gastric cancers.39, 40 Recent studies have demonstrated that serum levels of VEGF in patients with exudative macular degeneration may differ between ranibizumab users and bevacizumab users. Carneiro and colleagues found that prior to injection of 3 rounds of ranibizumab or bevacizumab, serum concentrations of each VEGF were similar among a group of patients with exudative AMD; however, after 3 months of injections, VEGF levels in the bevacizumab-treated patients were significantly lower than those in the patients receiving ranibizumab.41 This research suggests that bevacizumab may have more effects on the cardiovascular system than ranibizumab does. Although clinical trials comparing the effectiveness and safety of bevacizumab with ranibizumab are ongoing, we are unaware of any study adequately powered to directly compare rates of these uncommon but serious side effects. Nevertheless, because these side effects are associated with significant morbidity and mortality, we explored the impact on varying CVA rates on the ICER in a sensitivity analysis. We found that the annual risk for CVA would need to be at least 1.5% greater (1–2 more individuals developing CVA per 100 receiving injections) among patients receiving bevacizumab relative to ranibizumab for ranibizumab to be the more cost-effective option.
No universally agreed-on cutoff exists to determine which treatments are cost-effective, but researchers have suggested that we, in the U.S., should be willing to spend $100,000 or more.42 In Great Britain, NICE recently decided not to endorse ranibizumab for the treatment of CSDME because it did not meet their established cost-effectiveness threshold of £20,000–£30,000 ($31,500–$47,000 USD).43
Our study has several limitations. The DRCRnet trial only captured level of effectiveness, need for additional interventions, and side effects over 2 years’ duration. Extrapolating the findings of this trial beyond year 2 can be challenging because little is known about the longer-term natural history of CSDME among patients receiving these particular interventions. While sensitivity analyses were performed to address the uncertainty of the various model parameters beyond year 2, if these model inputs varied beyond these ranges, this could impact our findings. Second, the DRCRnet trial included only patients who physicians thought would benefit from laser treatment, and clinical trials participants may differ systematically from other patients in their health behavior, which could affect the generalizability of the findings to other groups. Another limitation is an assumption we made that BCVA is an acceptable surrogate for the impact of CSDME on overall HRQL. Visual needs vary from patient to patient, and different levels of BCVA could affect the overall HRQL of patients differently. Unfortunately, the DRCRnet trial collected no additional information on HRQL that we could incorporate into our models.
In conclusion, assuming bevacizumab and ranibizumab have equivalent effectiveness and a similar safety profile in the management of CSDME, we find that intravitreal bevacizumab confers the greatest value among the treatment options compared in our study. Intravitreal ranibizumab may be a reasonable alternative if bevacizumab is unavailable or if payers are willing to spend more than $71,000/QALY. Intravitreal triamcinolone confers the least value of the therapeutic options examined, mainly because of its side effects and the costs of managing them. Insurers and health policymakers should consider endorsing the use of intravitreal bevacizumab over other treatment options as first-line therapy for CSDME, as this may curtail some of the rapidly rising costs of managing patients with this condition.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01); Grant Number P30DK092926 from the National Institute of Diabetes and Digestive and Kidney Diseases; and an unrestricted grant from Research to Prevent Blindness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no proprietary or commercial interest in any material discussed in this manuscript
This article contains online-only material. We suggest that the following elements be made available online only: Table 1, Table 4, Appendix 1, Figure 2, Figure 3, Figure 4, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Isaman DJ, Messinger S, et al. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes Care. 2005;28:2856–2863. doi: 10.2337/diacare.28.12.2856. [DOI] [PubMed] [Google Scholar]
- 3.Grauslund J, Green A, Sjølie AK. Blindness in a 25-year follow-up of a population-based cohort of Danish type 1 diabetic patients. Ophthalmology. 2009;116:2170–2174. doi: 10.1016/j.ophtha.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Oliver-Fernandez A, Liu W, et al. The impact of diabetic retinopathy on health-related quality of life. Curr Opin Ophthalmol. 2005;16:155–159. doi: 10.1097/01.icu.0000161227.21797.3d. [DOI] [PubMed] [Google Scholar]
- 5.Scanlon PH, Martin ML, Bailey C, et al. Reported symptoms and quality-of-life impacts in patients having laser treatment for sight-threatening diabetic retinopathy. Diabet Med. 2006;23:60–66. doi: 10.1111/j.1464-5491.2005.01736.x. [DOI] [PubMed] [Google Scholar]
- 6.Hariprasad SM, Mieler WF, Grassi M, et al. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92:89–92. doi: 10.1136/bjo.2007.122416. [DOI] [PubMed] [Google Scholar]
- 7.Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010;26:1587–1597. doi: 10.1185/03007995.2010.482503. [DOI] [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report no. 4. Int Ophthalmol Clin. 1987;27(4):265–272. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–969. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen QD, Shah SM, Heier J, et al. READ-2 Study Group. Two-year outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology. 2010;117:2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas JB, Kreissig I, Söfker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 14.Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Brown GC, Brown MM, et al. The cost-effectiveness of grid laser photocoagulation for the treatment of diabetic macular edema: results of a patient-based cost-utility analysis. Curr Opin Ophthalmol. 2000;11:175–179. doi: 10.1097/00055735-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Dewan V, Lambert D, Edler J, et al. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2012;119:1679–1684. doi: 10.1016/j.ophtha.2012.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence. NICE unable to recommend drug to treat diabetic macular oedema. [Accessed March 12, 2012];2011 Jul 14; Available at: http://www.nice.org.uk/newsroom/pressreleases/RanibizumabForD MOFinalDraftGuidance.jsp.
- 18.Dooren JC, Whalen J. Study Compares Lucentis, Avastin. [Accessed May 27, 2012];The Wall Street Journal. 2011 Apr 28; Available at: http://online.wsj.com/article/SB10001424052748704463804576291572903925578.html. [Google Scholar]
- 19.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 20.Arias E. United States life tables, 2007. [Accessed November 10, 2012];Natl Vital Stat Rep. 2011 59(9):1–60. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_09.pdf. [PubMed] [Google Scholar]
- 21.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford: Oxford University Press; 2005. pp. 277–322. [Google Scholar]
- 22.Muennig P. Cost-Effectiveness Analysis in Health: A Practical Approach. 2nd ed. San Francisco, CA: Jossey-Bass; 2008. pp. 75–103. [Google Scholar]
- 23.Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. pp. 15–76. [Google Scholar]
- 24.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283:889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 25.Javitt JC, Aiello LP, Chiang Y, et al. Preventive eye care in people with diabetes is cost-saving to the federal government: implications for health-care reform. Diabetes Care. 1994;17:909–917. doi: 10.2337/diacare.17.8.909. [DOI] [PubMed] [Google Scholar]
- 26.Elman MJ, Aiello LP, Beck RW, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elman MJ, Bressler NM, Qin H, et al. Expanded 2- year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 29.U.S Department of Health and Human Services. [Accessed May 19, 2012];Average Medicare Fee Schedule [database online] 2011 Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSc hed/
- 30.Red Book. Montvale, NJ: Thomson Healthcare; 2008. pp. 1–1012. [Google Scholar]
- 31.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–223. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 32.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125:544–550. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 33.Aaberg TM, Jr, Flynn HW, Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperataive endophthalmitis surgery. A 10 year review of incidence and outcomes. Ophthalmology. 1998;105:1004–1010. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 34.Stein JD, Kim DD, Peck WW, et al. Cost-effectiveness of medication compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol. 2012;130:497–505. doi: 10.1001/archophthalmol.2011.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou H, Zhang X, Xu X, et al. Utility value and retinal detachment surgery [letter] Ophthalmology. 2011;118:601. doi: 10.1016/j.ophtha.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto F, Okamoto Y, Fukuda S, et al. Vision-related quality of life and visual function following vitrectomy for proliferative diabetic retinopathy. Arch Ophthalmol. 2008;145:1031–1036. doi: 10.1016/j.ajo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Torrance GW, Siegel JE, Luce BR. Framing and Designing the Cost-Effectiveness Analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. 54–81. [Google Scholar]
- 38.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 39.Kabbinavar F, Hurwitz H, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 40.Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: high incidence in patients receiving irinotecan- and bevacizumab-based therapy [letter] J Clin Oncol. 2005;23:2574–2576. doi: 10.1200/JCO.2005.81.908. [DOI] [PubMed] [Google Scholar]
- 41.Carneiro AM, Costa R, Falcão MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab [report online] Acta Ophthalmol. 2012;90:e25–e30. doi: 10.1111/j.1755-3768.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- 42.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 43.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–744. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.