Abstract
INTRODUCTION
Dietary fats must be digested into fatty acids and monoacylglycerols prior to absorption. In adults, colipase-dependent pancreatic triglyceride lipase (PTL) contributes significantly to fat digestion. In newborn rodents and humans, the pancreas expresses low levels of PTL. In rodents, a homologue of PTL, pancreatic lipase related protein 2 (PLRP2) and carboxyl ester lipase (CEL) compensate for the lack of PTL. In human newborns, the role for PLRP2 in dietary fat digestion is unclear. To clarify the potential of human PLRP2 to influence dietary fat digestion in newborns, we determined PLRP2 activity against human milk and infant formula.
METHODS
The activity of purified recombinant PLRP2, gastric lipase and CEL against fats in human milk and formula was measured with each lipase alone and in combination with a standard pH-stat assay.
RESULTS
Colipase added to human milk stimulated fat digestion. PLRP2 and CEL had activity against human milk and formula. Pre-digestion with gastric lipase increased PLRP2 activity against both substrates. Together, CEL and PLRP2 activity was additive with formula and synergistic with human milk.
CONCLUSIONS
PLRP2 can digest fats in human milk and formula. PLRP2 acts in concert with CEL and gastric lipase to digest fats in human milk in vitro.
Background
The digestion of dietary fats into fatty acids and monoacylglycerols is essential for efficient absorption of dietary fat by enterocytes. Digestion of dietary fats, mainly long-chain triglycerides, is accomplished by a variety of lipases (1). In adults, digestion begins in the stomach where gastric lipase releases 15–20% of fatty acids from dietary fat (2). The partially digested fats are released into the duodenum and mixed with bile salts and pancreatic lipases. Colipase-dependent pancreatic triglyceride lipase (PTL) and other pancreatic lipases complete digestion in the duodenum. Of these, PTL is the predominant.
In contrast to its pivotal role in adults, PTL does not contribute significantly to dietary fat digestion in newborns (3). In rodents, mRNA encoding PTL is undetectable at birth (4). Another pancreatic lipase, pancreatic lipase related protein 2 (PLRP2) compensates for the physiological deficiency of PTL in rodent neonates. mRNA encoding PLRP2 is expressed in the pancreas of rodent newborns (4). Importantly, suckling PLRP2-deficient mice have steatorrhea and poor weight gain (5). These studies provide compelling evidence for the essential role of PLRP2 in fat digestion in rodent neonates.
The evidence in humans is not as complete. Like rodents, mRNA encoding PLRP2 is present at adult levels in the pancreas of human newborns whereas the expression of mRNA encoding PTL is low or undetectable (6). Duodenal levels of lipase are lower in premature and term infants than in adults suggesting PTL secretion is diminished or absent (7–8). Taken together these observations suggest that PLRP2 may have an important role in dietary fat digestion in human newborns.
In this study, we provide additional support for PLRP2 mediating dietary fat digestion in human newborns by demonstrating activity of PLRP2 against physiological substrates present in the newborns’ diet. Previous kinetic studies of human PLRP2 have utilized prepared emulsions of a defined triglyceride and bile salt, a much less complex substrate than naturally occurring substrates ingested by newborns (9–10). A single study demonstrated the ability of PLRP2 to hydrolyze the fat globules from bovine milk, a more complex substrate but one that is not generally present in the newborn diet (11). Herein, we studied the ability of human PLRP2 to hydrolyze dietary fats contained in human breast milk fat globules and in infant formula emulsion particles. Because PLRP2 likely acts in concert with gastric lipase and carboxyl ester lipase (CEL), the lipase found in mother’s milk, we measured activity of PLRP2 alone and in combination with gastric lipase and CEL.
Results
Purification of human colipase, PLRP2 and CEL
Recombinant human proteins for these studies were expressed in Pichia pastoris and purified as described in Methods (10, 12–14). The purity of the proteins was assessed by SDS-PAGE. Each protein migrated as a single, predominant band (Figure 1). Several minor, faster-migrating bands are visible in the PLRP2 preparation. These bands are likely degradation products of PLRP2 since they cross-react with PLRP2 antisera (Data not shown).
Figure 1.
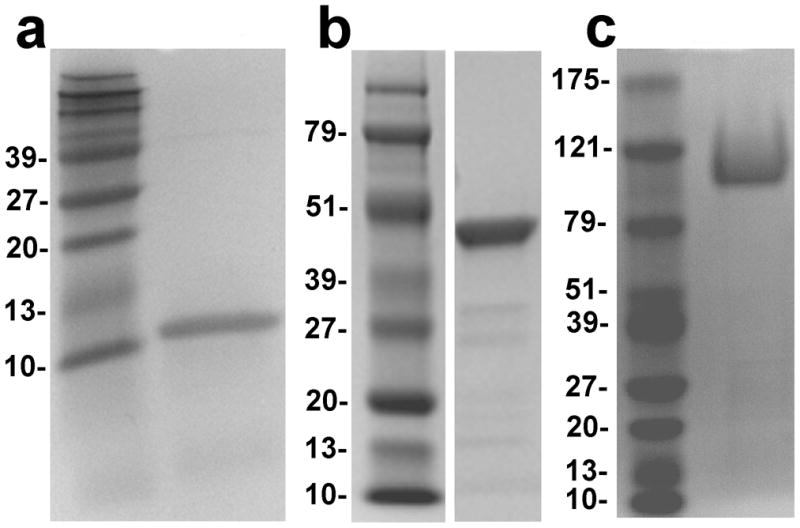
SDS-polyacrylamide gel electrophoresis of the recombinant proteins. The GelCode Blue stained gels are shown. (a) Human colipase on an 18% gel; (b) Human PLRP2 on a 10% gel; (c) Human CEL on a 7.5% gel. Molecular weight markers are included in each panel and the size in kDal is given on the right edge of each panel.
Effect of bile salt activation and freeze-thaw on lipase activity in human milk
To confirm that the mixture of bile salts activated CEL in human milk, we examined the effect of the bile salt mixture on the release of fatty acids using frozen human milk from several different donors. A 4 mM concentration of physiologic bile salts stimulated lipolysis at least 10-fold (P<0.001) (Figure 2a). The level of bile-salt-stimulated activity varied in human milk from individual donors and even with a single donor (Samples 3, 4 and 5). Next, we determined if freeze-thaw affected the lipase activity of human milk. Milk subjected to a single freeze-thaw cycle was compared with fresh milk from the same donor. Fresh human milk had significantly higher activity than did thawed frozen milk from the same donor presumably because freeze-thaw inactivated endogenous milk lipases (Figure 2b). For the rest of our studies we utilized either frozen or fresh human milk from a single donor.
Figure 2.
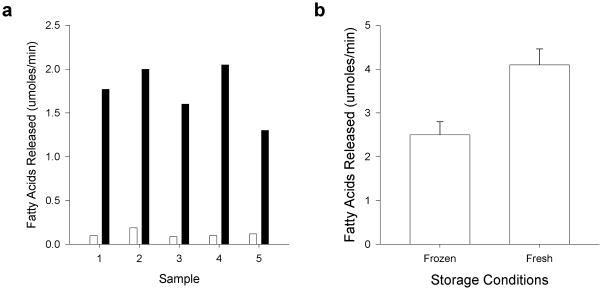
Activity of human milk lipases in the presence of bile salts and after freeze-thaw. (a) The release of fatty acids from human milk was measured in the pH-stat with and without a mixture of physiological bile salts as described in Methods. Frozen human milk was obtained from three separate donors. Sample 1 = donor 1; Sample 2 = donor 2; Samples 3, 4 and 5 = donor 3. Black bars, no bile salts; Grey bars, plus bile salts. The difference of the means of no bile salts and plus bile salts for all samples was significant, P<0.001. (b) The release of fatty acids from frozen milk stored less than one week and from fresh human milk. The milk was from donor 3. The assay was done with a mixture of physiological bile salts (4 mM) in the pH-stat as described in Methods. The difference between the means was significant, P = 0.001.
Effect of colipase and PLRP2 on the release of fatty acids from fresh human milk
We next determined if colipase and PLRP2 alone or in combination had any effect on the lipase activity in frozen human milk. When colipase was added to the incubation there was a 22% increase over no colipase with 5 μg of colipase (P = 0.05) and a 48% increase with 25 μg colipase (P = 0.002) (Figure 3a). The 20% difference between 5 and 25 μg was also significant (P = 0.039) indicating a concentration dependence of the colipase effect. When we added 25 μg of PLRP2 without colipase to frozen human milk, the activity (2.4 ± 0.2 μmole fatty acid/min) was not a significant difference in fatty acids released compared to milk alone (2.4 ± 0.2 μmole fatty acid/min). When both colipase and PLRP2 were added in a 5 to 1 molar ratio of colipase to PLRP2, the activity was higher than the activity of human milk alone (P = 0.006 for 15 μg sample versus milk alone) (Figure 3b). The difference between the assays with the combination and the assays with colipase alone was significant for the 10 μg and 15 μg samples.
Figure 3.
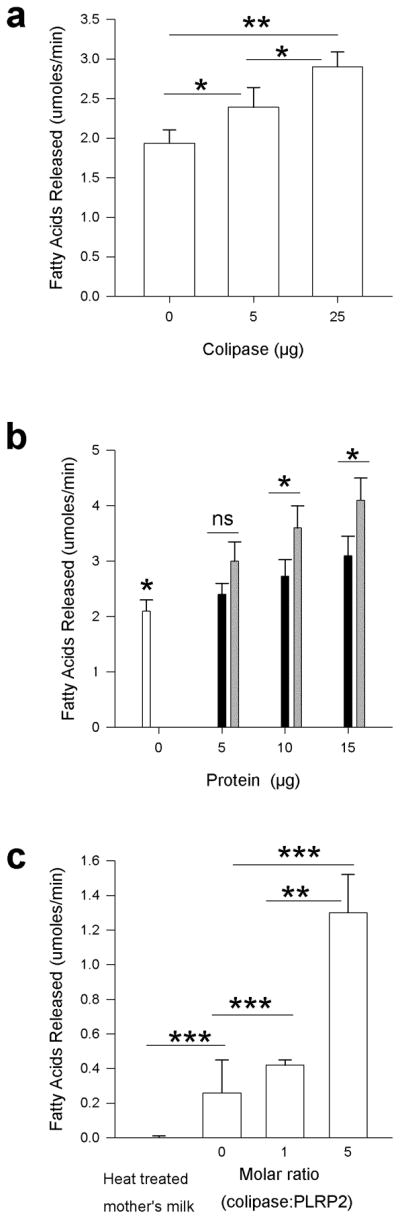
The influence of colipase and PLRP2 on lipolysis of human milk fats in the presence of bile salts. In each experiment activity was measured in the pH-stat in the presence of 4 mM bile salts as described in Methods. The P-value is given for the pair-wise comparisons between the different groups. (a) The release of fatty acids from frozen human milk after addition of various amounts of human colipase. *P≤0.05, **P≤0.01 (Student’s t-test). (b) Release of fatty acids from frozen human milk by the combination of PLRP2 and colipase at various concentrations. The amount of colipase or PLRP2 or both that was added to the incubation is indicated by the x-axis. White bar, human milk alone; Black bar, colipase alone; Gray bar, colipase and PLRP2; the same mass (μg) of colipase and PLRP2 were added as indicated. This resulted in a 5-fold molar ratio of colipase to PLRP2. *P≤0.05 (Student’s t-test). (c) Release of fatty acids from heat-treated human milk by 25 μg of human PLRP2 in the presence of a physiological mixture of bile salts (4 mM) and various concentrations of colipase. **P≤0.01; ***P≤0.001 (Student’s t-test).
To more clearly determine if PLRP2 hydrolyzes fats in human milk, we heat-treated fresh human milk to inactivate endogenous CEL. Heat treatment eliminated the activity from endogenous CEL even in the presence of colipase (Figure 3c). The release of fatty acids from heat-treated human milk was significantly increased by incubation with PLRP2 (P <0.001). The addition of colipase to the incubation increased the activity at both a 1:1 and 5:1 molar ratio to PLRP2 (P < 0.001 for each). The specific activities (U/mg) for 0, 1 and 5-fold molar ratio of colipase to PLRP2 were 8, 12, and 56, respectively.
Release of fatty acids from human milk by various lipases alone and in combination
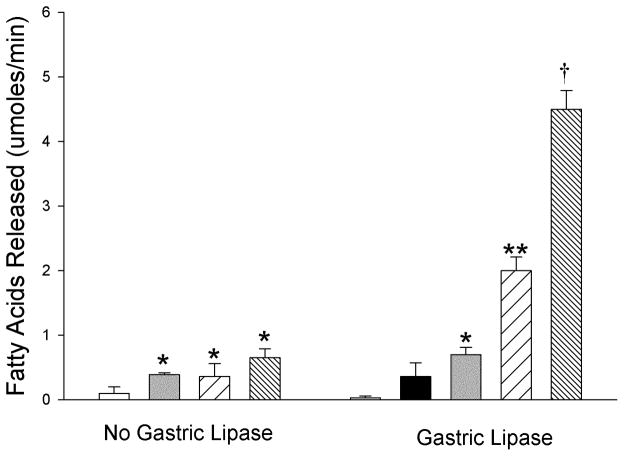
To determine if there was a synergistic effect of the individual lipases potentially involved in dietary fat digestion, we performed assays on heat-treated human milk with gastric lipase, CEL and PLRP2 alone or in combination in the presence of colipase and bile salts. We first tested the activity of CEL and PLRP2 alone and in combination (Figure 4). In each case, the addition of either lipase increased lipolysis compared to human milk alone. There was no difference in activity between CEL and PLRP2. The combination of CEL and PLRP2 did not have a significant additive or synergistic effect on the release of fatty acids.
Figure 4.
The dependence of human milk fat digestion on the combined action of gastric lipase, CEL and PLRP2. In each case, 25 μg of each lipase and 5 μg of colipase were added to the incubation except that no colipase was added during the pre-incubation with gastric lipase. The assays were done on heat-treated human milk with a physiological mixture of bile salts (4 mM). The values are the mean ± SD (n = 3). (No gastric lipase) White bar, human milk; Gray bar, CEL; Coarse hatched bar, PLRP2; Fine hatched bar CEL & PLRP2. There is a statistically significant difference among the mean values of the different conditions as determined by one-way ANOVA (F(3,8)= 12.63, P = 0.002). *P < 0.05 PLRP2 (0.36 ± 0.2) CEL (0.39 ± 0.03) and CEL & PLRP2 (0.65 ± 0.1) compared to mother’s milk by the Holms-Sidak post-hoc pairwise multiple comparisons method. (Gastric lipase) White bar, human milk; Black bar, gastric lipase; Gray bar, CEL; Coarse hatched bar, PLRP2; Fine hatched bar, CEL & PLRP2. There is a statistically significant difference among the mean values of the different conditions as determined by one-way ANOVA (F(4,10)= 270.15, P = <0.001). *P < 0.05 CEL (0.7 ± 0.1) compared to mother’s milk (0.03 ± 0.03), **P < 0.05 PLRP2 (2.0 ± 0.2) compared with CEL, gastric lipase and mother’s milk, †P < 0.05 CEL & PLRP2 (4.5 ± 0.3) compared with PLRP2, CEL, gastric lipase and mother’s milk by the Holms-Sidak post-hoc pairwise multiple comparisons method.
To determine if pretreatment with gastric lipase increased the activity of the pancreatic lipases, we repeated the assays after a 20 min pre-incubation with gastric lipase at pH 6.0 as described in the Methods (Figure 4). After the incubation with gastric lipase the pH was raised to 8.0 with NaOH. The mean activity in the sample containing only gastric lipase was not statistically different from the activity in heat-treated human milk alone. Adding CEL to the incubation increased the mean activity slightly. In contrast, adding PLRP2 to the incubation increased the average activity about 6-fold over gastric lipase alone. The average activity of the combination of CEL and PLRP2 was about 13-fold higher than the average activity for gastric lipase alone. The increase was greater than the combined activity of CEL and PLRP2 alone suggesting there is synergy in the activity of these two lipases after gastric lipase has acted on the substrate.
Release of fatty acids from formula by various lipases alone and in combination
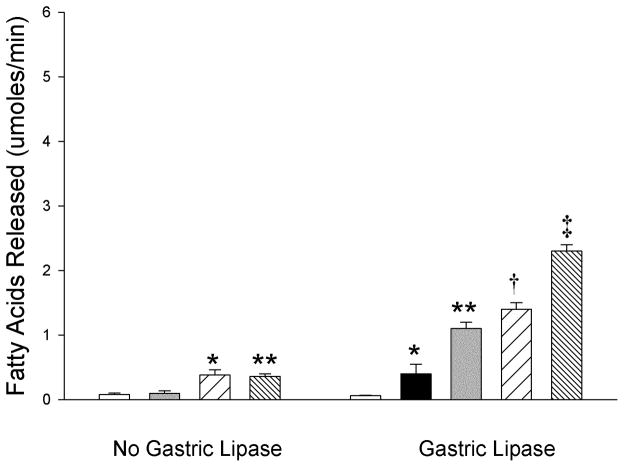
As formula is commonly used to substitute mother’s breast milk during infancy, we next investigated whether CEL and PLRP2 had activity against the lipids in an infant formula utilizing the same experimental design described above (Figure 5). Although the formula was brought to pH 8.0 prior to the start of monitoring in the pH-stat, the background was higher than with human milk. The background activity was not eliminated by heat treatment of the formula (Data not shown). Without prior digestion by gastric lipase, the activity of CEL was not statistically different from formula alone. In contrast, PLRP2 had low but detectable activity. The combination of CEL and PLRP2 had the same activity as PLRP2 alone. Preincubation with gastric lipase had a significant effect on the ability of both CEL and PLRP2 to digest the lipids in formula. CEL and PLRP2 had comparable activity when added alone and there was an additive and not a synergistic effect of incubating with both lipases.
Figure 5.
The dependence of formula fat digestion on the combined action of gastric lipase, CEL and PLRP2. In each case, 25 μg of each lipase and 5 μg of colipase were added to the incubation except that no colipase was added during the pre-incubation with gastric lipase. The assays were done on a human formula, Similac Advance 20, with a physiological mixture of bile salts (4 mM). The values are the mean ± SD (n = 3). (No gastric lipase) White bar, formula; Gray bar, CEL; Coarse hatched bar, PLRP2; Fine hatched bar CEL & PLRP2. There is a statistically significant difference among the mean values of the different conditions as determined by one-way ANOVA (F(3,8)= 32.05, P <0.001). *P < 0.05 PLRP2 (0.38 ± 0.08) compared with formula (0.08 ± 0.02) and CEL (0.1 ± 0.04), **P < 0.05 CEL & PLRP2 (0.36 ± 0.04) compared with formula and CEL by the Holms-Sidak post-hoc pairwise multiple comparisons method. (Gastric lipase) White bar, formula; Black bar, gastric lipase; Gray bar, CEL; Coarse hatched bar, PLRP2; Fine hatched bar, CEL & PLRP2. There is a statistically significant difference among the mean values of the different conditions as determined by one-way ANOVA (F(4,10)= 220..03, P <0.001). *P < 0.05 gastric lipase (0.4 ± 0.2) compared to formula (0.06 ± 0.01), **P < 0.05 CEL (1.1 ± 0.1) compared with gastric lipase and formula, †P < 0.05 PLRP2 (1.4 ± 0.1) compared with CEL, gastric lipase and formula, ‡P < 0.05 CEL & PLRP2 (2.3 ± 0.2) compared with PLRP2, CEL, gastric lipase and formula by the Holms-Sidak post-hoc pairwise multiple comparisons method.
Discussion
Our results show that human PLRP2 is able to hydrolyze the fats in human milk and in formula. Furthermore, the activity was increased by colipase. These results agree with the previously reported finding that PLRP2 released fatty acids from heat-treated or homogenized bovine milk and that colipase stimulated the activity (11). Although not surprising, the similar activity against human and bovine milk could not have been predicted with certainty. The composition of milk fat globules differs in protein and fat content. Even though the proteins in the milk of both species are highly conserved there are qualitative and quantitative differences in protein content (15). Importantly, there is variation in milk fat content. Compared to bovine milk, human milk has more 18:1 and 18:2 fatty acids, lower saturated fatty acids, much lower amounts of 4:0 to 8:0 fatty acids and more long-chain polyunsaturated fatty acids (16). The differences in lipid and protein content could influence substrate availability or the physical properties of the fat globule, either of which could affect PLRP2 activity.
The activity of PLRP2 is further enhanced by predigestion of both human milk and infant formula lipids by gastric lipase. In the absence of gastric lipase predigestion, the activity of both CEL and PLRP2 alone or in combination was much lower than after predigestion with gastric lipase. Interestingly, the activity of CEL against infant formula lipids was stimulated 11-fold by gastric lipase predigestion and only 5-fold with human milk fats as the substrate. The activity of PLRP2 was stimulated to the same extent by predigestion with gastric lipase regardless of the substrate. The stimulation of CEL activity by limited digestion with gastric lipase confirms early findings (17). This is the initial report to show that predigestion with gastric lipase increases activity of PLRP2. Presumably, the effect of gastric lipase is to produce fatty acids. Multiple studies have demonstrated that addition of fatty acids to the substrate initiates lipolysis by PTL (17–20). Similarly, adding fatty acids to bovine fat globules or to emulsions of a single triglyceride with bile salts activate the activity of human PLRP2 (10–11). These observations support the hypothesis that gastric lipase initiates PLRP2 activity by generating fatty acids.
When CEL and PLRP2 were both added to human milk after predigestion with gastric lipase, the activity was greater than the sum of the individual activities, suggesting that the two enzymes work synergistically when digesting human milk. A similar result was reported for the digestion of an artificial substrate, triolein, by the combination of CEL and PLRP2 using cellular uptake by Caco-2 cells as a read out (21). A slightly different effect was found with formula as the substrate. The combination of CEL and PLRP2 was not synergistic. Differences in the physicochemical properties of lipid globules in human milk and formula may explain this observation (22–24). Whether the differences in lipolysis of human milk and formula have a significant effect on fat digestion in vivo cannot be addressed by in vitro assays. Premature and Term infants fed formula have slightly lower coefficients of fat absorption than infants fed human milk, but the reason for the difference is not clear (3).
The observation that colipase stimulated lipase activity of human milk above the levels achieved by bile salts alone was unexpected. That colipase did not stimulate fatty acid release in heat-treated milk suggests that it affects the activity of CEL or lipoprotein lipase. Colipase is not predicted to interact with either lipase in the way that it interacts with PTL. CEL does not contain the β-sandwich sheet carboxyl terminal domain that is critical for the interaction of colipase with pancreatic lipases. The observation raises the possibility that colipase has another role in the digestion of dietary fats. In monolayer systems, colipase binds to the surface and alters the lateral distribution of phospholipids and diacylglycerols in the monolayer (25). Perhaps, colipase has surface-active properties that alter the physical aspects of the milk fat globules in a way that improves the activity of lipases. Intriguingly, this observation raises the possibility that colipase stimulates PLRP2 activity through effects on the substrate rather than by anchoring PLRP2 to the substrate interface as proposed for the colipase-PTL complex. This could explain the findings that PLRP2 lipases have activity in the absence of colipase and colipase stimulates that activity to varying degrees depending on the substrate and PLRP2 species (26).
Although the results demonstrate that PLRP2 can hydrolyze fats in human milk, extrapolation to in vivo hydrolysis of human milk is not directly possible from this study. It is difficult to recreate in vivo conditions with an in vitro system and these experiments were not intended to do so. Whereas the pH of 6.0 chosen for gastric lipase incubations is reasonably close to the gastric pH after ingestion of a meal, the pH of 8.0 for the CEL and PLRP2 assays is higher than the pH in the duodenum after a meal which ranges between 6.0 and 6.5 in adults and may be lower in preterm and term infants (2, 27–28). The higher pH was used to increase the sensitivity of the assay with long-chain triglycerides (29). The concentration of bile salts used in the assay is in the range reported for premature and term infants but some infants have concentrations lower than 4 mM (30). Importantly, the amount of each lipase in the gastric or duodenal contents in human newborns is not known with certainty. One study measured a range of 5μg/ml to 80 of gastric lipase in the stomach of premature infants (31). A second study reported of 2 to 4 μg/ml (32). The amount of gastric lipase we added to the assays (1.6 μg/ml) was slightly lower than the reported range. There are no reports of PLRP2 levels in human newborns. A recent study estimated the content of PLRP2 in pancreatic secretions of human adults (around 200 ng/ml) was lower than the concentration of PLRP2 included in our assays (1.6 μg/ml) (33). The concentration we utilized is identical to the concentration of PLRP2 used in the study on bovine milk (11). There are also no reliable estimates of CEL concentrations in the duodenal contents of human newborns. Similarly, the concentration of colipase in the pancreatic secretions of human infants is not known; it is likely that colipase is secreted in excess of PLRP2. In human adults, colipase and PTL are present in a 1:1 molar ratio and the concentration of PLRP2 is about 4-fold lower (27). Thus, the 5-fold molar ratio of colipase to PLRP2 in this study seems reasonable. Lastly, proteases are present in the stomach and duodenum during digestion and proteolysis may influence lipolysis. Even with these caveats, our results are consistent with the hypothesis that the concerted action of gastric lipase, CEL and PLRP2 is required for the efficient digestion of human milk and formula fats.
Heat treatment and freezing human milk may alter the physicochemical properties of milk fat globules by altering the size of the particles and perhaps the organization of the lipids in the droplet (34). The fat content and composition is not significantly affected by heating (34–35). In this study, we used frozen and heat-treated human milk predominantly. Because the physicochemical properties of the substrate influence lipase activity, these treatments may alter the activity of PLRP2 and the other lipases. Even so, heat-treated and frozen human milk is fed to newborns and our observation that PLRP2 can hydrolyze fats from heat-treated and frozen human milk pertains to what happens in practice. We also showed that PLRP2 can release fatty acids from fresh human milk.
Our conclusion that PLRP2 digests dietary fats in human milk and formula raises an interesting speculation about the effect of a nonsense polymorphism in the gene encoding PLRP2. Several studies from different investigators report a high allele frequency (0.33 to 0.50) of this polymorphism (36–37). In an earlier study, we showed that the polymorphism results in a truncated form of PLRP2 that has low activity and is poorly secreted (10). The combination of the results from this study and the earlier study suggests that neonates who are homozygous for the nonsense polymorphism will be PLRP2 deficient and may have decreased dietary fat absorption. Homozygosity for the PLRP2 polymorphism may account for the broad range of fecal fat concentrations found in newborns since the lack of PLRP2 would likely decrease the efficiency of dietary fat digestion in humans as it does in mice (38). If true, fat absorption and nutrition in these newborns may be improved by pancreatic enzyme replacement therapy.
Methods
Materials
Fresh or frozen human breast milk was donated by women with infants in the Neonatal Intensive Care Unit at Magee Women’s Hospital of University of Pittsburgh Medical Center. Mothers on medications were excluded from donation. Fresh milk samples were used within 24 hours of collection. Fresh milk samples from a single donor were used for most experiments. Exceptions are noted in the figure legend. The protocol was approved by the Institutional Review Board of the University of Pittsburgh. Similac Advance 20 calorie infant formula (fat (long-chain safflower, soy and corn oil) 1.11 g, carbohydrate (lactose and galactooligosaccharides) 2.17 g, protein (non-hydrolyzed nonfat milk and whey) 0.42 g in 29.6 ml) was used in some assays. Bile salts were obtained from Sigma (St. Louis, MO). Other chemicals were obtained from standard sources.
Protein methods
The recombinant protein PLRP2, CEL, and colipase (Col) were expressed in Pichia pastoris and purified as previously described (10, 12, 14). The recombinant CEL contained 16 tandem repeats in the hypervariable region. Gastric lipase (GL) was generously provided by Dr. Frederic Carriere at the Laboratoire d’Enzymologie Interfaciale et Physiologie de la Lipolyse in Marseille, France (39). To determine the purity and integrity of the recombinant proteins, 6 μg of each protein was resolved by 7.5, 10 and 18% SDS-PAGE for CEL, PLRP2 and colipase, respectively, and followed by staining with GelCode Blue Stain Reagent (Pierce, Rockford, IL).
Lipase assay method
For some experiments, the endogenous CEL in human milk was inactivated by heat treatment at 73 °C for 20 minutes. Where indicated, a 200 mM mixture containing 84 mM of taurocholate, 44 mM of glycocholate, 52 mM of taurochenodeoxycholate and 20 mM of glycochenodeoxycholate was added to a final concentration of 4 mM (17). The release of fatty acids by the various lipases was determined by continuous titration to pH 8.0 with 50 mM NaOH in a Titra Lab TIM 854 pH-stat at room temperature. The assay contained 3 mL of human milk or formula and 12 mL reaction buffer (1 mM Tris-Cl, pH 8.0, 2.0 mM CaCl2, and 0.15 M NaCl). The amount of bile salts and various lipases in the assay are given in the legends. Assays with single lipases were performed over 15 to 30 minutes. Preincubation with gastric lipase was performed at room temperature for 20 minutes using the pH-stat to maintain the pH at 6.0. The release of fatty acids was measured by back-titrating to pH 8.0 (40). Subsequently, the appropriate amounts of bile salt, lipase and colipase were added and the reaction monitored continuously at pH 8.0 for 15 to 30 minutes. The reactions were linear over the assay period. The results are expressed as μmoles fatty acid released/min. Values are presented as means ± SD of at least 3 determinations.
Statistical methods
Statistical analysis was performed with SigmaStat software (version 3.5, Systat Software, Point Richmond, CA). Pairwise comparisons were analyzed by Student’s t test. Significance was considered P < 0.05. Multiple pairwise comparisons were done by one-way analysis of variance (ANOVA) using the Holm-Sidak method for the pairwise multiple comparisons with alpha = 0.05 and an overall significance level of 0.05.
Acknowledgments
We thank Dr. Ada Youk for advice on statistical methods.
Statement of Financial Support
The studies were supported by National Institutes of Health Grant DK-080820 (to M.E.L.)
References
- 1.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 2.Carriere F, Barrowman JA, Verger R, Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–88. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care. 2010;13:314–20. doi: 10.1097/MCO.0b013e328337bbf0. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Lindquist S, Lowe M, Noppa L, Hernell O. Bile Salt-Stimulated Lipase and Pancreatic Lipase-Related Protein 2 Are the Dominating Lipases in Neonatal Fat Digestion in Mice and Rats. Pediatr Res. 2007;62:537–41. doi: 10.1203/PDR.0b013e3181559e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe ME, Kaplan MH, Jackson-Grusby L, D’Agostino D, Grusby MJ. Decreased neonatal dietary fat absorption and T cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J Biol Chem. 1998;273:31215–21. doi: 10.1074/jbc.273.47.31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Sanchez D, Figarella C, Lowe ME. Discoordinate expression of pancreatic lipase and two related proteins in the human fetal pancreas. Pediatr Res. 2000;47:184–8. doi: 10.1203/00006450-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Boehm G, Bierbach U, Senger H, et al. Activities of lipase and trypsin in duodenal juice of infants small for gestational age. J Ped Gastroenterol Nutri. 1991;12:324–7. doi: 10.1097/00005176-199104000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Boehm G, Borte M, Muller H, Moro G, Minoli I. Activities of trypsin and lipase in duodenal aspirates of preterm infants: influence of dietary protein and fat composition. Am J Clin Nutr. 1995;61:524–7. doi: 10.1093/ajcn/61.3.524. [DOI] [PubMed] [Google Scholar]
- 9.Eydoux C, De Caro J, Ferrato F, et al. Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells. J Lipid Res. 2007;48:1539–49. doi: 10.1194/jlr.M600486-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Xiao X, Mukherjee A, Ross LE, Lowe ME. Pancreatic Lipase-related Protein-2 (PLRP2) Can Contribute to Dietary Fat Digestion in Human Newborns. J Biol Chem. 2011;286:26353–63. doi: 10.1074/jbc.M111.249813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berton A, Sebban-Kreuzer C, Rouvellac S, Lopez C, Crenon I. Individual and combined action of pancreatic lipase and pancreatic lipase-related proteins 1 and 2 on native versus homogenized milk fat globules. Mol Nutr Food Res. 2009;53:1592–602. doi: 10.1002/mnfr.200800563. [DOI] [PubMed] [Google Scholar]
- 12.Cordle RA, Lowe ME. Purification and characterization of human procolipase expressed in yeast cells. Prot Exp Purif. 1998;13:30–5. doi: 10.1006/prep.1998.0873. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Lowe ME. Human pancreatic triglyceride lipase expressed in yeast cells: purification and characterization. Protein Expr Purif. 1998;13:36–40. doi: 10.1006/prep.1998.0874. [DOI] [PubMed] [Google Scholar]
- 14.Sahasrabudhe AV, Solapure SM, Khurana R, et al. Production of recombinant human bile salt stimulated lipase and its variant in Pichia pastoris. Protein Expr Purif. 1998;14:425–33. doi: 10.1006/prep.1998.0974. [DOI] [PubMed] [Google Scholar]
- 15.Lemay DG, Lynn DJ, Martin WF, et al. The bovine lactation genome: insights into the evolution of mammalian milk. Genome Biol. 2009;10:R43. doi: 10.1186/gb-2009-10-4-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen RG, Ferris AM, Lammi-Keefe CJ, Henderson RA. Lipids of bovine and human milks: a comparison. J Dairy Sci. 1990;73:223–40. doi: 10.3168/jds.S0022-0302(90)78666-3. [DOI] [PubMed] [Google Scholar]
- 17.Bernback S, Blackberg L, Hernell O. The complete digestion of human milk triacylglycerol in vitro requires gastric lipase, pancreatic colipase-dependent lipase, and bile salt-stimulated lipase. J Clin Invest. 1990;85:1221–6. doi: 10.1172/JCI114556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernback S, Blackberg L, Hernell O. Fatty acids generated by gastric lipase promote human milk triacylglycerol digestion by pancreatic colipase-dependent lipase. Biochim Biophys Acta. 1989;1001:286–93. doi: 10.1016/0005-2760(89)90113-6. [DOI] [PubMed] [Google Scholar]
- 19.Gargouri Y, Pieroni G, Riviere C, et al. Importance of human gastric lipase for intestinal lipolysis: an in vitro study. Biochim Biophys Acta. 1986;879:419–23. doi: 10.1016/0005-2760(86)90234-1. [DOI] [PubMed] [Google Scholar]
- 20.Borgstrom B. Importance of phospholipids, pancreatic phospholipase A2, and fatty acid for the digestion of dietary fat: in vitro experiments with the porcine enzymes. Gastroenterol. 1980;78:954–62. [PubMed] [Google Scholar]
- 21.Andersson EL, Hernell O, Blackberg L, Falt H, Lindquist S. BSSL and PLRP2: key enzymes for lipid digestion in the newborn examined using the Caco-2 cell line. J Lipid Res. 2011;52:1949–56. doi: 10.1194/jlr.M015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen RG, Ferris AM, Lammi-Keefe CJ. Lipids in human milk and infant formulas. Annu Rev Nutr. 1992;12:417–41. doi: 10.1146/annurev.nu.12.070192.002221. [DOI] [PubMed] [Google Scholar]
- 23.Michalski MC, Briard V, Michel F, Tasson F, Poulain P. Size distribution of fat globules inhuman colostrum, breast milk, and infant formula. J Dairy Sci. 2005;88:1927–40. doi: 10.3168/jds.S0022-0302(05)72868-X. [DOI] [PubMed] [Google Scholar]
- 24.Straarup EM, Lauritzen L, Faerk J, Hoy Deceased CE, Michaelsen KF. The stereospecific triacylglycerol structures and Fatty Acid profiles of human milk and infant formulas. J Pediatr Gastroenterol Nutr. 2006;42:293–9. doi: 10.1097/01.mpg.0000214155.51036.4f. [DOI] [PubMed] [Google Scholar]
- 25.Momsen MM, Dahim M, Brockman HL. Lateral packing of the pancreatic lipase cofactor, colipase, with phosphatidylcholine and substrates. Biochemistry. 1997;36:10073–81. doi: 10.1021/bi9703857. [DOI] [PubMed] [Google Scholar]
- 26.Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–16. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Carriere F, Renou C, Lopez V, et al. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterol. 2000;119:949–60. doi: 10.1053/gast.2000.18140. [DOI] [PubMed] [Google Scholar]
- 28.Klumpp TG, Neale AV. The gastric and duodenal contents of normal infants and children. Am J Dis Child. 1930;40:1215–29. [Google Scholar]
- 29.Lowe ME. Assays for pancreatic triglyceride lipase and colipase. In: Doolittle MH, Reue K, editors. Methods in Molecular Biology: Lipase and Phospholipase Protocols. Totowa NJ: Humana Press Inc; 1998. pp. 59–70. [DOI] [PubMed] [Google Scholar]
- 30.Boehm G, Braun W, Moro G, Minoli I. Bile acid concentrations in serum and duodenal aspirates of healthy preterm infants: effects of gestational and postnatal age. Biol Neonate. 1997;71:207–14. doi: 10.1159/000244419. [DOI] [PubMed] [Google Scholar]
- 31.Roman C, Carriere F, Villeneuve P, et al. Quantitative and qualitative study of gastric lipolysis in premature infants: do MCT-enriched infant formulas improve fat digestion? Pediatr Res. 2007;61:83–8. doi: 10.1203/01.pdr.0000250199.24107.fb. [DOI] [PubMed] [Google Scholar]
- 32.Armand M, Hamosh M, Mehta NR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–37. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Eydoux C, Aloulou A, De Caro J, et al. Human pancreatic lipase-related protein 2: tissue localization along the digestive tract and quantification in pancreatic juice using a specific ELISA. Biochim Biophys Acta. 2006;1760:1497–504. doi: 10.1016/j.bbagen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Mizuno K, Itabashi K. The freeze-thaw process and long intervals after fortification denature human milk fat globules. Am J Perinatol. 2012;29:283–8. doi: 10.1055/s-0031-1295659. [DOI] [PubMed] [Google Scholar]
- 35.Fidler N, Sauerwald TU, Demmelmair H, Koletzko B. Fat content and fatty acid composition of fresh, pasteurized, or sterilized human milk. Adv Exp Med Biol. 2001;501:485–95. doi: 10.1007/978-1-4615-1371-1_60. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Hegele RA. DNA polymorphisms of lipase related genes. J Hum Genet. 2003;48:443–6. doi: 10.1007/s10038-003-0051-1. [DOI] [PubMed] [Google Scholar]
- 37.Hancock AM, Witonsky DB, Ehler E, et al. Human adaptations to diet, subsistence, and ecoregion are due to subtle shifts in allele frequency. Proc Natl Acad Sci U S A. 2010;107 (Suppl 2):8924–30. doi: 10.1073/pnas.0914625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJ. Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr. 1970;23:1299–313. doi: 10.1093/ajcn/23.10.1299. [DOI] [PubMed] [Google Scholar]
- 39.Carriere F, Moreau H, Raphel V, et al. Purification and biochemical characterization of dog gastric lipase. Eur J Biochem. 1991;202:75–83. doi: 10.1111/j.1432-1033.1991.tb16346.x. [DOI] [PubMed] [Google Scholar]
- 40.Gargouri Y, Pieroni G, Riviere C, et al. Kinetic assay of human gastric lipase on short- and long-chain triacylglycerol eulsions. Gastroenterol. 1986;91:919–25. doi: 10.1016/0016-5085(86)90695-5. [DOI] [PubMed] [Google Scholar]