Abstract
Background
Hypoxia is an important disease mechanism in prematurity, childhood asthma and obesity. In children, hypoxia results in chronic inflammation.
Methods
We investigated the effects of hypoxia (Hx) (12% O2) during postnatal day 2 to 20 in rats. Control groups were normoxic (Nc), and normoxic growth restricted (14 pup liters) (Gr).
Results
Hypoxia decreased growth similar Gr. Hx increased plasma TNFα and IL-6 and decreased IGF-I and VEGF. Hypoxia resulted in right ventricular (RV) hypertrophy but disproportionate decrements in limb skeletal muscle (SM) growth. miR206 was depressed in the hypertrophied RV of Hx rats while increased in growth retarded SM. Hx resulted in a decreased RV mRNA for myostatin but had no effect on SM myostatin. The mRNA for hypoxia sensitive factors such as HIFα was depressed in the RV of Hx rats suggesting negative feedback.
Conclusion
The results indicate that Hx induces a proinflammatory state that depresses growth regulating mechanisms and that tissues critical for survival, such as the heart, can escape from this general regulatory program to sustain life. This study identifies accessible biomarkers for evaluating the impact of interventions designed to mitigate the long-term deleterious consequences of hypoxia that all too often occur in babies born prematurely.
INTRODUCTION
There are emerging data supporting the concept of critical periods of growth when relatively brief physiologic perturbations can lead to detrimental functional, genomic, and even epigenetic changes in affected tissues (1). In this context, prematurely born newborns are particularly vulnerable because they are developing rapidly and because many premature newborns are exposed to profound environmental stresses such as hypoxia (2). Not surprisingly, premature babies are at high risk of developing growth and body composition abnormalities later in life that include failure-to-thrive, obesity, and osteopenia (3, 4).
A remarkable feature of hypoxia associated growth retardation early in life is that certain tissues such as the heart, actually increase in size relative to body mass even in the context of overall reductions in somatic growth (5). This seeming disparity in individual tissue responses provides an opportunity to examine key regulatory mediators of simultaneous growth inhibition and compensation. By understanding the mechanisms through which hypoxia early in life alter growth, we might identify potential targets that could improve outcomes at critical periods of growth and development in premature babies.
The goal of this study was to test the hypotheses that a) exposure to hypoxia early in life will alter the expression level of selected genes that are involved in growth and inflammatory processes in heart and skeletal muscles of neonatal rats, and b) the altered expression pattern will differ between heart and skeletal muscle. As shown in the Results, we focused on selected genes as well as growth and inflammatory mediators known to play a role in the regulation of heart and skeletal muscle as well as on key microRNAs, the latter increasingly demonstrated to play a role in gene regulation in muscle (6). We measured key anabolic mediators and inflammatory cytokines since there is evidence from this and other laboratories that growth in early life is regulated in part through the balance of growth and inflammatory mediators, the latter known to be stimulated by hypoxia (7, 8). Finally, given the increasing data that gender influences gene expression responses to physiological perturbations very early in life, we hypothesized that the phenotypic, genomic, and mediator response to hypoxia would be modified by gender in the neonatal rats.
METHODS
Pregnant Sprague-Dawley (SD) rats were purchased from Charles River (Wilmington, Ma.). Immediately post partum, the litters were randomly cross fostered and gender balanced. At post-partum day 3, four litters were randomized to the hypoxia treatment group (Hypoxia-exposed), were culled to four pups per litter (total 8 male, 8 female) and housed, with the dam, in standard cages placed in a normobaric chamber Biospherix ProOx 360 (Lacona, NY). The small litter size was adopted to ensure adequate nutrition and minimize the maternal stress associated with full litter size in the hypoxic environment (9). The 4 litters randomized to the normoxic control (Nc) groups were also culled to four pups (total 8 male, 8 female) and housed in standard cages in the same room as the chamber. The protocols used in this study were approved by the University of California-Irvine Institutional Animal Care and Use Committee.
To induce hypoxia, a feedback controller senses O2 levels in the chamber and feeds N2 into the chamber to maintain the preset level of O2 (12%). We have modeled hypoxia at an FiO2 of 12% that represents moderate hypoxic exposure, equivalent to the ambient FiO2 found at 4000 m and quite compatible with life. In humans, inhalation of 12.5% O2 has been reported to decrease systemic PO2 to below 50 Torr (10). Using the alveolar gas equation this exposure should produce approximately 80–85% oxygen saturation of hemoglobin in the rat. We and others have found that this level of hypoxia is “tolerated” by the young rats (i.e., the vast majority survive) (9). The chamber used in this study allows exchange with the environment so that pressure remains unchanged. Humidity and CO2 were monitored and regulated.
To provide a comparison group that experienced growth restriction without hypoxia, three additional litters were created consisting of fourteen pups per dam (total 21 male, 21 female). This treatment is known to result in significant growth impairment (11). The diet-restricted groups were also housed in standard cages in the same room as the chamber. Previous studies indicated that hypoxia exposure is associated with some degree of reduced food intake, but not of sufficient magnitude to account for the decrements in growth seen with hypoxia (9).
Tissue Collection & Analysis
The study was terminated on day 21 postpartum. The rats were euthanized using Pentosol solution. After the induction of deep anesthesia, but prior to the cessation of breathing, blood was collected from the left ventricle via the diaphragm using a heparinized syringe. The ventricles were removed and weighed. The soleus, plantaris, medial gastrocnemius (MG) muscles of both legs were dissected free of connective tissue, weighed. All tissues were snap frozen and stored at −80°C for later analysis.
Heart and Skeletal Muscle Protein, Myofibrillar Protein and DNA
Tissue samples were homogenized in 20 ml of buffer per gram tissue. The homogenization buffer contained 250 mM sucrose, 100 mM KCl, 5 mM EDTA, and 10 mM Tris HCl, pH 7.0. Myofibrillar proteins were quantitatively extracted from a known volume of the total homogenate suspended into a known volume of 100 mM KCl, 10 mM Tris, and 1 mM EDTA, pH 7.4. Protein concentration in the homogenate and myofibril suspension was determined using the Biorad Protein assay with gamma globulin as a standard. Muscle protein and myofibril content were calculated based on the homogenized muscle piece weight and total muscle weight. Muscle total DNA concentration calculation was based on total DNA concentration in the total homogenate and was determined by a fluorometric assay using the DNA-specific fluorescent Hoechst 33258 dye (12).
In each case the data provided includes both the content and concentration for the protein, myofibrillar protein, RNA and DNA of the tissues examined. This was done to provide an appreciation of both the changes in size that occurred in response to the treatment (content values) and relevant concentrations so that the reader can determine if these values remained in a normal physiological range.
Complete Blood Count
Complete blood count with differential for hemoglobin and white blood cell analysis were obtained using Hemavet 950 (Drew Scientific Waterbury, CT).
Plasma Analysis
Circulating cytokines (TNF-α and IL-6), growth factors (IGF-I, Growth Hormone (GH), and VEGF) and sex hormones (testosterone and estradiol) levels were measured using commercially available ELISA kits manufactured by R&D Systems (Minneapolis, MN).
RNA Analysis
Total RNA
Total RNA was extracted from pre-weighed frozen tissue samples using the TRI Reagent according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, OH). Total RNA concentration was determined by optical density at 260nm. The tissue total RNA concentration was calculated based on total RNA yield and the weight of the analyzed sample.
mRNA analysis
One microgram of total RNA was reverse transcribed into cDNA using the SuperScript II RT from Invitrogen (Grand Island, NY) and a mix of oligo dT and random primers.
Cardiac MHC mRNA (myosin heavy chain), IGF-I, IGFBP4, IGF BP5, Myostatin, MURF1, Atrogin, Cyclin D1, and Nos3 mRNA expression were analyzed using an end point RT-PCR approach as described previously (12). For all these mRNAs, except for Nos3, PCR primers sequence is as reported previously (12–14). Nos3 primers were designed using Primer Select software (DNAStar Madison, WI) and NM_021838 reference sequence in NCBI. Nos3 mRNA primers seq was 5′>3′: Fwd: GATTCTGGCAAGACCGATTACACGAC : Rev: CCGCGGCCAGCTCTGTCC to amplify a 228 bp. In addition to the above mRNA markers, HIF1α, HYOU1, VEGF mRNA were analyzed using Taqman Real-Time PCR assays (Applied Biosystems, Carlsbad, Ca.) and was normalized to endogenous control beta actin (ACTB).
Rationale
Analysis of MHC phenotype provides important insights on the adaptation of striated muscle tissue relative to contractile characteristics and economy. IGF-I is a powerful regulator of tissue growth. Modulation of this system is accomplished both via changes in IGF-I expression and it’s bioavailability as determined, in part, by the IGF binding protein family. Myostatin is a powerful, negative, regulator of muscle growth. MURF1 and Atrogin are muscle specific E3 ligases that can modulate muscle size and adaptation via the targeting of proteins for proteolysis. Cyclin D1 is a key regulator of entry into the cell cycle. Nos3 is important for the generation of NO. In muscle NO signaling is implicated in a number of processes such as angiogenesis. VEGF is also a critical regulator of angiogenesis that functions in conjunction with hypoxia sensitive proteins such as HIF1α and HYOU1.
MicroRNA Analysis
TaqMan assays were carried out on miR-1, miR-133a and miR206. Reverse transcriptase (RT) reactions were carried out using the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer’s instructions. Real-Time PCR analysis was performed with the Applied Biosystems 7900HT Sequence Detection System by using TaqMan Universal PCR Master Mix and Assays-on-Demand microRNA probes (Applied Biosystems). All reactions were run in duplicate. The cycle threshold for each sample was determined using SDS software version 2.3 (Applied Biosystems) and was normalized to endogenous control U6 snRNA.
Statistical Analysis
Between groups analysis was conducted using a One-Way ANOVA with Bonferroni’s multiple comparisons test post test using PRISM software (Graphpad, La Jolla, CA). Graphical representations of the data include 95% confidence limits. Tabular data includes Mean ± Standard Error. For all statistical tests the significance level was set at 0.05.
RESULTS
Body Weight, Muscle Mass and Phenotype, and Organ Mass
Total Body Mass
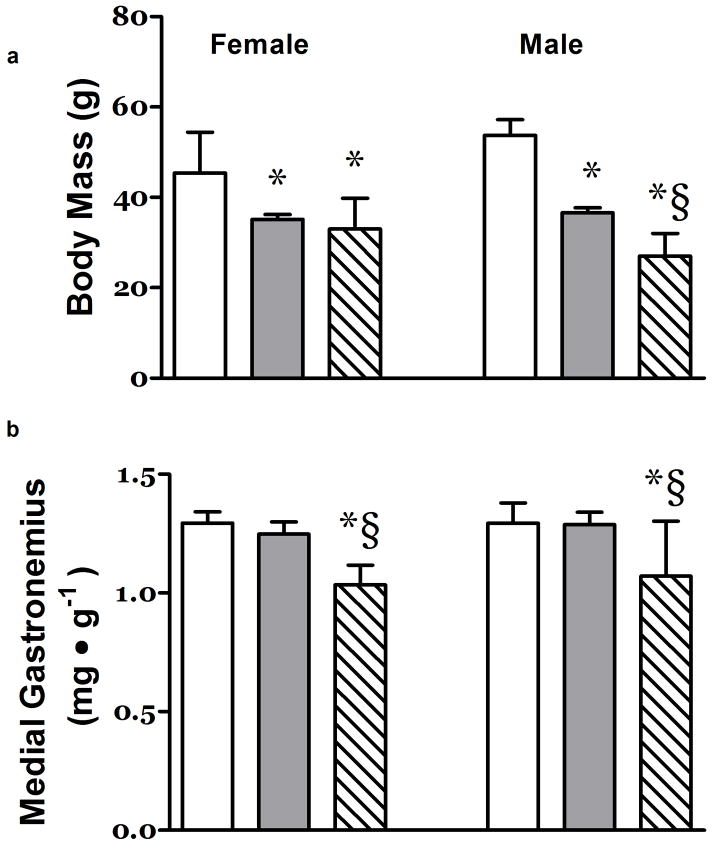
At 21 days of age, the body mass of hypoxia exposed pups was significantly smaller than the control animals and similar to that of the Gr group (Figure 1a). The growth deficits imposed either by hypoxia or large litter size appeared to be more pronounced in the male pups (Figure 1a).
Figure 1. Effects of Hypoxia and Litter Size on Body and Muscle Mass.
a) Both large litter size in normoxia (Gr – Grey Column) and exposure to hypoxia (Hx – Hatched Column) (litter size 4) resulted in depressed body mass relative to pups maintained in normoxia (Nc - White Column) (litter size 4) at 21 days postpartum in rats. b) The relative mass of the leg muscle Medial Gastrocnemius and the concentration of RNA in that muscle were depressed at 21 days post partum in the Hx neonates. The left three columns are data from female rats, the right 3 columns are data from the male rats. N ≥ 7 *, P<0.05 vs. Nc; §, P<0.05 vs. Gr.
Skeletal Muscle
The relative mass of mixed fiber type locomotor skeletal muscles such as the plantaris and MG were significantly depressed to a similar extent in both male and female Hx rats (Figure 1b and data not shown).
Right Ventricle (RV)
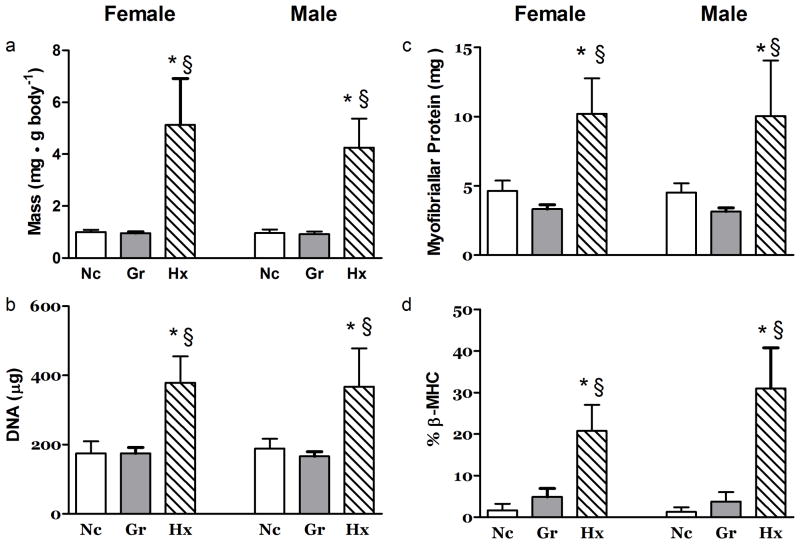
Hx but not Gr rats experienced a remarkable adaptation in the heart in which the relative (mg/g body) mass of right ventricles were increased ~5x fold (Figure 2a.) The DNA content of the RV was increased ~2 fold in the Hx rats (Figure 2b.) while the DNA concentration was not different from the Nc group (Table 1). In Gr rats, the DNA concentration of the RV was elevated ~30% relative to that of the control and hypoxia exposed animals (Table 1). The total- and myofibrillar-protein content of the right ventricles of Hx rats was increased ~2 fold (Figure 2c & Table 1). However, the concentration of total protein in the RV was similar in all groups (Supplementary Table 1 (online)). In contrast to content, the myofibrillar protein concentration in the RV from female Hx rats was 14% lower (P<0.05) than in the Nc group (Table 1).
Figure 2. Effects of Hypoxia and Litter Size on the Right Ventricle.
The right ventricular a) mass, b) DNA content, c) myofibrillar protein content and d) β-myosin heavy chain percent were dramatically altered to support the increased demands of the hypoxic treatment at 21 days post partum. N ≥ 7. *, P<0.05 vs. Nc; §, P<0.05 vs. Gr.
Table 1.
Treatment effects in the right ventricle
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nc | Gr | Hx | Nc | Gr | Hx | |
|
| ||||||
| Protein Content (mg) | 10.1±0.5 | 7.8±0.2 | 26.8±2.8*§ | 12.2±0.8 | 8.8±0.4 | 27.6±4.5*§ |
| Myofibrillar Protein Conc. (mg g−1) | 93.0±2.6 | 89.7±2.8 | 79.9±2.6* | 81.7±4.1 | 85.7±2.2 | 81.1±3.1 |
| DNA Conc. (mg.g−1) | 2.83±0.16 | 3.80±0.14 | 2.47±0.16 | 2.73±0.06 | 3.65±0.07 | 2.57±0.19 |
| RNA Content (μg) | 134±8 | 83±4 | 359±54*§ | 132±5 | 93±3 | 302±54*§ |
| α-MHC (% total) | 98.4±0.7 | 95.1±0.9 | 79.2±2.7*§ | 98.8±0.5 | 96.3±1.0 | 69.0±3.9*§ |
| miR133a (AU) | 1.30±0.17 | 1.88±0.16 | 1.15±0.23§ | 1.01±0.06 | 1.12±0.12 | 0.98±0.11 |
n ≥ 7,
P<0.05 vs. Nc;
P<0.05 vs. Gr.;
Conc.: concentration; AU: Arbitrary units
As part of the adaptation to hypoxia, the RV myosin heavy chain (MHC) phenotype of the hypoxia-exposed rats was shifted toward substantial expression of the β-MHC isoform (Figure 2d) reflecting a shift to a metabolically more economical phenotype (15). There were concomitant decreases (P<0.05) in the expression of the α-MHC isoform (Table 1).
Left Ventricle (LV)
As with the RV, relative LV mass was significantly increased (~2 fold) in Hx rats relative to Nc or Gr (Table 2). The LV mass of the Gr rats was appropriate to the size of the animal, i.e., relative mass was not different from that of Nc. The much smaller absolute size of the Gr LV was reflected in the lower protein and DNA contents (Table 2).
Table 2.
Treatment effects in the left ventricle
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nc | Gr | Hx | Nc | Gr | Hx | |
|
| ||||||
| Mass (mg g body −1) | 3.4±0.08 | 3.3±0.06 | 7.2±0.71*§ | 3.4±0.08 | 3.3±0.04 | 8.8±1.5*§ |
| Protein Content (mg) | 174±5.8 | 112±2.0* | 230±18*§ | 184±7.4 | 124±3.6* | 196±26§ |
| Myofibrillar Protein (mg) | 13.6±0.56 | 8.13±0.25* | 16.19±1.4§ | 14.6±0.75 | 9.39±0.27* | 14.91±1.9§ |
| DNA Content (μg) | 471±19 | 365±17* | 611±36*§ | 522±8 | 394±12* | 506±39§ |
| RNA Conc. (mg g−1) | 2.5±0.07 | 2.5±0.05 | 2.5±0.05 | 2.3±0.07 | 2.4±0.08 | 2.4±0.08 |
| α-MHC (% total) | 97.8±0.6 | 95.8±1.5 | 83.2±2.9*§ | 97.1±0.8 | 95.1±1.1 | 77.3±5.9*§ |
| β-MHC (% total) | 2.1±0.6 | 4.3±1.5 | 16.8±2.9*§ | 2.9±0.8 | 4.9±1.1 | 22.7±5.8*§ |
| HIF1α mRNA (AU) | 0.90±0.03 | 0.92 +0.03 | 0.76±0.03*§ | 0.95±0.02 | 0.93±0.03 | 0.87±0.07 |
| HYOU1 mRNA (AU) | 0.93±0.05 | 0.98±0.04 | 0.68±0.05*§ | 0.85±0.06 | 0.95±0.04 | 0.62±0.07*§ |
| VEGF mRNA (AU) | 0.87±0.04 | 0.86±0.02 | 0.69±0.02*§ | 1.02±0.03 | 1.01±0.04 | 1.00±0.12 |
| Myostatin mRNA mg−1 | 88.5±12.5 | 116.1±10.1 | 39.3±5.9*§ | 55.8±10.8 | 75.6±3.5 | 25.0±6.5*§ |
| IGFBP5 mRNA mg−1 | 57.2±5.9 | 58.7±4.3 | 66.3±13.0 | 41.3±6.4 | 41.2±5.4 | 19.9±4.2*§ |
| miR1 (AU) | 1.01±0.06 | 1.04±0.05 | 0.98 ±0.04 | 1.13±0.06 | 1.09±0.07 | 0.83±0.08*§ |
n ≥ 7,
P<0.05 vs. Nc;
P<0.05 vs. Gr.;
Conc.: concentration; AU: Arbitrary units.
Relative to controls, the total protein, myofibrillar protein and DNA content of the LV was elevated in the female Hx rats but not the males (Table 2). In both genders, the protein and DNA contents of the Hx LV were greater than that of the diet-restricted rats reflecting the increased size of this organ.
In contrast to the content values, the concentrations of protein and myofibrillar protein were similar across groups and genders in the LV (Supplementary Table 2 (online)). Similar to the RV, the concentration of DNA in the LV was slightly greater with Gr (Supplementary Table 2 (online)). However, in the LV, significant differences were only seen in the female Gr rats.
The LV of the Hx rats experienced a shift in β-MHC phenotype that was qualitatively similar to that seen in the RV (Table 2). There was a complimentary decrease (P<0.05) in the expression of the α-MHC isoform in the LV (Table 2)
Growth Factors, Inflammatory Mediators, and Leukocytes
Plasma estradiol was lower in the Nc males relative to Nc females (e.g., Nc: 30±3 vs. 40±4 pg•ml−1; P=0.04, Student’s t-test). Estradiol appeared to be or was significantly elevated in the Hx and Gr males (Table 3). There were no differences in plasma testosterone between the Nc male and female animals (Table 3). Plasma testosterone was significantly depressed in the female Hx rats relative to Nc females (Table 3) .
Table 3.
Treatment effects on plasma
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nc | Gr | Hx | Nc | Gr | Hx | |
|
| ||||||
| Monocytes (K μL−1) | 0.38±0.08 | 0.20±.011 | 0.23±0.06 | 0.34±0.11 | 0.27±0.11 | 0.90±0.30*§ |
|
|
||||||
| Neutrophils (K μL−1) | 1.35±0.25 | 0.76±0.18 | 0.77±0.12 | 1.45±0.39 | 1.08±0.31 | 2.68±0.53*§ |
|
|
||||||
| TNFα (pg ml−1) | 2.56±0.23 | 3.05±0.13 | 3.86±0.27* | 2.68±0.15 | 2.91±0.10 | 3.96±0.25*§ |
|
|
||||||
| IL-6 (pg ml−1) | 14.0±1.5 | 16.0±2.8 | 27.2±2.0*§ | 13.5±1.5 | 18.7±1.6 | 30.2±3.7*§ |
|
|
||||||
| Hemoglobin (g.dL−1) | 12.1±0.4 | 12.6±0.2* | 13.4±0.1*§ | 13.5±0.1 | 15.1±0.2* | 16.0±0.2*§ |
|
|
||||||
| Testosterone (ng ml−1) | 1.58±0.22 | 1.07±0.11 | 0.82±0.09* | 1.52±0.14 | 1.12±0.08 | 0.95±0.35 |
|
|
||||||
| Estradiol (pg ml−1) | 40.3±3.8 | 37.0±2.7 | 40.0±8.0 | 30.2±2.5 | 42.1±2.4* | 47.4±12.0 |
n ≥ 7,
P<0.05 vs. Nc;
P<0.05 vs. Gr.
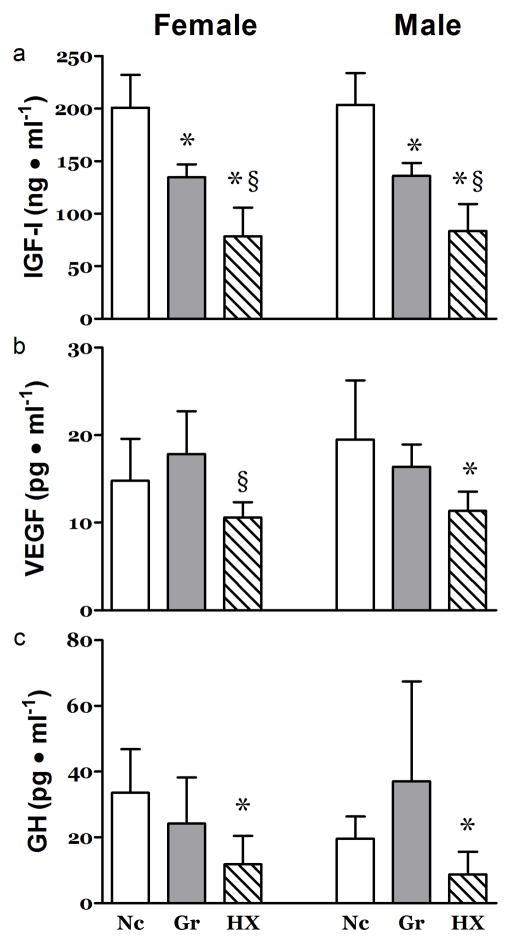
Circulating levels of IGF-I were lower than control in both Hx and Gr animals (Figure 3a). Further, IGF-I in Hx rats was significantly lower than in Gr animals (Figure 3a). VEGF and GH were reduced in the hypoxia-exposed but not diet-restricted pups (Figure 3b&c).
Figure 3. Effects of Hypoxia and Litter Size on Circulating Growth Mediators.
At 21 days post partum, plasma concentrations of a) IGF-I were depressed in both the Gr and Hx neonates. Plasma concentrations of b) VEGF and c) GH were depressed only in the Hx groups. n ≥ 7, *, P<0.05 vs. Nc; §, P<0.05 vs. Gr.
In Hx rats, the concentrations of monocytes and neutrophils were elevated in the male but not the female (Table 3). TNF-α and IL-6 were significantly elevated in the hypoxia exposed pups (Table 3). Hemoglobin concentrations were elevated in both the hypoxia-exposed and diet-restricted pups with greater changes seen in the hypoxia group (Table 3).
Gene Expression
Heart
In the RV, the levels of several hypoxia sensitive mRNAs were significantly altered. The Hx animals experienced decreases in Hypoxia Inducible Factor 1α (HIF1α) and Hypoxia Up-Regulated-1 (HYOU1) in both genders (Figure 4a & b) and decreased VEGF in the females (Figure 4c). However, the mRNA for the Platelet Derived Growth Factor Receptor-β (PDGFRβ), known to be critical for angiogenic adaptation in overloaded hearts, was increased in the RV of the Hx animals (Figure 4d).
Figure 4. Effects of Hypoxia and Litter Size on Hypoxia Sensitive mRNA in the Right Ventricle.
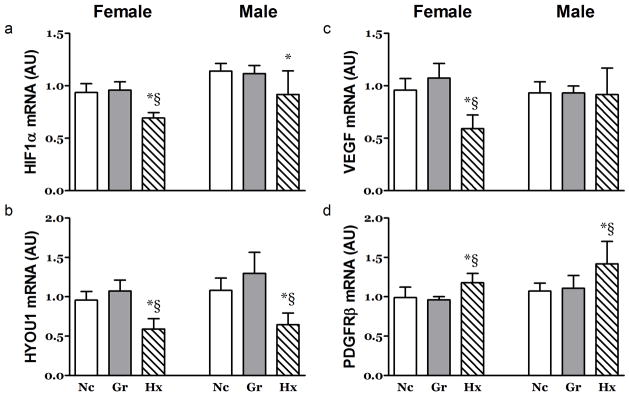
The Abundance of mRNA for a) HIF1α, b) HYOU1, c) VEGF and d) PDGFRβ was altered in the in the right ventricle at 21 days post partum. An exception to this was seen for VEGF in the Hx male neonates. n ≥ 7, *, P<0.05 vs. Nc; §, P<0.05 vs. Gr. AU; arbitrary units.
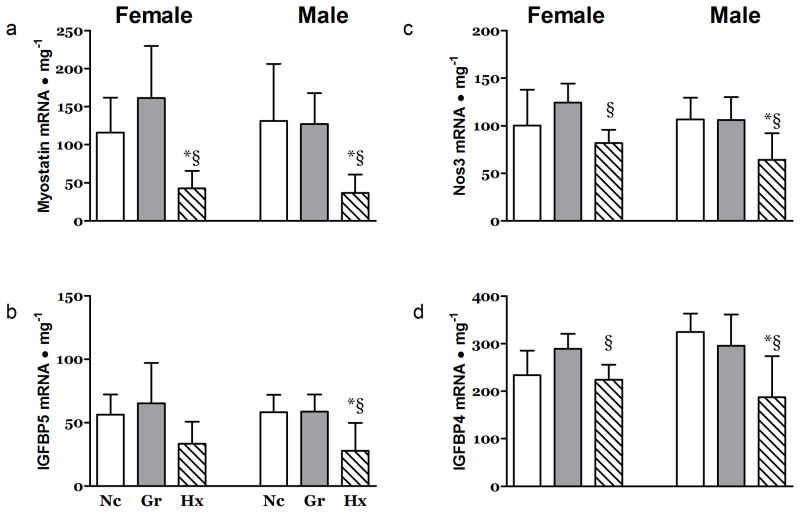
Hypoxia also impacted the mRNA levels of several growth related factors in the heart. The levels of mRNA for myostatin, a powerful negative regulator of muscle growth, and IGF-I binding protein-5 (IGFBP-5), a binding protein sensitive to IGF-I levels, were depressed in both the RV (Figure 5a&b) and LV (Table 2) of Hx animals. No differences were observed in the levels of mRNA for IGFBP4, Cyclin D1 and Nos3 (Supplementary Table 1 (online)).
Figure 5. Effects of Hypoxia and Litter Size on Growth Related mRNA in the Right Ventricle and Skeletal Muscle.
The abundance of mRNA for a) Myostatin and b) IGFBP5 was significantly depressed in the right ventricles of the Hx neonates at 21 days post partum. The abundance of mRNA for c) Nos3 and d) IGFBP4 was depressed in the MG muscle. n ≥ 7, *, P<0.05 vs. Nc; §, P<0.05 vs. Gr.
In the LV, the levels of several hypoxia sensitive mRNAs were significantly altered primarily in female rats. The female Hx animals experienced decreases in HIF1α and VEGF mRNA while that of HYOU1 was decreased ~26% in both genders (Table 2). The mRNA for the PDGFRβ was not different across treatments (Supplementary Table 2 (online)).
Skeletal Muscle
The mRNA concentration for Nos3, a pro-angiogenic regulator (16) and IGFBP4 were significantly depressed in the MG muscles from Hx rats of both genders (Figure 5c&d). In male Hx rats the mRNA levels for IGFBP5 were also significantly lower than that found in the Nc and Gr animals (Table 4). The mRNA of muscle ring finger-1 (MURF-1) and Atrogin-1, muscle specific E3 ligases often associated with muscle atrophy (17), were not significantly different between groups (Supplementary Table 3 (online)). Interestingly, the mRNA for myostatin was not different across groups (Table 4). No treatment associated changes were seen in MHC isoform expression (Not shown). In the MG muscle, the mRNA concentrations for HIF1α, VEGF, HYOU1 and PDGFRβ were similar across groups (Supplementary Table 3 (online)).
Table 4.
Treatment effects in the medial gastrocnemius muscle
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nc | Gr | Hx | Nc | Gr | Hx | |
|
| ||||||
| RNA Conc. (mg g−1) | 2.28±0.06 | 2.54 ±0.08 | 2.26±0.06 | 2.38±0.06 | 2.50±0.06 | 2.02±0.05 |
| IGF-I mRNA mg−1 | 200±26 | 241 ±13 | 199±16 | 219±16 | 208±19 | 170±21 |
| IGFBP5 mRNA mg−1 | 169±17 | 218±15 | 188±19 | 220±19 | 222±24 | 107±22*§ |
| Myostatin mRNA mg−1 | 282.3±28.0 | 356.7±23.4 | 320.3±25.3 | 297.8±16.4 | 321.6±15.0 | 217.9±36.2 |
| miR133a (AU) | 1.07±0.16 | 0.40±0.07* | 0.73±0.06 | 0.94±0.08 | 0.75±0.10 | 1.15±0.10§ |
n ≥ 7,
P<0.05 vs. Nc;
P<0.05 vs. Gr.;
Conc.: Concentration; AU: Arbitrary units.
MicroRNAs
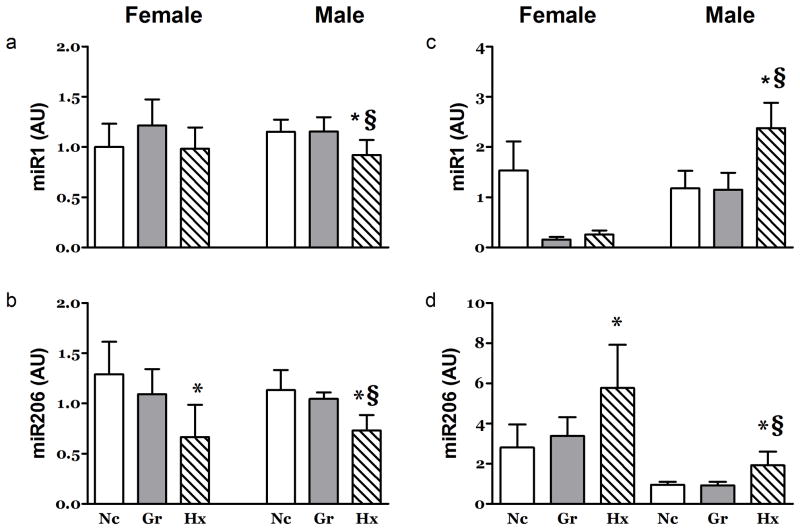
Levels of microRNAs known to be important regulators of gene expression in the heart were affected by exposure to hypoxia. In the RV, miR206 was depressed in both genders as was that of miR1 in male Hx rats (Figure 6a&b). The expression of miR133a was increased in the RV of female Gr rats relative to the Hx group (not shown), no other differences were observed in the RV. Only one significant change in miR abundance was observed in the LV. The expression of miR1 was decreased 27% in the LV of male but not female Hx rats (Table 2).
Figure 6. Effects of Hypoxia and Litter Size on microRNA Expression in Heart and Skeletal Muscle.
At 21 days post partum, the abundance of a) miR1 was depressed in the RV of male neonates. At this time point, the abundance of b) miR206 was depressed in the RV of both genders. In MG muscle miR1 (c) was depressed in both the Gr and Hx female neonates. In contrast, the miR206 (d) was increased in both genders of Hx neonates. n ≥ 7, *, P<0.05 vs. Nc; §, P<0.05 vs. Gr. AU: arbitrary units.
Analysis of candidate microRNAs commonly reported to be important in skeletal muscle demonstrated various responses that were often gender specific. MG muscle miR1 was significantly elevated in male Hx rats while miR1 was repressed in females in the Hx and Gr groups (Figure 6c). The expression of miR206 was elevated in the MG muscles of Hx rats in both genders (Figure 6b). The miR133a was repressed in female Gr rats (Table 4).
DISCUSSION
In this study, we succeeded in comparing two types of somatic growth inhibition, one with hypoxia and one with large litter size, in neonatal rats. The comparison permitted us to shed new light on molecular mechanisms of both growth stimulation, in the hearts of hypoxia-exposed rats, and growth inhibition in the skeletal muscles. We identified, for the first time, potential hypoxia specific gene mediators of growth such as decreased cardiac myostatin and IGFBP5 expression in hypoxia-exposed neonatal rats. We identified the potential pivotal role played by the microRNA miR-206 as a hypoxia sensitive regulator of growth in cardiac muscle in neonatal rats. Our studies further elucidated the unique role that hypoxia plays in promoting inflammatory mediators, a phenomenon that is potentially linked to the long term consequences of hypoxia early in life. Finally, our studies suggest that the impact of moderate hypoxia early in life may alter growth to a greater extent through secondary mediators, such as inflammatory cytokines, than through direct effects of hypoxia on known regulatory genes such as HYOU1, HIF1α, and VEGF. These results are encouraging as we have previously found evidence that such secondary effects (such as inflammation) can be attenuated by physical activity (13, 18).
It is conceivable that the paradoxical growth effects that we observed, namely, a robust increase in relative heart muscle size in the hypoxia exposed rats despite their smaller size, resulted from somewhat antagonistic simultaneous effects of hypoxia exposure and increased muscle work. Even under the conditions of moderate hypoxia imposed in the current study, cardiac work would be increased: in the right heart likely caused by hypoxia-induced pulmonary vasoconstriction (19); in the left heart through the adaptive response of increasing systemic cardiac output to maintain tissue oxygen delivery in light of reduced arterial blood oxygenation. Skeletal muscle, in contrast, would not be similarly stimulated by hypoxia.
In the heart, an important finding was the decreased expression of VEGF, either as protein present (circulation) or message (tissues), associated specifically with the hypoxia exposure. VEGF plays a key role in the angiogenesis that occurs in skeletal muscle in response to exercise training (20) and heart muscle in response to loading and hypoxia (21). VEGF gene expression is sensitive to hypoxia through mediators such as HIF1α (20). In the current study, both the circulating and tissue levels of message for VEGF and/or VEGF regulatory mediators (e.g., HIF1α and IGF-I (22) were actually depressed indicating that these regulatory feedback pathways may have been invoked during hypoxia exposure. These data strongly suggest that in future studies functional indices of angionesis, such as capillary density, should be examined.
This potential differential mechanism is highlighted in the comparison of skeletal and cardiac muscle. In contrast to the heart, the changes in VEGF or its regulatory genes were not influenced by hypoxia in skeletal muscle. This result is similar to that reported by He et al. (23) in adult rats where hypoxia per se had no effect in muscle expression of HIF1α and VEGF. Interestingly, these authors found increased expression of the mRNA for these mediators in adult animals that were exposed to hypoxia in combination with exercise suggesting that an additional stressor is necessary to induce adaptations in this tissue (23).
Developmental growth of the heart is modulated both by mechanical factors and mediators such as IGF-I (24). In the case of the IGF-I system, the results seen in the current study (Figure 3) are consistent with observations that a decrease in IGFBP5 expression may promote growth/hypertrophy (25). However, in contrast to hypoxia imposed in adult rats (26), the RV hypertrophy seen in the current study did not appear to be driven by an increase in the mRNA for IGF-I itself.
Similar to the heart, skeletal muscle developmental growth is coordinated, in part, by circulating mediators such as IGF-I (27). In that context, the restrained growth of the leg skeletal muscles investigated was consistent with the depressed circulating levels of growth factors (Figure 3). In the current study, the mRNA for IGFPB4 was depressed by hypoxia in both genders. This IGF binding protein is commonly considered to function as a negative regulator of IGF-I. However, IGFBP4 gene knockout has been reported to decrease prenatal growth (28) suggesting that the decrease observed in the current study may be consonant with the observed growth inhibition.
In contrast to studies imposing hypoxia on adult rats (29), the limb skeletal muscles of neonates in this study did not demonstrate enhanced levels of myostatin mRNA suggesting that this mediator did not participate in the hypoxia induced growth retardation. Interestingly, the enhanced growth seen in the heart of hypoxia treated rats did appear to be a function, at least in part, of a decrease in myostatin mRNA (Figure 5, Table 2).
An additional mechanistic contrast between the response of the neonatal heart and skeletal muscles to hypoxia is seen in the levels of mRNA for angiogenic regulators. As noted above, in the heart, VEGF mRNA levels were depressed in the Hx females (Figure 4, Table 2) while Nos3 mRNA was unchanged (Supplementary Table 1 (online)). In skeletal muscle, VEGF mRNA was unaffected by hypoxia (Supplementary Table 3 (online)) while Nos3 mRNA levels were depressed in both females and males (Figure 5).
With regard to miR expression, the most interesting and clear cut result was seen for miR206. Hypoxia but not growth restriction uniformly increased the expression of this miR in skeletal muscle, a tissue that experienced a profound decrement in growth. In contrast, the RV, which escaped the somatic anti-growth signaling program, had decreased miR206 expression. This result suggests that, in a developmental setting, miR206 may be a critical regulator in striated muscle.
In skeletal muscle, the up regulation of miR206 is consistent with a decrease in satellite cell proliferation and indications of increased differentiation (30). This response would be expected to strongly limit total muscle size during development, i.e., fewer myofibers. The role of miR-206 in the development of the heart is less clear. In mature animals, miR-206 expression is increased in response to injury and appears to contribute to the proliferation of progenitor cells.
In both skeletal and cardiac muscle miR-206 appears to function via the down regulation of tissue inhibitor of metalloproteinases-3 (TIMP3) (31). TIMP3 in turn, appears to be a critical regulator of muscle regeneration(31). In the present study, the Hx RV demonstrated a marked decrease in miR206 expression, while the concentration of DNA was similar to that in RV of the Nc animals. This suggests that the down regulation of miR206 biased cardiac adaptation towards hypertrophy and away from myogenesis possibly as a strategy to match cardiomyocyte growth with vascularization (32).
The results from a number of studies have indicated that early in life hypoxia results in lasting changes in the cardiovascular system and metabolism(5, 9, 33). For example, Del Duca et al reported that, in rats, 10 days of neonatal exposure to hypoxia resulted in differential gene expression in the left ventricle in adulthood (9). In that study, genes regulating a range of functions such as apoptosis, metabolism and vascular remodeling were affected by neonatal hypoxia. This research group found that the brief period of hypoxia exposure during this critical period of development had lasting effects on cardiomyocyte function, morphology and viability (9, 33).
In the current study, exposure to hypoxia resulted in increased levels of circulating inflammatory mediators (Table 3) and, in male rats, an elevation the number of monocytes and neutrophils in the circulation (Table 3). The activation of inflammatory processes by hypoxia has been reported in a number of previous studies (34). In an interesting study using intravital microscopy, Dix et al. reported that inflammation in microcirculatory beds was increased in response to systemic but not local hypoxia (35). Subsequent studies reported that alveolar macrophages mediate inflammatory responses in tissues remote from the lung (36). These results demonstrate that inflammatory responses to hypoxia are regulated via global mechanisms with the potential to affect all of the somatic systems.
In general, proinflammatory cytokines entrain a number of intracellular processes that would be anti-anabolic in muscle (37). TNFα is thought to exert negative effects on cardiac muscle growth and function (37). However, some studies suggest a more complex relationship in which TNFα may play a role in myogenesis following muscle injury (32).
Similar to TNFα, IL-6 has been reported to play a variety of roles, both positive and negative, in cardiac pathology (32, 38). However, in the heart, the pathological effects of IL-6 expression are generally associated with hypertrophy.
It is well established that physiological insults, such as malnutrition or inflammation, during fetal life can adversely and profoundly affect subsequent growth and development (1). As noted above, it is also well established that exposure to hypoxia induces systemic inflammation (39, 40). There is some evidence that early in life hypoxia results in lasting changes in the cardiovascular system and metabolism (5, 9, 33). However, little is known about the long-term effects of hypoxia and attendant inflammation experienced during critical periods of postnatal life. This is a critical gap in our knowledgebase since chronic diseases of childhood like asthma and obesity may not only impair day-to-day health in affected individuals but may adversely impact health throughout the lifespan (41). The results from the current study point to mechanisms that may mediate lifelong changes resulting from a hypoxic episode experienced during a critical period of development. Such knowledge can be used to identify accessible biomarkers for evaluating the impact of interventions designed to mitigate the long-term deleterious consequences of hypoxia that all too often occur in babies born prematurely.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health, Bethesda, MD, USA (NIH)- 2P01HD048721-06, and the UCI Institute for Clinical and Translational Science, Irvine, CA, USA NIH- UL1 TR000153
The authors would like to thank Cherryl Nugas, Ming Zeng and Paul Bodell for their technical assistance.
Footnotes
The authors certify that there are no potential perceived conflicts of interest or financial disclosures related to this work.
References
- 1.Petry CJ, Ozanne SE, Hales CN. Programming of intermediary metabolism. Mol Cell Endocrinol. 2001;185:81–91. doi: 10.1016/s0303-7207(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 2.Esquer C, Claure N, D’Ugard C, Wada Y, Bancalari E. Role of Abdominal Muscles Activity on Duration and Severity of Hypoxemia Episodes in Mechanically Ventilated Preterm Infants. Neonatology. 2007;92:182–6. doi: 10.1159/000102056. [DOI] [PubMed] [Google Scholar]
- 3.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: Insights into adult cardiovascular disease. Life Sci. 2011;89:417–21. doi: 10.1016/j.lfs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Eliakim A, Nemet D. Osteopenia of prematurity - the role of exercise in prevention and treatment. Pediatr Endocrinol Rev. 2005;2:675–82. [PubMed] [Google Scholar]
- 5.Rohlicek CV, Viau S, Trieu P, Hébert TE. Effects of neonatal hypoxia in the rat on inotropic stimulation of the adult heart. Cardiovasc Res. 2005;65:861–8. doi: 10.1016/j.cardiores.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Williams AH, Liu N, vanRooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–9. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheett TP, Mills PJ, Ziegler MG, Stoppani J, Cooper DM. Effect Of Exercise On Cytokines And Growth Mediators In Prepubertal Children. Ped Res. 1999;46:429–34. doi: 10.1203/00006450-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci. 2008;286:E84–E93. doi: 10.2527/jas.2007-0483. [DOI] [PubMed] [Google Scholar]
- 9.Del Duca D, Wong G, Trieu P, et al. Association of neonatal hypoxia with lasting changes in left ventricular gene expression: an animal model. J Thorac Cardiovasc Surg. 2009;138:538–46. doi: 10.1016/j.jtcvs.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 10.Naeije R, Hallemans R, Melot C, et al. Eicosanoids and hypoxic pulmonary vasoconstriction in normal man. Bull Eur Physiopathol Respir. 1987;23:613–7. [PubMed] [Google Scholar]
- 11.Agnish ND, Keller KA. The rationale for culling of rodent litters. Fundam Appl Toxicol. 1997;38:2–6. doi: 10.1006/faat.1997.2318. [DOI] [PubMed] [Google Scholar]
- 12.Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric and eccentric resistance exercise prevents unloading induced muscle atrophy in rats. J Appl Physiol. 2007;103:1644–54. doi: 10.1152/japplphysiol.00669.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol. 2009;106:443–53. doi: 10.1152/japplphysiol.90831.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad F, Qin AX, Bodell PW, Jiang W, Giger JM, Baldwin KM. Intergenic transcription and developmental regulation of cardiac myosin heavy chain genes. Am J Physiol. 2008;294:H29–H40. doi: 10.1152/ajpheart.01125.2007. [DOI] [PubMed] [Google Scholar]
- 15.Han Y-S, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–96. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hoier B, Nordsborg N, Andersen S, et al. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol. 2011;590:595–606. doi: 10.1113/jphysiol.2011.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass D. PI3 Kinase Regulation of Skeletal Muscle Hypertrophy and Atrophy. Curr Top Microbiol Immunol. 2011;346:267–78. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 18.Buchowicz B, Yu T, Nance DM, Zaldivar FP, Cooper DM, Adams GR. Increased rat neonatal activity influences adult cytokine levels and relative muscle mass. Ped Res. 2010;68:399–404. doi: 10.1203/PDR.0b013e3181f2e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 20.Wagner P. The critical role of VEGF in skeletal muscle angiogenesis and blood flow. Biochem Soc Trans. 2011;39:1556–9. doi: 10.1042/BST20110646. [DOI] [PubMed] [Google Scholar]
- 21.Leychenko A, Konorev E, Jijiwa M, Matter M. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS ONE. 2011;6:e29055. doi: 10.1371/journal.pone.0029055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinovsky ED. The multifunctional role of IGF-1 in peripheral nerve regeneration. Neurol Res. 2004;26:204–10. doi: 10.1179/016164104225013851. [DOI] [PubMed] [Google Scholar]
- 23.He Z, Feng L, Zhang L, Lu Y, Xu J, Lucia A. Effects of Hypoxic Living and Training on Gene Expression in an Obese Rat Model. Med Sci Sports Exerc. 2012;44:1013–20. doi: 10.1249/MSS.0b013e3182442d82. [DOI] [PubMed] [Google Scholar]
- 24.Hudlicka O, Brown M. Postnatal growth of the heart and its blood vessels. J Vasc Res. 1996;33:266–87. doi: 10.1159/000159155. [DOI] [PubMed] [Google Scholar]
- 25.Salih DA, Tripathi G, Holding C, et al. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci. 2004;23:4314–9. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell-Jones DL, Leach RM, Ward JPT, Thomas CR. Insulin-like growth factor-I gene expression is increased in the right ventricular hypertrophy induced by chronic hypoxia in the rat. J Mol Endocrinol. 1993;10:99–102. doi: 10.1677/jme.0.0100099. [DOI] [PubMed] [Google Scholar]
- 27.Adams GR, McCue SA, Bodell PW, Zeng M, Baldwin KM. Effects of spaceflight and thyroid deficiency on hindlimb development. I. Muscle mass and IGF-I expression. J Appl Physiol. 2000;88:894–903. doi: 10.1152/jappl.2000.88.3.894. [DOI] [PubMed] [Google Scholar]
- 28.Ning Y, Schuller AGP, Conover CA, Pintar JE. Insulin-Like Growth Factor (IGF) Binding Protein-4 Is Both a Positive and Negative Regulator of IGF Activity in Vivo. Mol Endocrinol. 2008;22:1213–25. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayot M, Rodriguez J, Vernus B, et al. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol. 2011;332:38–47. doi: 10.1016/j.mce.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Chen J-F, Tao Y, Li J, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190:867–79. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Chen S-E, Jin B, et al. TIMP3: a physiological regulator of adult myogenesis. J Cell Sci. 2010;123:2914–21. doi: 10.1242/jcs.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–80. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Del Duca D, Tadevosyan A, Karbassi F, et al. Hypoxia in early life is associated with lasting changes in left ventricular structure and function at maturity in the rat. Int J Cardiol. 2010;156:165–73. doi: 10.1016/j.ijcard.2010.10.135. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez NC, Wood JG. Alveolar hypoxia-induced systemic inflammation: what low PO(2) does and does not do. Adv Exp Med Biol. 2010;662:27–32. doi: 10.1007/978-1-4419-1241-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dix R, Orth T, Allen J, Wood JG, Gonzalez NC. Activation of mast cells by systemic hypoxia, but not by local hypoxia, mediates increased leukocyte-endothelial adherence in cremaster venules. J ApplPhysiol. 2003;95:2495–502. doi: 10.1152/japplphysiol.00735.2003. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez NC, Allen J, Blanco VG, Schmidt EJ, vanRooijen N, Wood JG. Alveolar macrophages are necessary for the systemic inflammation of acute alveolar hypoxia. J Appl Physiol. 2007;103:1386–94. doi: 10.1152/japplphysiol.00312.2007. [DOI] [PubMed] [Google Scholar]
- 37.Saini A, Al-Shanti N, Stewart CE. Waste management - cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. 2006;17:475–86. doi: 10.1016/j.cytogfr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–39. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Dix R, Orth T, Allen J, Wood JG, Gonzalez NC. Activation of mast cells by systemic hypoxia, but not by local hypoxia, mediates increased leukocyte-endothelial adherence in cremaster venules. J Appl Physiol. 2003;95:2495–502. doi: 10.1152/japplphysiol.00735.2003. [DOI] [PubMed] [Google Scholar]
- 40.Tamura DY, Moore EE, Partrick DA, Johnson JL, Offner PJ, Silliman CC. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 2002;17:269–73. doi: 10.1097/00024382-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res. 2007;61:653–9. doi: 10.1203/pdr.0b013e31805d8a8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.