Abstract
We report a facile synthesis of glycoprotein-based glyco-ligands and their binding with influenza hemagglutinin and human galectin-3. Human serum albumin (HSA) was used as the scaffold and an Asn-linked complex type N-glycan prepared from chicken eggs was used as the glycan building block. It was found that Cu(I)-catalyzed alkyne–azide cycloaddition reaction (click chemistry) between the alkyne-labeled glycan and the azide-tagged HSA led to an efficient formation of the glycoconjugates. The density of glycan ligands on the protein scaffold was readily varied by changing the molar ratios of the two reactants. Binding studies indicated that the sialylated and desialylated multivalent glycoligands could selectively bind to influenza hemagglutinin and human galectin-3, respectively, with high affinity. In the two glycan–lectin interactions, a clear multivalent effect was observed. Moreover, a cell-based assay showed that the synthetic multivalent glyco-ligands could efficiently inhibit the attachment of galectin-3 to human prostate cancer and lung cancer cell lines. This study suggests that the synthetic glycoprotein-based glyco-ligands can be useful for different applications, including blocking the function of galectin-3 in cancer metastasis.
Keywords: Glycoprotein, Conjugation, Glyco-ligand, Galectin-3, Hemagglutinin, Multivalent inhibitor, Influenza, Cancer metastasis
1. Introduction
Protein–carbohydrate recognition plays an important role in many physiological and disease processes, such as viral infection, cancer metastasis, and immune recognition.1-6 In biological systems, protein–carbohydrate recognition is often characterized by multivalent interactions between glycan clusters in glycoconjugates (e.g., glycoproteins) and glycan-binding proteins (e.g., lectins) with multiple carbohydrate recognition domains.6-13 Thus, multivalent interactions involving simultaneous binding of multiple sites represent a common strategy that has arisen in evolution for enhancing the strength and specificity between the two recognition parties.7 This shared process also provides important clues for guiding the design of efficient multivalent glyco-ligands as inhibitors for interventions of key host–pathogen interactions.13,14
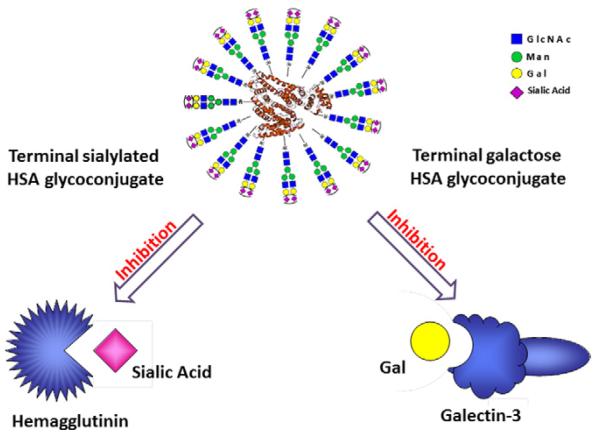
Influenza hemagglutinin (HA) is a glycoprotein found on the viral surface that is responsible for virus entry by binding to the sialic acid (SA) ligands (e.g., sialoglycoproteins) on the host cell membrane.7,15,16 On the other hand, galectin-3 (Gal3), a lectin that recognizes b-galactoside-terminating glycoconjugates such as the Thomsen-Friedenreich (TF) antigen (Galβ1,3GalNAc-Thr/Ser) plays an active role in cancer progression and metastasis. Gal3 may exert its effects via different mechanisms, including mediation of cell adhesion between endothelial and cancer cells, mediation of tumor cell homotypic aggregation, regulation of intracellular antiapoptotic function, and modulation of cell migration.10,17-22 It has been demonstrated that Gal3 plays an important role in the preferential adhesion of the prostate cancer cell to bone marrow endothelial cells,23 and over-expression of Gal3 occurs in many aggressive cancers, strongly suggesting a key participation of Gal3 in neoplastic progression and metastasis.24,25 These discoveries have stimulated extensive interest in recently years aiming at the design of effective inhibitors targeting the protein–carbohydrate interactions mediated by influenza HA and cancer-associated Gal3. For example, multivalent sialylated glycoconjugates, built on various templates including small-molecule scaffolds, polypeptides, polysaccharides, polyacrylamides, and nanoparticles, have been previously reported as inhibitors for competing the binding between SA and HA.7,26-30 Different Gal3 inhibitors that mimic the natural carbohydrate ligands have also been prepared and tested for targeting cancer metastasis.20,31-34 Despite these progresses, there is still an urgent need for improved design to address issues of the relatively low activity observed in some classes of the multivalent ligands, the cytotoxicity of polyacrylamide-based inhibitors, and the unwanted immunogenicity of protein-based ligands. We report herein the design and synthesis of biocompatible glycoprotein-based glyco-ligands by employing natural N-glycans as the carbohydrate ligands and endogenous human serum albumin (HSA) as the carrier protein (Fig. 1), which should be well tolerated in biological systems and are no or less immunogenic than foreign-protein based multivalent ligands carrying synthetic oligosaccharides. The glycoconjugates were assembled using the copper-catalyzed alkyne–azide cycloaddition reaction between an alkyne-labeled glycan and an azide-modified HSA. Binding studies show that the synthetic sialylated glycoproteins are high-affinity ligands for the influenza HA while the asialoglycoproteins are high-affinity ligand for Gal3. A preliminary cell-based assay indicates that the asialoglycoproteins act as potent inhibitors capable of efficiently blocking the attachment of Gal3 to cancer cells including the PC3 and A549 cancer cell lines.
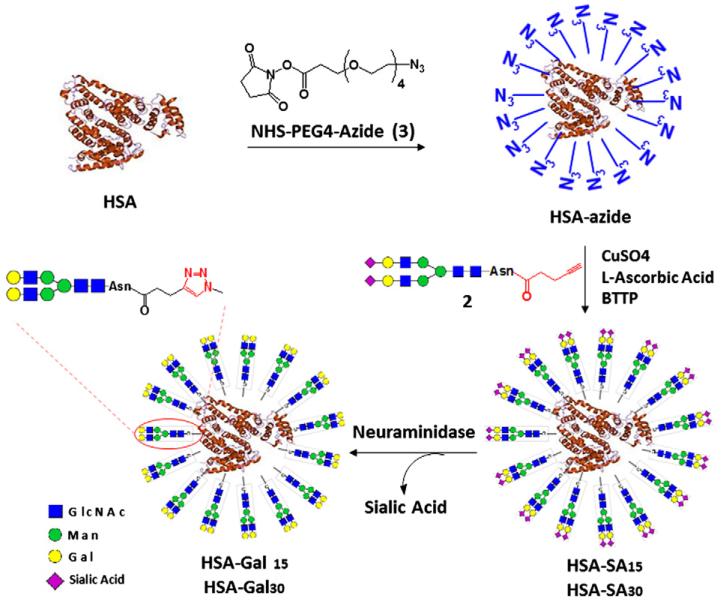
Figure 1.
Design of multivalent HSA-based neoglycoproteins.
2. Results and discussion
2.1. Design of the glycoprotein-based glyco-ligands
Our design strives to use biocompatible natural carrier protein and natural N-glycan ligands to construct multivalent gly-co-ligands as antiviral or anticancer agents that are expected to be of low cellular toxicity and be of no or low immunogenicity. For this reason, we chose to make a glycoconjugate using an endogenous human protein, human serum albumin (HSA) as the scaffold and a natural bi-antennary complex-type N-glycan as the carbohydrate ligand (Fig. 1). For the conjugation, we decided to apply the bio-orthogonal Cu(I)-catalyzed azide-alkyne cycloaddition reaction as the key step, which has been shown to be highly selective and efficient for biomolecule conjugation in water.35-38
For this purpose, an asparagine-linked sialylated complex-type bi-antennary glycan (SCT-Asn), prepared from the sialoglycopeptide (SGP) isolated from chicken egg yolk,39,40 was used as starting material and was labeled with an alkyne group at the Asn moiety, while the HSA was tagged with azide functionality to provide the two partners for conjugation. One advantage for this reaction design is that the density of glycan ligands loaded can be readily controlled by changing the ratio of ligands to the carrier in the reaction to provide glycoproteins with varied multivalency. The sialylated glycoconjugates are expected to act as inhibitors against SA/HA interactions in influenza infection. On the other hand, the terminal sialic acids (SA) can be easily removed by neuraminidase treatment to expose the galactose moieties in the non-reducing end, yielding a terminal galactosyl-HSA glycoconjugate for inhibition of Gal3 (Fig. 1). This design presents an efficient approach to attain multivalent glycoproteins with desired terminal carbohydrate moieties for different targets.
2.2. Synthesis of alkyne-labeled complex type N-glycan
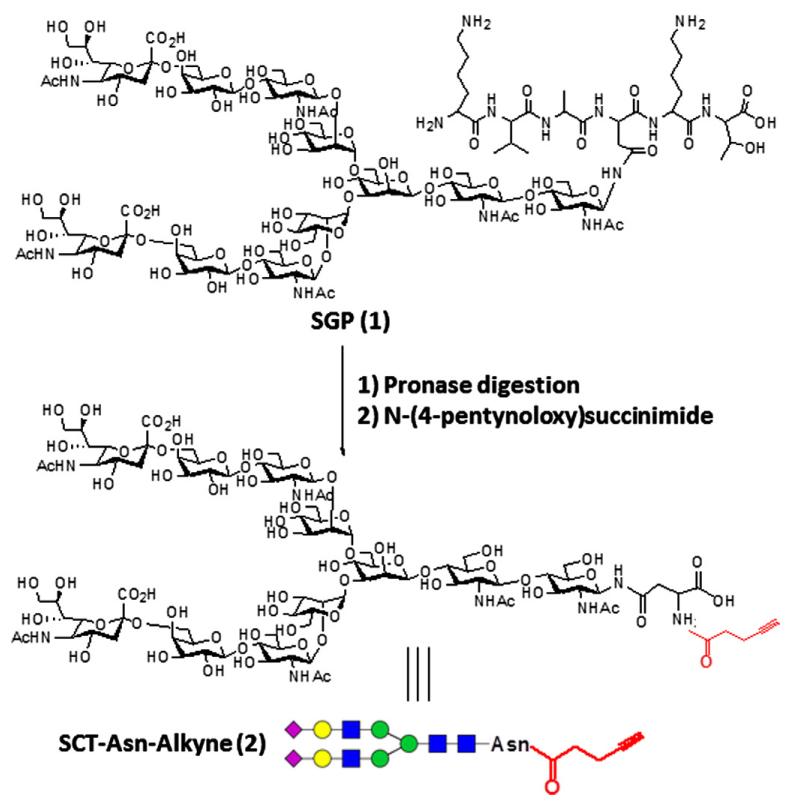
A sialoglycopeptide (SGP), Lys-Val-Ala-Asn(Sia2Gal2Glc-NAc2Man3GlcNAc2)-Lys-Thr, was purified from chicken egg yolks and used as the starting material for the preparation of the alkyne-labeled glycan ligand. The isolation of the SGP (Scheme 1) glycopeptide followed the previously described procedures39,40 with some modifications. Egg yolks were separated from egg white and treated with aqueous phenol. After removal of pellet by centrifugation, the supernatant was washed with chloroform to remove the phenol and lipids. The residue was subject to size-exclusion chromatography and reverse-phase HPLC to give the sialoglycopeptide (1). The product was characterized by HPLC, HPAEC-PAD, ESI-MS, and NMR, and showed properties identical to the authentic SGP.
Scheme 1.
Synthesis of alkyne-labeled N-glycan (2).
To synthesize the alkyne-labeled N-glycan, SGP was thoroughly digested with pronase to remove the peptide portion, leaving the asparagine (Asn)-linked N-glycan (SCT-Asn). The process was monitored by high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) (Dionex). The retention time (tR) of SCT-Asn was 24.8 min under the HPAEC conditions (see Section 4), which was well separated from the SGP peak (tR = 19.8 min) under the same chromatographic condition. After the digestion was complete, the residue was subject to size-exclusion (Sephadex G-25) chromatography to give the SCT-Asn. The identity of SCT-Asn was confirmed by ESI-MS analysis (calcd for SCT-Asn, M = 2338.10 Da; found (m/z): 1169.48 [M+2H]2+ and 780.43 [M+3H]3+). An alkyne group was then introduced into SCT-Asn by selective reaction of the free Asn amino group with an activated ester, N-(4-pentynoyloxy)succinimide in an aqueous solution. The resulting SCT-Asn-alkyne was purified by reverse-phase HPLC in 71% yield, and its identity was confirmed by ESI-MS (calcd for SCT-Asn-alkyne, M = 2418.18 Da; found (m/z), 1209.90 [M+2H]2+ and 807.21 [M+3H]3+).
2.3. Synthesis of HSA-based glycoproteins
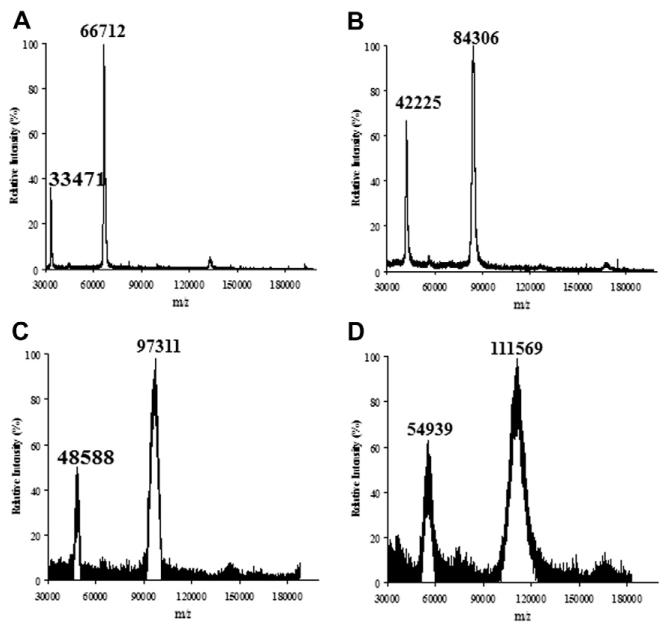
For conjugation with the alkyne-labeled glycan via click chemistry, we sought to incorporate azide groups on human serum albumin (HSA) taking advantage of free lysine residues on the protein surface (Scheme 2). The mature HSA protein consists of 585 amino acids with 60 lysine residues. Treatment of HSA with an excess of the activated azide derivative, NHS-PEG4-azide (3), in an aqueous NaHCO3 solution resulted in the introduction of multiple azide groups in the protein. The modified protein (HSA-azide) was purified by gel filtration. Comparison of the MALDI-TOF MS spectra of HSA and HSA-azide (Fig. 2, panels A and B) indicated that there was a gain of ca. 17 kDa for the azide-tagged HSA, suggesting an incorporation of about 60 azide moieties (with the linker) into HSA. This data implicates that almost all free amino groups in the HSA were reacted to the azide-containing reagent.
Scheme 2.
Synthesis of HSA-based neoglycoproteins.
Figure 2.
MALDI-TOF MS spectra of HSA and the synthetic glycoconjugates. (A) HSA; (B) HSA-azide; (C) HSA-Gal15; (D) HSA-Gal30.
The conjugation of SCT-Asn-alkyne and HSA-azide was achieved via the Cu(I)-catalyzed alkyne–azide cycloaddition reaction. A solution of the alkyne and azide in a phosphate buffer (pH 7.5) was incubated together with CuSO4, l-ascorbic acid, and a newly reported copper ligand, BTTP.41 To achieve varied degree of ligand loading, different molar ratios of SCT-Asn-alkyne to the HSA-azide were applied. After reaction, the resultant HSA-SA glycoconjugates were purified by size-exclusion chromatography. Treatment of the HSA-SA glycoconjugates with neuraminidase gave the corresponding asialoglycoproteins, HSA-Gal (Scheme 2). An initial attempt to measure the MALDI-TOF MS of the sialylated glycoprotein (HSA-SA) failed to obtain any meaningful data. This might be due to signal suppression by multiple negatively charged sialic acid (SA) units on the glycoconjugate surface.42,43 We then attempted to quantify the sialic acid (SA) contents in the glycoconjugates as a way to estimate the numbers of N-glycan chains introduced. Thus, HSA-SA was treated with neuraminidase and the released SA was quantified by HPAEC-PAD analysis. Our data indicated that there were about 15 and 30 SA units attached per HSA when the alkyne–glycan and HSA-azide were used in a molar ratio of 35:1 and 70:1 for the click reaction, respectively. The low and high glycan-loading glycoproteins were named as HSA-SA15 and HSA-SA30, respectively. Since each complex type N-glycan carries two SA units, these results suggest that the glycoproteins, HSA-SA15 and HSA-SA30, would contain about 8 and 15 N-glycan chains per HSA, respectively. In contrast to the sialylated glycoconjugates, the MALDI-TOF MS spectra of the asialoglycoproteins, HSA-Gal15 and HSA-Gal30, were successfully measured and the data was consistent with the SA quantification results (Fig. 2). Furthermore, HSA-Gal glycoconjugates were treated with a β-galactosidase to liberate the terminal galactose that was quantified by HPAEC-PAD. It was found that there were about 16 Gal units on HSA-Gal derived from HSA-SA15 and about 32 Gal units on HSA-Gal derived from HSA-SA30. These galactose quantification data were consistent with the results of the SA quantification and MALDI-TOF MS analysis of the glycoproteins.
2.4. Binding of the synthetic glycoproteins with influenza hemagglutinin (HA) and Gal3
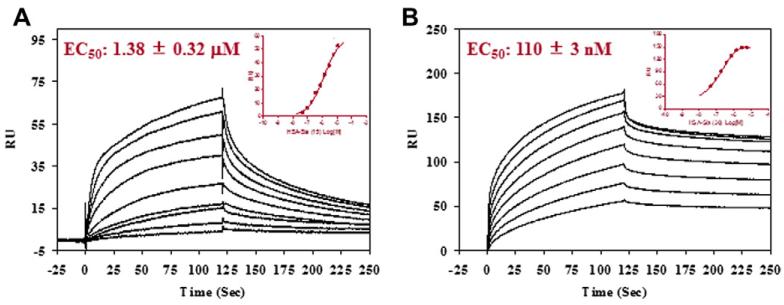
The affinity of HSA-SA and HSA-Gal for HA and Gal3, respectively, was measured by surface plasmon resonance (SPR) technology. HA or Gal3 was immobilized on a CM5 chip through an amine coupling method. Then, glycoproteins (HSA-SA or HSA-Gal) at various concentrations were injected and the responses were measured. The chip was regenerated by 3 M MgCl2 after each injection cycle. The data were plotted and analyzed using the GraphPad Prism4 software to obtain the EC50. It was found that the parent sialoglycopeptide (SGP) has only very weak affinity for the HA, with an estimated EC50 value being larger than 2 mM. In contrast, the multivalent sialoglycoprotein, HSA-SA, showed significantly enhanced affinity for HA (Fig. 3). An analysis of the binding data gave an EC50 of 1.4 μM and 110 nM for the low- and high-density ligand clusters, HSA-SA15 and HSA-SA30, respectively. This will correspond to an EC50 of 11 and 1.6 μM for the HSA-SA15 and HSA-SA30, respectively, based on the glycan units. The high-density ligand cluster demonstrated over sevenfold higher affinity for HA than the low-density ligand cluster per glycan unit. Moreover, both are much better ligands than the single complex type N-glycan. These results clearly show the multivalent effects on the interactions between the sialoglycoproteins and HA.
Figure 3.
SPR analysis of the binding of the sialoglycoproteins with immobilized influenza hemagglutinin. (A) HSA-SA15, starting concentration at 12 μM with 1:2 serial dilutions; (B) HSA-SA30, starting concentration at 6 μM with 1:2 serial dilutions. Inset, fitting of the SPR data.
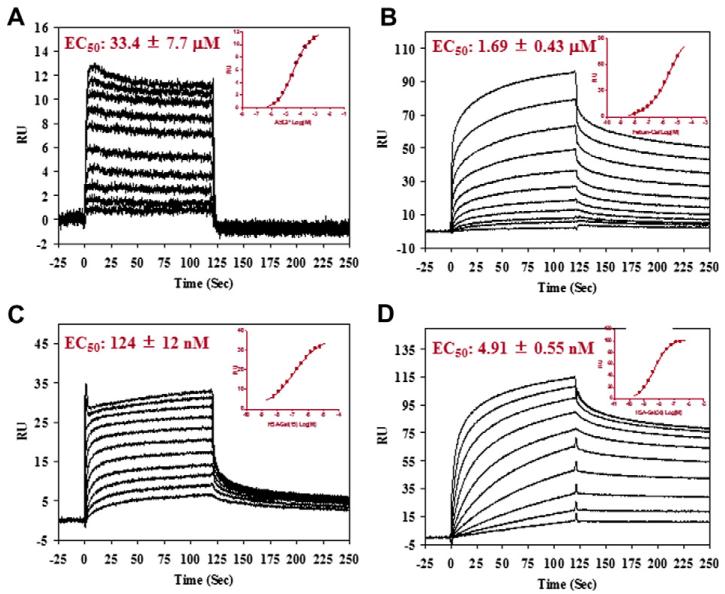
The HSA-Gal glycoproteins were assessed on their binding with Gal3 immobilized on a SPR CM5 chips (Fig. 4). Again, multivalent effects were also demonstrated in the interaction between the asialoglycoprotein HSA-Gal and Gal3. The EC50 value of HSA-Gal30 was 4.91 nM. As HSA-Gal30 containing about 15 N-glycans, the EC50 normalized to each glycan unit would be 73 nM, which would be about 460-fold better than the monomeric asialoglycopeptide (SGP) (EC50 = 33 μM). The EC50 for the asialo-fetuin (containing 3 N-glycans) and the HSA-Gal15 (containing about 8 N-glycans) were found to be 1.7 μM and 124 nM, respectively. When normalized by the glycan units attached, these would give a glycan unit-corrected EC50 of 5 and 1 μM, respectively. While the asialofetuin demonstrated only 6.6-fold enhancement of affinity in comparison with the SGP (EC50 = 33 μM), the synthetic HSA-Gal15 (containing about 8 N-glycans) showed 30-fold increase in affinity per glycan unit in comparison with the SGP carrying a single N-glycan. Notably, the synthetic glycoprotein HSA-Gal30 (glycan-corrected EC50, 73 nM) demonstrated 58-fold higher affinity for Gal3 than the asialofetuin (a natural asialoglycoprotein, glycan-corrected EC50, 5 μM). All these binding data demonstrated the successful design of the multivalent glycoconjugates and the power of glycoside clustering effect.
Figure 4.
SPR analysis of the binding of the asialoglycoproteins with immobilized galectin-3. (A) de-sialylated SGP starting concentration at 800 μM with 1:2 serial dilutions; (B) asialofetuin, starting concentration at 10 μM with 1:2 serial dilutions; (C) HSA-Gal15, starting concentration at 6 μM with 1:2 serial dilutions; (D) HSA-Gal30, starting concentration at 250 nM with 1:2 serial dilutions. Inset, fitting of the SPR data.
2.5. Inhibition of the attachment of Gal3 to immobilized cancer cells by HSA-Gal30
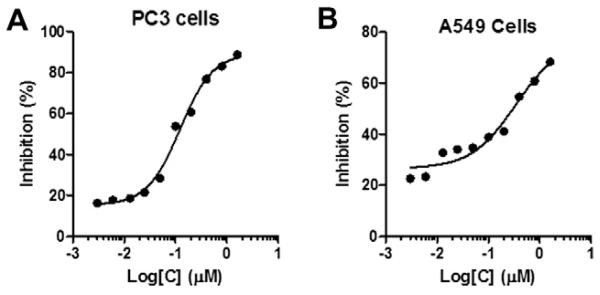
To evaluate whether the synthetic asialoglycoproteins could actually inhibit the adhesion of Gal3 to cancer cells (Fig. 5) for functions, we performed a cell-based assay using HSA-Gal30 as the inhibitor. Briefly, cancer cells, including the PC3 prostate cancer cell and the A549 alveolar basal epithelial adenocarcinoma cell, were immobilized and the binding of Gal3 to the immobilized cells in the presence or absence of HSA-Gal30 at various concentrations was assessed by ELISA. Our preliminary data showed that HSA-Gal30 was able to block the attachment of Gal3 to both cancer cells in a dose-dependent manner (IC50 for PC3 cells, 122 nM; IC50 for A549 cells, 338 nM). This result suggests that the synthetic HSA-based glyco-ligands may find applications for blocking the function of Gal3 in cancer cell aggregation, adhesion and metastasis, thus providing a potentially useful approach to cancer treatment.
Figure 5.
Inhibition of the attachment of galectin-3 to immobilized cancer cells by HSA-Gal30. The cells were immobilized and fixed on micro-plastic plates and incubated with galectin-3 in the presence or absence of the inhibitor. The bound galectin-3 was probed by ELISA with mouse anti-galectin-3 antibody and goat anti-mouse IgG-HRP. Panel A, PC3 prostate cancer cells; Panel B, A549 human lung cancer cells.
3. Conclusion
This paper describes an efficient synthesis of a new class of multivalent glyco-ligands and their interactions with two biologically important targets: the influenza hemagglutinin (HA) involved in viral entry and the human galectin-3 (Gal3) associated with immune regulation and cancer metastasis. The efficient incorporation of alkyne and azide groups into the glycan and protein scaffold, respectively, followed by their conjugation via the highly efficient click chemistry provides a general approach to the synthesis of neoglycoproteins. Our binding studies demonstrate a clear multivalent effect on the interactions between the synthetic glyco-ligands with both HA and Gal3. The observed high affinity of the sialo- and asialo-glycoproteins for HA and Gal3, respectively, suggests that these synthetic glyco-ligands may be further developed as potent inhibitors to block viral entry or cancer metastasis. Our preliminary cell-based assay indicated that the asialoglycoprotein, HSA-Gal30 was able to block the binding of Gal3 to cancer cells, underscoring the potential of these novel multivalent glyco-ligands for therapeutic intervention. Future studies will be directed to a detailed mechanistic analysis of the inhibition of the multivalent glyco-ligands for viral entry and cancer metastasis.
4. Experimental
4.1. Materials
N-(4-Pentynoyloxy)succinimide was prepared following the previously described procedure.44,45 NHS-PEG4-azide (3) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Neuraminidase was purchased from New England Biolabs (Ipswich, MA). The unit definition was following the company’s manual. Influenza hemagglutinin was purchased from AXXORA LLC (Farmingdale, NY). Recombinant Gal3 was expressed following the reported procedures.22 All other reagents were purchased from Sigma–Aldrich (St. Louis, MO) and used as received.
4.2. HPLC analysis
Analytic RP-HPLC was performed on a Waters 626 HPLC instrument with a Symmetry 300 C18 column (5.0 μm, 4.6 × 250 mm) at 40 °C. The column was eluted with a linear gradient of 0–30% MeCN containing 0.1% TFA for 30 min at a flow rate of 1.0 mL/min. Preparative HPLC was performed with a Waters 600 HPLC instrument of a Waters C18 column (Symmetry 300, 7.0 μm, 19 × 300 mm). The column was eluted with a suitable gradient of MeCN–H2O containing 0.1% TFA at a flow rate of 12 mL/min.
4.3. HPAEC-PAD analysis
High-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) was performed on a DIONEX DX600 chromatography system (DIONEX Corporation, Sunnyvale, CA) equipped with an electrochemical detector (ED50) and an anion exchange column (CarboPac PA100, 4 × 250 mm). The mobile phase (flow rate, 1.0 mL/min) was composed of deionized water (eluent A), 1 M NaOAc (eluent B), and 0.2 M NaOH (eluent C).The gradient used was as follows: 0 min, 50% eluent A, 0% eluent B, 50% eluent C; 5.0 min, 50% eluent A, 0% eluent B, 50% eluent C; and 25.0 min, 35% eluent A, 15% eluent B, and 50% eluent C.
4.4. Mass spectrometric analysis
ESI-MS spectra were measured on a Waters Micromass ZQ-4000 single-quadrupole mass spectrometer. The MALDI-TOF MS was performed on an Autoflex II MALDI-TOF mass spectrometer (Bruker Daltonics, Billerica, MA). The instrument was calibrated by using ProteoMass Peptide MALDI-MS calibration kit (MSCAL2, Sigma/Aldirich). The matrix of sinapinic acid was used for the HSA and HSA conjugates.
4.5. Preparation of sialoglycopeptide (SGP) from egg yolks
24 fresh chicken eggs were obtained from market and the egg yolks were separated from egg white. The egg yolks (400 mL) were diluted with an equal volume of water. The solution was mixed with 80 mL of 90% aqueous phenol and stirred vigorously for 2 h at 4 °C. The emulsion was diluted with 400 mL of water and centrifuged at 6000 rpm for 30 min. The supernatant was washed with ethyl acetate and chloroform to remove the phenol and then centrifuged again and lyophilized. The residue was subject to gel filtration by a Sephadex G-25 column. The column was eluted with 0.1 M AcOH, and the fractions were analyzed by thin layer chromatography (TLC) and HPAEC-PAD (Dionex). The SGP fractions were combined and concentrated, and the residue was subject to preparative HPLC purification to give the pure SGP as a white powder. Analytic RP-HPLC: tR = 20.7 min; HPAEC-PAD: tR = 19.8 min; ESI-MS: calcd, M = 2864.17, found, 1433.78 [M+2H]2+, 956.48 [M+3H]3+, 717.73 [M+4H]4+.
4.6. Preparation of SCT-Asn-alkyne (2)
SGP (60.6 mg, 21.1 μmol) in a Tris–HCl buffer (3 mL, 50 mM, pH 7.5) containing. CaCl2 (10 mM) and 0.1% NaN3 were digested by incubation with pronase (10 mg, 5.5 units/mg) at 37 °C for 5 days. The pH was monitored during the digestion and adjusted to pH 7.5 by adding aqueous Na2CO3. The reaction was monitored by HPAEC-PAD (Dionex) until all the SGP was converted to SCT-Asn. The solution was lyophilized and the residue was subject to gel filtration with a Sephadex G-25 column. The column was eluted with 0.1 M acetic acid and product fractions were combined and lyophilized. A total of 33 mg of SCT-Asn was obtained (yield 55%) as a white solid. HPAEC-PAD: tR = 24.8 min; ESI-MS: calcd, M = 2338.10, found, 1169.48 [M+2H]2+, 780.43 [M+3H]3+. To prepare the SCT-Asn-alkyne derivative, the obtained SCT-Asn (28.0 mg, 12.0 μmol) was dissolved in aqueous sodium bicarbonate (0.2 M, pH 8.0, 351 μL) and a solution of N-(4-pentynoyloxy)-succinimide (3.5 mg, 43.9 μmol) in MeCN (351 μL) containing MeOH (35 μL) was added. The mixture was incubated at room temperature for 2 h. The residue was subject to preparative RP-HPLC for purification to give the SCT-Asn-alkyne (2) (20.0 mg, yield 71.4%). Analytic RP-HPLC: tR = 19.5 min; ESI-MS: calcd, M = 2418.18, found, 1209.90 [M+2H]2+, 807.21 [M+3H]3+. 1H NMR (D2O, 400 MHz) δ 5.12 (s, 1 H, H-1 of β-Man), 5.04 (d, 1 H, J = 9.6 Hz, H-1 of β-GlcNAc), 4.94 (s, 1 H, H-1 of α-Man), 4.77 (s, 1 H, H-1 of α-Man), 4.63–4.58 (m, 3 H, H-1 of three β-GlcNAc), 4.48–4.43 (m, 2 H, H-1 of two β-Gal), 4.25 (s, 1 H, H-2 of β-Man), 4.19 (s, 1 H, H-2 of α-Man), 4.11 (s, 1 H, H-2 of α-Man), 2.87–2.84 (m,1 H, Asn-β-CH2), 2.69–2.62 (m, 2 H, H-3eq of NeuAc), 2.58–2.51 (m, 4 H, CH2CH2-alkyne), 2.38–2.35 (m,1 H, Asn-β-CH2), 2.07 (s, 3 H, Ac), 2.06 (s, 3 H, Ac), 2.02 (s, 3 H, Ac), 2.0 (s, 3 H, Ac), 1.75 (t, 2 H, J = 12.2 Hz, H-3ax of NeuAc).
4.7. Preparation of HSA-azide and HSA glycoconjugates
Human Serum Albumin (HSA) (10 mg) and NHS-PEG4-azide (3) (4 mg) in a NaHCO3 buffer (50 mM, pH 8.0, 2 mL) were incubated at room temperature for 5 h. The reaction mixture was loaded to a Sephadex G-50 gel filtration column and the column was eluted with Tris–Cl buffer (10 mM, pH 8.0). The product fractions were combined and concentrated to yield HSA-azide (12 mg). MALDI-TOF MS: M = 84,306 Da.
4.8. Conjugation of SCT-Asn-alkyne and HSA-azide—preparation of moderate glycan loading conjugate
A mixture of SCT-asn-alkyne (12.3 mg, 5.1 μmol), HSA-azide (12.3 mg, 0.144 μmole), CuSO4·5H2O (0.8 μmol), l-ascorbic acid (1.6 μmol), and BTTP (5.0 mg) in a phosphate buffer (50 mM, pH 7.5, 3 mL) was incubated at room temperature overnight. The mixture was then directly applied to a Sephadex G-50 column for gel filtration. The column was eluted with Tris–Cl buffer (10 mM, pH 8.0). The product fractions were combined and concentrated to afford HSA-SA15 (12 mg). The glycoconjugate was characterized by SA quantification and MALDI-TOF MS after treated with neuraminidase. HSA-SA15 (3 mg) in a phosphate buffer (50 mM, pH 5.5, 500 μL) was incubated with neuraminidase (200 U) at 37 °C overnight. The released sialic acid was quantified by HPAEC-PAD. The reaction mixture was subject to a Sephadex G-50 gel filtration to give the HSA-Gal15 (3 mg). MALDI-TOF MS: M = 97,311 Da.
4.9. Conjugation of SCT-Asn-alkyne and HSA-azide—high loading of glycan ligand
A mixture of SCT-asn-alkyne (8 mg, 3.3 μmol), HSA-azide (3 mg, 45.4 nmol), CuSO4·5H2O (1.0 μmol), l-ascorbic acid (2.0 μmol), and BTTP (2 mg) in a phosphate buffer (50 mM, pH 7.5, 100 μL) was incubated at room temperature overnight. The mixture was then subject to gel filtration with a Sephadex G-50 column. The column was eluted with Tris–Cl buffer (10 mM, pH 8.0). The product fractions were combined and concentrated to afford HSA-SA30 (3 mg). The glycoconjugate was characterized by SA quantification and MALDI-TOF MS after treatment with neuraminidase. HSA-SA30 (3 mg) in a phosphate buffer (50 mM, pH 5.5, 500 μL) was incubated with neuraminidase (200 U) at 37 °C overnight. The released sialic acid was quantified by HPAEC-PAD. The reaction mixture was subject to a Sephadex G-50 gel filtration to give the HSA-Gal30 (3 mg). MALDI-TOF MS: M = 111.569 Da.
4.10. Surface plasmon resonance (SPR) measurement
Surface plasmon resonance measurement was carried on a BIA-core T100 (GE Healthcare) instrument. Influenza hemagglutinin and Gal3 were immobilized on individual CM5 chips until reaching 1000 response units. A reference channel was immobilized with ethanolamine, respectively. Immobilizations were carried out at protein concentrations of 25 μg/mL in 10 mM acetate (pH 5.0) by using an amine coupling kit supplied by the manufacturer. In all instances, analytes were carried out at 25 °C in 10 mM HEPES, pH 7.4 containing 150 mM NaCl and 0.005% surfactant P20 at a flow rate of 20 μL/min. The association time was 120 s and dissociation time was 200 s. The surface was regenerated by 3 M MgCl2 solution. Data were analyzed with GraphPad Prism4 for EC50 calculation.
4.11. Inhibition of Gal3 binding to immobilized cancer cells
Binding of human Gal3 to A549 and PC-3 cells and its inhibition by HSA-Gal30 was determined in triplicate on 96-well polystyrene plates (Corning Inc., Corning, NY). For this purpose, A549 and PC3 cells (2 × 105 cells/100 μL/well) were seeded with complete DMEM medium in the presence of 10% FBS and incubated overnight at 37 °C. Attached cells (about 90% confluent) were washed three times with PBS (1 ×) and treated with neuraminidase (from Clostridium perfringens; 0.3 unit/106 cells) in serum-free medium for 1 h at 37 °C. In initial experiments both untreated and neuraminidase-treated cells were tested for Gal3 binding. After removal of the supernatant, the treated or untreated cells were fixed with 4% paraformaldehyde in PBS for 20 min at rt followed by three washes with PBS containing 0.1 M glycine. The fixed cells were blocked with 3% BSA in PBS for 30 min at rt and washed once with PBS (1×). The cells were washed again with 10 mM β-mercaptoethanol (ME) in PBS (PBS-ME; 1×) and immediately incubated with Gal3 (control) or a mixture of Gal3 and varying amounts of test inhibitor that had been pre-incubated for 1 h at 4 °C. After incubation for 1 h at 4 °C, the plates were washed with PBS/ME and incubated with a monoclonal anti-Gal3 antibody (1:1000) for 50 min at rt. After washing with cold PBS-Tween 20 buffer for three times, plates were incubated with goat anti-mouse IgG/HRP (1:2000) for 50 min at rt. The plates were washed three times with cold PBS-Tween 20 buffer and developed with 100 ml of TMB substrate. The reaction was stopped with 1 M HCl and read at 450 nm. From the mean value of absorbance, an inhibition curve was drawn.
Acknowledgments
We thank Professor Peng Wu for kindly providing BTTP that was used as catalyst for the click chemistry; Dr. Mohammed N. Amin, Dr. Joseph V. Lomino, and other members of the Wang Lab for technical assistance and helpful discussions. This work was supported by the National Institutes of Health (NIH Grants R01 GM080374 to L.X.W. and 5R01GM070589-06 to G.R.V.).
References and notes
- 1.Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwek RA. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 3.Paulson JC, Blixt O, Collins BE. Nat. Chem. Biol. 2006;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 4.Vasta GR. Nat. Rev. Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinovich GA, Toscano MA. Nat. Rev. Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 6.Vasta GR. Adv. Exp. Med. Biol. 2012;946:21–36. doi: 10.1007/978-1-4614-0106-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammen M, Choi SK, Whitesides GM. Angew. Chem., Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Cloninger MJ. Curr. Opin. Chem. Biol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- 9.Wolfenden ML, Cloninger MJ. Bioconjugate Chem. 2006;17:958–966. doi: 10.1021/bc060107x. [DOI] [PubMed] [Google Scholar]
- 10.Pieters RJ. ChemBioChem. 2006;7:721–728. doi: 10.1002/cbic.200600011. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman N. Chem. Soc. Rev. 2009;38:3463–3483. doi: 10.1039/b815961k. [DOI] [PubMed] [Google Scholar]
- 12.Lee RT, Lee YC, Glycoconj J. 2000;17:543–551. doi: 10.1023/a:1011070425430. [DOI] [PubMed] [Google Scholar]
- 13.Deniaud D, Julienne K, Gouin SG. Org. Biomol. Chem. 2011;9:966–979. doi: 10.1039/c0ob00389a. [DOI] [PubMed] [Google Scholar]
- 14.Chabre YM, Roy R. Adv. Carbohydr. Chem. Biochem. 2010;63:165–393. doi: 10.1016/S0065-2318(10)63006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaverin NV, Matrosovich MN, Gambaryan AS, Rudneva IA, Shilov AA, Varich NL, Makarova NV, Kropotkina EA, Sinitsin BV. Virus Res. 2000;66:123–129. doi: 10.1016/s0168-1702(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 16.Matrosovich M, Klenk HD. Rev. Med. Virol. 2003;13:85–97. doi: 10.1002/rmv.372. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara S, Raz A. Methods Enzymol. 2006;417:273–289. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- 18.Nakahara S, Raz A. Cancer Metastasis Rev. 2007;26:605–610. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nangia-Makker P, Nakahara S, Hogan V, Raz AJ. Bioenerg. Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glinsky VV. Cancer Metastasis Rev. 2006;25:531–540. doi: 10.1007/s10555-006-9029-8. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed H, Banerjee PP, Vasta GR. Biochem. Biophys. Res. Commun. 2007;358:241–246. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed H, Cappello F, Rodolico V, Vasta GR. Transl. Oncol. 2009;2:146–156. doi: 10.1593/tlo.09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehr JE, Pienta KJ. J. Natl. Cancer Inst. 1998;90:118–123. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 24.Califice S, Castronovo V, Van Den Brule F. Int. J. Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- 25.Inufusa H, Nakamura M, Adachi T, Aga M, Kurimoto M, Nakatani Y, Wakano T, Miyake M, Okuno K, Shiozaki H, Yasutomi M. Int. J. Oncol. 2001;19:913–919. doi: 10.3892/ijo.19.5.913. [DOI] [PubMed] [Google Scholar]
- 26.Marra A, Moni L, Pazzi D, Corallini A, Bridi D, Dondoni A. Org. Biomol. Chem. 2008;6:1396–1409. doi: 10.1039/b800598b. [DOI] [PubMed] [Google Scholar]
- 27.Oka H, Onaga T, Koyama T, Guo CT, Suzuki Y, Esumi Y, Hatano K, Terunuma D, Matsuoka K. Bioorg. Med. Chem. 2009;17:5465–5475. doi: 10.1016/j.bmc.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Hidari KI, Murata T, Yoshida K, Takahashi Y, Minamijima YH, Miwa Y, Adachi S, Ogata M, Usui T, Suzuki Y, Suzuki T. Glycobiology. 2008;18:779–788. doi: 10.1093/glycob/cwn067. [DOI] [PubMed] [Google Scholar]
- 29.Makimura Y, Watanabe S, Suzuki T, Suzuki Y, Ishida H, Kiso M, Katayama T, Kumagai H, Yamamoto K. Carbohydr. Res. 2006;341:1803–1808. doi: 10.1016/j.carres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Papp I, Sieben C, Sisson AL, Kostka J, Bottcher C, Ludwig K, Herrmann A, Haag R. ChemBioChem. 2011;12:887–895. doi: 10.1002/cbic.201000776. [DOI] [PubMed] [Google Scholar]
- 31.Nangia-Makker P, Conklin J, Hogan V, Raz A. Trends Mol. Med. 2002;8:187–192. doi: 10.1016/s1471-4914(02)02295-5. [DOI] [PubMed] [Google Scholar]
- 32.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. J. Natl. Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 33.Fort S, Kim HS, Hindsgaul O. J. Org. Chem. 2006;71:7146–7154. doi: 10.1021/jo060485v. [DOI] [PubMed] [Google Scholar]
- 34.Glinskii OV, Sud S, Mossine VV, Mawhinney TP, Anthony DC, Glinsky GV, Pienta KJ, Glinsky VV. Neoplasia. 2012;14:65–73. doi: 10.1593/neo.111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertozzi CR. Acc. Chem. Res. 2011;44:651–653. doi: 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 37.Ning X, Guo J, Wolfert MA, Boons GJ. Angew. Chem., Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 39.Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Biochim. Biophys. Acta. 1997;1335:23–32. doi: 10.1016/s0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Singh S, Zeng Y, Song H, Wang LX. Bioorg. Med. Chem. Lett. 2005;15:895–898. doi: 10.1016/j.bmcl.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 41.Besanceney-Webler C, Jiang H, Zheng T, Feng L, Soriano Del Amo D, Wang W, Klivansky LM, Marlow FL, Liu Y, Wu P. Angew. Chem., Int. Ed. 2011;50:8051–8056. doi: 10.1002/anie.201101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alley WR, Jr., Novotny MV. J. Proteome Res. 2010;9:3062–3072. doi: 10.1021/pr901210r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hortin GL. Clin. Chem. 2006;52:1223–1237. doi: 10.1373/clinchem.2006.069252. [DOI] [PubMed] [Google Scholar]
- 44.Salmain M, Vessieres A, Butler IS, Jaouen G. Bioconjugate Chem. 1991;2:13–15. doi: 10.1021/bc00007a002. [DOI] [PubMed] [Google Scholar]
- 45.Ochiai H, Huang W, Wang LX. J. Am. Chem. Soc. 2008;130:13790–13803. doi: 10.1021/ja805044x. [DOI] [PMC free article] [PubMed] [Google Scholar]