Abstract
Animals deficient for connexin 45 (Cx45), Cx43, or Cx40 and Cx37 all suffer embryonic or post-natal lethal vascular phenotypes. We developed an in vitro model of blood vessel assembly to dissect the specific roles of these connexins in this process. Previously, we showed that heterocellular gap junction channel formation between endothelial and mesenchymal cells is required for TGF-β activation and endothelial-induced mural cell differentiation, and that Cx43-containing channels support these processes. Developmental studies suggest that Cx45 is required for mural cell development during embryogenesis, although its exact role was not delineated.
OBJECTIVE
The focus of this study was to investigate the role of Cx45 in endothelial-induced mural cell differentiation.
METHODS AND RESULTS
We created mural cell precursors that stably express only Cx45 in Cx43-deficient mesenchymal cells (ReCx45), and used our in vitro model of blood vessel assembly to assess the capacity of this Cx to support endothelial-induced mural cell differentiation. Lucifer Yellow dye injection and dual whole-cell patch clamping revealed that functional gap junctions exhibiting properties of Cx45-containing channels formed amongst ReCx45 transfectants, and between ReCx45 and endothelial cells. Heterocellular Cx45-containing gap junction channels enabled TGF-β activation, and promoted the upregulation of mural cell-specific proteins in the mesenchymal precursors.
CONCLUSION
These studies reveal a critical role for Cx45 in the regulation of endothelial-induced mural cell differentiation, which is consistent with the phenotype of Cx45-deficient embryos that exhibit dysregulated TGF-β and lack mural cell development.
Keywords: Gap junction, connexin, mural cell development, TGF-β, endothelial cell
Blood vessels are composed predominantly of two cell types: endothelial cells that form the luminal lining and mural cells (vascular smooth muscle cells and pericytes) that make up the surrounding medial layer. During blood vessel formation, endothelial tubes form first and govern the subsequent formation of the vessel wall via release of platelet-derived growth factor-B (PDGF-B), which acts as a chemoattractant and mitogen for mural cell precursors derived from the surrounding mesenchyme.1,2 Upon contact with endothelial cells, newly recruited mesenchymal progenitor cells are induced toward a mural cell fate2 by endothelial cell-mediated activation of transforming growth factor-beta (TGF-β).3-5 Although the process of TGF-β activation in response to heterocellular interactions is unclear,6, 7 gene-targeting experiments indicate that TGF-β8 signaling via activation of activin-like kinase (ALK) receptors9 plays a critical role in vascular development. Thus, local mesenchymal progenitors are recruited by endothelial cells to differentiate into the mural cell layer(s) in developing vessels, likely resulting in tissue-specific functional and regulatory properties of mural cells.10
Observations from genetically altered mice suggest that gap junctions play a critical role in vascular development,11-13 and we showed more specifically that gap junction channel formation between endothelial cells and recruited mesenchymal cells is required for their endothelial-induced differentiation into a mural cell phenotype.3 Gap junctions are aggregates of intercellular channels that allow the diffusion of second messengers, ions and metabolites to the cytoplasm of adjoining cells.14 Gap junction channels that form between vascular cells are composed of one or more connexin (Cx) proteins that include Cx37, Cx40, Cx43 and Cx45.3, 15-17 In the adult arterial vasculature, endothelial cells of large vessels predominantly express Cx37 and Cx40, whereas Cx43 expression is largely in the microvasculature and mural cells.15, 18 Although Cx45 is modestly co-expressed with Cx43 in the medial layer of adult vessels,19 it does not appear to be a major contributor to gap junction function in postnatal vasculature.18, 20, 21 However, Cx45 is highly expressed in the developing vasculature in both endothelial and smooth muscle cells22, 23 where it appears to be critical for mural cell investment of endothelial cell tubes; Cx45-deficient mice die mid-gestation due, in part, to failure of vascular smooth muscle to form.11 Interestingly, although Cx43 is often co-expressed with Cx45 during development,22, 23 it cannot compensate for loss of Cx45 during early stages of blood vessel formation. Similarly, Cx45 does not compensate for lack of Cx43 at later stages of development; mice deficient for Cx43 die peri-natally from severe cardiovascular malformations.12 Thus, Cx43 and Cx45 are both critical for proper vascular development, although their exact function(s), distinct or similar, at various stages of development have not been clearly delineated.
In previous studies, we demonstrated that Cx43 is highly expressed by endothelial cells, as well as mesenchymal cells that function as mural cell precursors.3 Furthermore, Cx43-mediated coupling between endothelial and mesenchymal cells promotes activation of TGF-β that enables endothelial-induced mural cell differentiation.3 In this study, we investigated whether Cx45, which is also expressed by endothelial and mesenchymal cells, although to a lesser extent,3 also regulates mural cell differentiation via similar mechanisms. We generated mesenchymal cells that stably express Cx45 (ReCx45) in parental cells that lack Cx43 (derived from Cx43−/− mice), and found that they formed functional gap junctions amongst themselves and with endothelial cells. Heterocellular Cx45-containing gap junctions supported TGF-β activation and promoted the upregulation of mural cell-specific proteins in the mesenchymal cells. These results reveal a critical, and previously undefined, role for Cx45 in the regulation of mural cell differentiation that likely underlie the lethal defects observed in Cx45-deficient embryos, which exhibit dysregulated TGF-β and lack mural cell development.11
Methods
Extended methods are provided in Supplemental Material.
Cell Culture
Cx43-expressing (Cx43wt) and Cx43-deficient (Cx43−/−) mesenchymal cells3 were used. Cx43−/− mesenchymal cells were also transfected with full-length Cx45 (ReC45) (see below). Endothelial and smooth muscle cells were isolated from bovine tissue. In some experiments, cells were plated on 0.25 - 1 μg/cm2 fibronectin (Sigma #F4759) at 37°C.
Generation of Cx45-Expressing Mesenchymal Cells
Cx45 cDNA in a pCl-neo vector was stably transfected into Cx43−/− mesenchymal cells (ReCx45). Individual clones of stable transfectants were selected for further study.
Assessment of Gap Junction Communication
Gap junction intercellular communication was assessed by scrape loading, dual dye injection, and whole cell patch clamp, as detailed in Supplemental Methods.
Assessment of Endothelial-Induced Mural Cell Differentiation
Endothelial and mesenchymal cells were cultured alone or in co-culture for 48h, and Western blot and immunofluorescent techniques were used to assess mural cell differentiation as described.3
Measurement of Activated TGF-β
An established bioassay24 was employed to measure levels of activated TGF-β, as described.3
Treatment with Exogenous TGF-β1 or TGF-β Blocking Antibodies
Cells were exposed for 48 hr to either 1ng/mL human TGF-β1 (R&D #100-B-001) or 10μg/mL TGF-β blocking antibodies that target all isoforms of TGF-β (Genzyme #1836-01) as described.3
Assessment of Extracellular Matrix Deposition
Solo cultures or co-cultures of PKH26-labeled endothelial cells and unlabeled mesenchymal cells were plated for 48 hr onto glass coverslips and assessed for fibronectin (BD #610077, 1:250) or fibrillin (Santa Cruz #sc-7540, 1:100) expression by immunofluorescence. Five random fields from 4-12 experiments were quantified for ECM deposition.
Results
We previously showed that Cx43-deficient mesenchymal cells fail to form functional gap junctions with endothelial cells and undergo mural cell differentiation upon heterocellular contact. Re-expression of Cx43 in Cx43−/− mesenchymal cells restored gap junction formation, TGF-β activation and endothelial-induced mural cell differentiation.3 Observations from the Cx45 knock-out animal suggest that this connexin is necessary for mural cell development during embryogenesis.11 Using our in vitro model, we investigated whether Cx45 plays a direct role in endothelial-induced mural cell differentiation.
Stable Expression of Cx45 in Cx43−/− Mesenchymal Cells
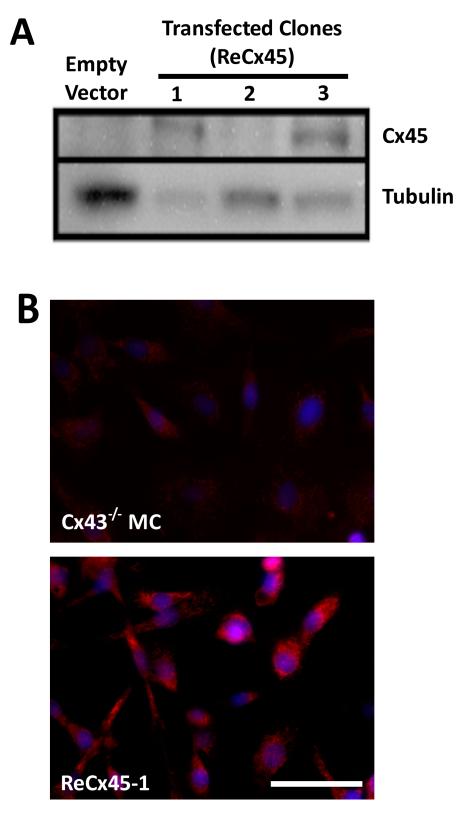
To determine whether Cx45 can regulate endothelial-induced mural cell differentiation, we generated mesenchymal cells that expressed only Cx45 (ReCx45). The Cx43−/− mesenchymal cells represent an excellent model as its primary connexin has been removed,12, 25 and the remaining low levels of Cx45 are not detectable by Western blot analysis,25 and do not support heterocellular communication of injected dyes or mural cell differentiation.3 In this study, full-length Cx45 cDNA was transfected into Cx43−/− mesenchymal cells and stable puromycin-resistant clones were selected. Two clones (ReCx45-1 and ReCx45-3) expressed ~45kDa protein detected by anti-Cx45 antibodies (Figure 1A). ReCx45-1 expressed the highest levels of Cx45 and ReCx45-3 expressed levels that were ~90% of ReCx45-1; both of these clones were used for all subsequent studies. No Cx45 expression was evident in Cx43−/− mesenchymal cells transfected with empty vector or in the ReCx45-2 clone (Figure 1A). Immunofluorescent analysis of ReCx45-1 (and ReCx45-3, not shown) revealed abundant Cx45 signal compared to parental Cx43−/− cells (Figure 1B).
Figure 1. Stable expression of Cx45 in Cx43−/− mesenchymal cells.
A) Western blot analyses demonstrated that Cx43−/− cells transfected with the empty vector did not express detectable Cx45 protein. Stably transfected clones, ReCx45-1 and ReCx45-3, expressed significantly higher levels of Cx45 protein, whereas the ReCx45-2 clone did not. B) Immunocytochemistry of ReCx45-1 (and Re45-3, not shown) cells revealed significant Cx45 expression (red), which was not observed in parental Cx43−/− mesenchymal cells (scale = 50μm, nuclei = blue).
Heterotypic Communication between Cx45 Mesenchymal and Endothelial Cells
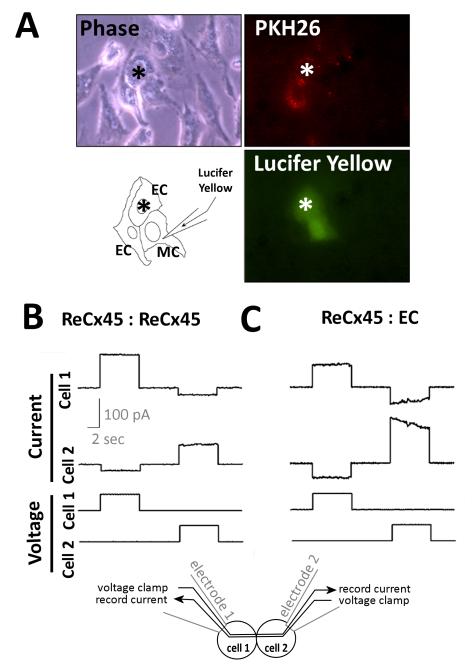
To determine whether Cx45 mediates intercellular communication between mesenchymal and endothelial cells, dye coupling and dual whole cell voltage clamp techniques were employed. Homocellular dye coupling was assessed in confluent monolayers of ReCx45 mesenchymal cells using a scrape loading assay (data not shown).26 Uptake of the gap junction-permeable Lucifer Yellow dye was significantly increased (by ~25%, n=3-6) compared to connexin- and communication-deficient Cx43−/− mesenchymal cells. To determine if dye coupling was retained in ReCx45 cells when co-cultured with endothelial cells, we performed microinjection of Lucifer Yellow. The heterocellular nature of assessed cell pairs was ensured by labeling one cell type with a fluorescent dye (either PKH26 or DiI) and by selecting cell pairs that included a fluorescent and a non-fluorescent cell for analysis. No dye- or electrical-coupling could be detected in co-cultures of endothelial cells with parental Cx43−/− mesenchymal cells.3 In contrast, 20 of 102 (19.6%) ReCx45 cells were dye-coupled to at least one neighboring endothelial cell (Figure 2A).
Figure 2. ReCx45 cells formed functional gap junctions with themselves and with EC that support intercellular dye and electrical coupling.
A) PKH26-labeled (red) endothelial cells (EC) were co-cultured with ReCx45 mesenchymal cells, and heterocellular pairs or clusters of cells were assessed for gap junction coupling. In the representative dye injection reproduced here, the ReCx45 mesenchymal cell was microinjected with Lucifer Yellow dye that transferred to a neighboring recipient EC (*) within 2 min. B) Representative voltage clamp records of homocellular ReCx45 and heterocellular ReCx45:EC cell pairs revealed coupling, downward deflections in the current traces, and in the ReCx45:EC trace asymmetric voltage dependence characteristic of heterotypic channel formation.
Since Cx45 comprised junctions are cation-selective27 and thus, in general, poorly permeated by anionic dyes, this level of dye-coupling might be indicative of a higher incidence of electrical coupling. Thus, we used dual whole-cell voltage clamp techniques to better assess the extent of coupling of ReCx45 cells amongst themselves and with endothelial cells. In these studies, each cell of a pair is voltage clamped with an electrode, and the cells are alternately stimulated with 0 to 40 mV. If functional gap junctions exist between the two cells, current will be will be detectable in the adjoining (non-stimulated) cell and used to calculate the macroscopic junctional conductance (gj = recorded current divided by transjunctional voltage difference) (diagram at the bottom of Figure 2), as described. This approach revealed that 100% of ReCx45 pairs (n=8) were electrically coupled. Mean macroscopic conductance of ReCx45 pairs was 1.5 ± 0.4 nS, which is consistent with Cx45-containing gap junction channels (Figure 2B). When heterocellular pairs were assessed (n=20), 50% of ReCx45-EC pairs were coupled with a mean conductance of 1.1 ± 0.6 nS (Figure 2C). Thus, the combined dye injection and electrophysiologic (Figure 2) data demonstrate that ReCx45 mesenchymal cells form functional gap junctions amongst themselves and with endothelial cells.
Cx45 Supports Endothelial-induced Mural Cell Differentiation
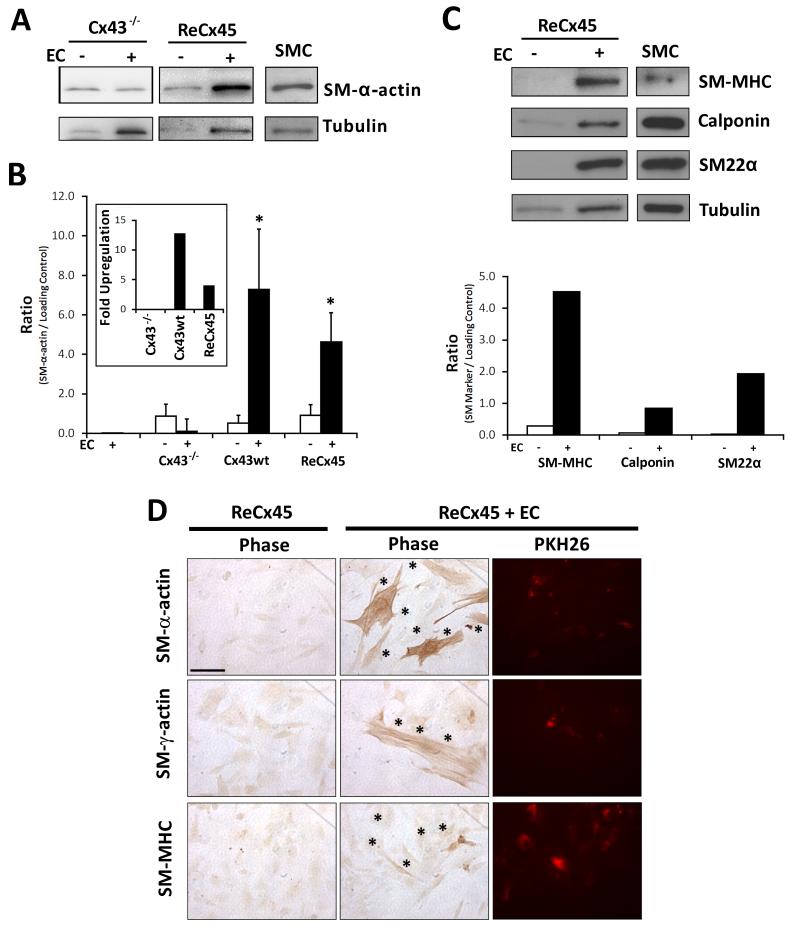
To determine whether Cx45 expression and formation of functional heterocellular gap junctions supports endothelial-induced mural cell differentiation, ReCx45 mesenchymal cells (of either clone) were co-cultured with endothelial cells and total protein was isolated and analyzed for expression of mural cell-specific markers. We observed an upregulation of SM-α-actin expression in ReCx45 mesenchymal cells upon co-culture with endothelial cells (vs. total protein from mesenchymal cells alone) (Figure 3A-B), a response observed in Cx43wt, but lost in Cx43−/−, mesenchymal cells (previous studies3 and Figure 3A-B). Consistent with previous studies3, SM-α-actin was not detected in endothelial cells (Figure 3B); therefore its upregulation in endothelial-mesenchymal co-cultures reflected changes in mesenchymal cell protein expression. Expression of other mural cell-specific markers (calponin, SM22α, and SM-MHC) was also increased in ReCx45 cells upon co-culture with endothelial cells (Figure 3C). Furthermore, we visualized expression of mural cell-specific markers by immunocytochemistry. Compared to solo cultures of ReCx45, expression of SM-α-actin, SM-γ-actin and SM-MHC was increased in ReCx45-3 (and ReCx45-1, not shown) cells in co-culture with fluorescently-labeled endothelial cells and was limited to mesenchymal cells (Figure 3D). These data indicate that endothelial-induced mural cell differentiation is restored by expression of Cx45 in Cx43−/− mesenchymal cells.
Figure 3. ReCx45 mesenchymal cells undergo mural cell differentiation in co-culture with EC.
A) As previously found3, expression of SM-α-actin was not upregulated in Cx43−/− mesenchymal cells co-cultured with EC. However, its expression was elevated in ReCx45 mesenchymal cells co-cultured with EC vs. cultured alone. As described in Methods, twice the total protein is loaded in co-culture lanes to permit comparison of SM marker expression from the mesenchymal cell fraction against total protein isolated from mesenchymal cell solo cultures. B) Densitometric analysis (n=3-6) revealed significant (*, Students’ T-test) upregulation of SM-α-actin in co-cultures of Cx43wt control (13-fold, inset) or ReCx45 (5-fold, inset) mesenchymal cells vs. solo culture, that was not observed in co-cultures of EC and Cx43−/− mesenchymal cells; additionally, no SM-α-actin expression was detected in total protein isolated from EC solo cultures. C) Upregulation of other mural cell-specific markers (calponin, SM22α, and SM-MHC) was also observed in ReCx45 mesenchymal cells upon co-culture with EC. D) Increased expression of mural cell-specific markers (SM-α-actin, SM-γ-actin, and SM-MHC) in co-culture of ReCx45 mesenchymal cells with PKH36-labelled EC (*) was limited to mesenchymal cells (scale bar = 50μm).
Co-culture of ReCx45 Mesenchymal and Endothelial Cells Enables TGF-β Activation
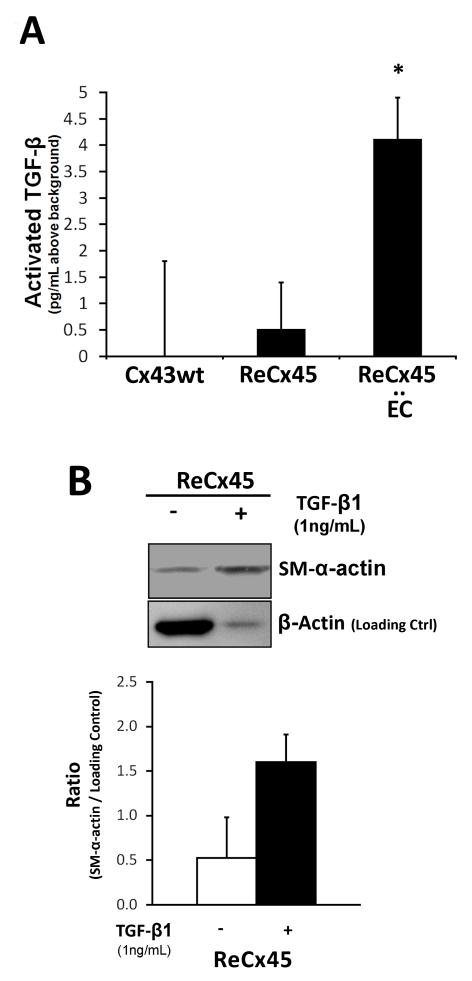
To determine whether Cx45-containing gap junction channels mediate TGF-β activation to enable mural cell differentiation, activated TGF-β was measured in ReCx45 cells in solo culture and in co-culture with endothelial cells. Activated TGF-β was significantly elevated in ReCx45 and endothelial cell co-cultures compared to solo cultures of either Cx43wt or ReCx45 mesenchymal cells (Figure 4A). Furthermore, as was previously shown for Cx43−/− and Cx43wt mesenchymal cells,3 exogenous TGF-β1 upregulated SM-α-actin expression in ReCx45 cells (Figure 4B). Collectively, these data demonstrate that heterocellular Cx45-containing gap junctions formed between mesenchymal and endothelial cells promote the activation of TGF-β, which mediates endothelial-induced mural cell differentiation.
Figure 4. TGF-β was activated in EC-ReCx45 mesenchymal cell co-cultures and exogenous TGF-β upregulated mural cell proteins in ReCx45 mesenchymal cells.
A) TGF-β activation was significantly (*, p<0.05 Students’ T-test) elevated in co-cultures of ReCx45 mesenchymal cells and EC, compared to levels in ReCx45 and Cx43wt mesenchymal cells alone. B) 48h treatment of ReCx45 solo cultures with 1ng/mL human TGF-β upregulated SM-α-actin (3-fold), compared to untreated cells even when the latter sample is over-loaded to visualize baseline expression levels (loading control = β-actin; n = 2; error bars = st. dev).
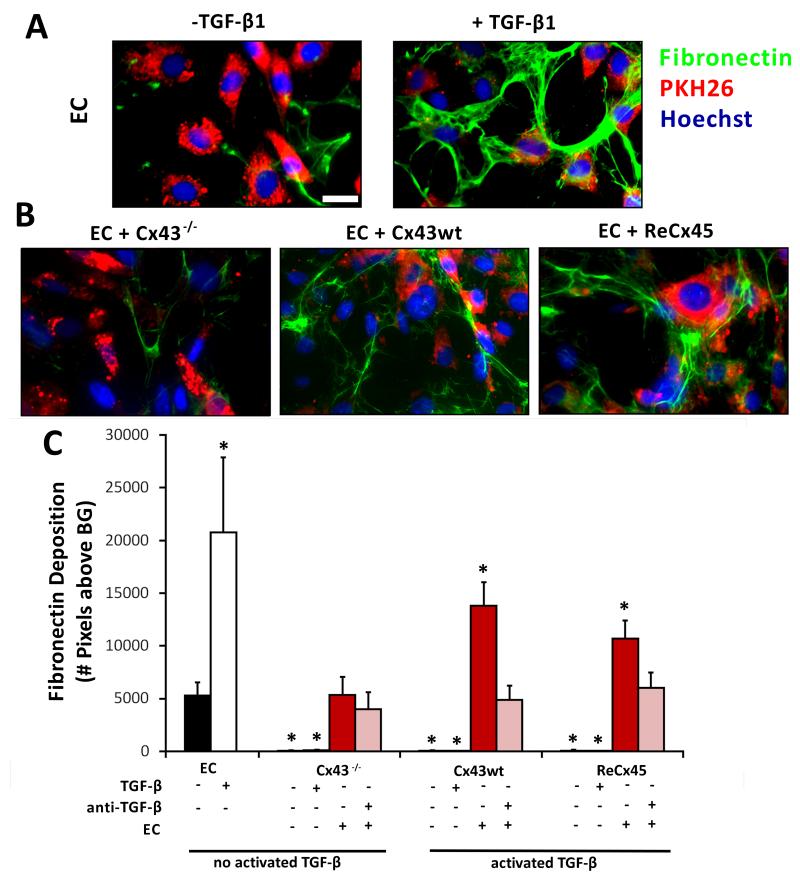
Co-culture of ReCx45 Mesenchymal and Endothelial Cells Upregulates Endothelial ECM Deposition Downstream of TGF-β Activation
TGF-β activation depends in part upon binding of latent TGF-β to the extracellular matrix (ECM), and subsequent cleavage of the TGF-β latency peptide by extracellular proteases.28 Therefore, it is possible that gap junction channel formation between endothelial and mesenchymal cells promotes ECM production/deposition and this, in turn, enables TGF-β activation. To test this, we first examined the expression of ECM proteins in solo cultures and co-cultures of endothelial and mesenchymal cells. We found that fibronectin (and fibrillin, not shown) was expressed by endothelial cells (Figure 5A), but not by mesenchymal cells (Figure 5C), in solo culture, and persisted when endothelial cells were co-cultured with either Cx-expressing or Cx-deficient mesenchymal cells (Figure 5B). Therefore, gap junction channel formation was not required for ECM production/deposition in endothelial-mesenchymal cell co-cultures.
Figure 5. Endothelial ECM deposition was upregulated in EC-ReCx45 mesenchymal co-cultures downstream of TGF-β activation.
A) Endothelial cells (PKH26-labelled, red) produced basal levels of the ECM protein fibronectin (green) that were enhanced in response to 1ng/mL hTGF-β (scale bar = 20μm). B) Fibronectin expression was present in all EC-mesenchymal cell co-cultures and elevated in co-cultures of EC and Cx43wt or ReCx45 mesenchymal cells, but not in EC-Cx43−/− mesenchymal cell co-cultures. C) Quantification of ECM deposition revealed that fibronectin was similarly expressed by endothelial cells (and not mesenchymal cells) in solo culture and in co-culture with Cx43−/− mesenchymal cells, and significantly upregulated in response to exogenous TGF-β and co-culture with Cx43wt or ReCx45 mesenchymal cells. Treatment of co-cultures of EC and Cx43wt or ReCx45 mesenchymal cells with a pan-TGF-β blocking antibodies reduced fibronectin expression to basal levels. Mesenchymal cells fail to significantly express fibronectin when cultured alone or in response to exogenous TGF-β. Asterisks (*) indicate significant differences (ANOVA/Tukey-Kramer) vs. EC mono-culture (black bar) (n=4-12).
Nonetheless, fibronectin (and fibrillin, not shown) deposition was upregulated in endothelial cells co-cultured with Cx43- or Cx45-expressing mesenchymal cells, but not with Cx43−/− mesenchymal cells that do not form functional gap junctions with endothelial cells (Figure 5B). The increased expression of ECM proteins in endothelial cells co-cultured with mesenchymal cells (Figure 5B) was similar to that observed when endothelial cells were treated with exogenous TGF-β1 (Figure 5A), and neutralization of TGF-β in co-cultures suppressed the upregulation of ECM proteins (Figure 5C). Furthermore, culturing Cx43−/− or ReCx45 mesenchymal cells onto fibronectin (Supplemental Figure I) did not promote upregulation of mural cell-specific proteins. Thus, ECM proteins are upregulated in endothelial-mesenchymal co-cultures downstream of TGF-β activation, but do not appear to be sufficient to promote TGF-β activation and mural cell differentiation in the co-cultures.
Collectively, these data demonstrate that heterocellular Cx45-containing gap junctions formed between mesenchymal and endothelial cells promote TGF-β activation and enable endothelial-induced mural cell differentiation, in a process that is not dependent upon the production/deposition of fibronectin or fibrillin. However, ECM proteins upregulated in response to gap junction-mediated TGF-β activation likely contribute to subsequent steps of blood vessel assembly/stabilization including regulation of cell growth.29
Discussion
We previously demonstrated that formation of gap junction channels is required for endothelial-mesenchymal cell contact-induced TGF-β activation, which promotes mesenchymal cell differentiation toward a mural cell phenotype.3 We also showed that Cx43-containing gap junctions between endothelial and mesenchymal cells mediate these processes.3 In this study, we investigated whether Cx45 plays a similar role in the regulation of endothelial-induced mural cell differentiation.
We found that Cx45 expression in Cx43-deficient mesenchymal cells restores formation of functional heterocellular gap junctions with endothelial cells and enables TGF-β activation, presumably by stimulating release of an enzyme or activator that frees active TGF-β from its inactivating latency-associated peptide (LAP30), thereby promoting the upregulation of mural cell-specific proteins in the mesenchymal cells. These findings are consistent with previous in vivo studies of Cx45-deficient embryos that exhibit dysregulated TGF-β signaling and lack mural cell development.11
Still unclear, however, is why Cx43, which can support these same processes and is frequently co-expressed with Cx45 in the embryonic and adult vasculature,22, 23 does not compensate for lack of Cx45 at early stages of vascular development. This observation suggests that Cx45, specifically, regulates endothelial-induced mural cell differentiation during embryogenesis. This idea is further supported by the fact that Cx43-deficient embryos exhibit normal blood vessel formation and mural cell development during gestation, despite dying shortly after birth from severe defects in cardiovascular function.12 Thus, both Cx43 and Cx45 are required for normal vascular development, and may play similar roles in vivo, but at different stages of development and perhaps through different intracellular mechanisms.
Although we found that both Cx43- and Cx45-containing gap junctions formed between endothelial and mesenchymal cell precursors are capable of supporting TGF-β activation in heterocellular co-cultures, it is not clear how these connexins regulate this process. One possibility is that gap junction channel formation/function leads to upregulation of ECM proteins that are required for binding the latent TGF-β peptide complex for subsequent cleavage of the inhibitory peptide by extracellular proteases to activate this growth factor.28 However, we found that fibronectin and fibrillin, which have previously been associated with TGF-β activation in endothelial cells31 and other cell types28, 32 are present in endothelial-mesenchymal cell co-cultures that do not form heterocellular gap junctions, yet TGF-β is not activated.3 These ECM proteins also failed to stimulate mural cell differentiation in ReCx45 or Cx43−/− mesenchymal cells which when cultured alone we previously showed express latent but not activated TGF-β,3 and also failed to restore mural cell differentiation in co-cultures of Cx-deficient mesenchymal cells and endothelial cells. Therefore, endothelial-mesenchymal heterocellular gap junction channel formation/function must regulate other intercellular signaling processes that are critical for TGF-β activation.
Historically, connexins, as components of intercellular channels, were thought to mediate the intercellular exchange of soluble signals that regulate cellular processes and behaviors in a variety of cells,33, 34 including vascular cells.15 However, connexins can also exert channel-independent functions and stimulate, and/or respond to, intracellular signaling cascades via regulation (i.e. phosphorylation) of their cytoplasmic regions.35, 36 Thus, comparisons between the selective properties of Cx43 and Cx45 channels, as well as the regulatory regions of the proteins themselves, could provide clues as to how they mediate the activation of TGF-β and enable mural cell differentiation.
Interestingly, Cx45 and Cx43 form homomeric channels with notable differences in permeability and selectivity. Cx45 channels are cation-selective27, 37 whereas Cx43 channels are not charge selective.37, 38 In addition, Cx43 channels support higher permeability than Cx45 channels. Based upon the selective properties of Cx45 vs. Cx43 junctions, it appears that if a gap junction-traversing signal is required for TGF-β activation, then this signal may be a small positively charged molecule, perhaps calcium. Interestingly, intracellular calcium levels regulate many cellular processes including secretion of enzymes known to activate TGF-β, such as cathespin B, plasmin, thrombospondin and metalloproteinase 2 and 9.30, 39,40 Alternatively, if TGF-β activation requires dye (as well as ion) permeable gap junctions channels comprised of either Cx43 or Cx45, the communicated signal may be a larger signaling molecule, such as 1,4,5-inositol phosphate 3 (IP3), a Ca2+-activating signaling molecule important in TGF-β signaling during mural cell differentiation.41 Clearly additional studies are needed to determine whether exchange of a signaling molecule through functional channels is necessary and, if so, what the traversing signal might be.
The carboxyl terminal domains of both Cx4342 and Cx4543, 44 are targets for phosphorylation by a variety of kinases and serve as binding sites for several intracellular proteins. The consequences of phosphorylation are multiple, and include acute and longer-term regulation of channel function, as well as channel-independent protein-protein interactions. Comparison of the amino acid sequences of Cx45 and Cx43 carboxyl terminal domains suggests, and functional studies confirm,43, 45, 46 that these proteins are likely targeted by some of the same, as well as distinct, protein kinases (Supplemental Figure II and Supplemental Table I). For example, there are no high probability consensus sites for MAPK-dependent phosphorylation in Cx45 but several in Cx43; in contrast, both connexins share a serine-rich region at the ends of their C-terminal tails containing several high-probability PKC consensus sites (Cx43: S364, S368 and S372; Cx45: S378, S381, and S385). As PKC is central in transducing growth factor signals to their ultimate “growth” effects, and this kinase has been shown to regulate the dye and electrical permeability of Cx43,47 these conserved putative PKC-targeted serines may underlie the requirement for Cx43 in TGF-β activation and endothelial-induced mural cell differentiation and the ability of Cx45 to restore this response in the absence of Cx43.
As suggested above, TGF-β activation could also occur as a consequence of intracellular signaling events initiated by the formation of either Cx43- or Cx45-comprised gap junctions, but independent of intercellular diffusion of soluble signals through open channels. As an example, during cardiomyocyte differentiation, Cx43 channel formation is required for and induces differentiation by competing with SMAD2/3 for binding sites on β-tubulin.48 As Cx43 expression increases, SMAD2/3 is released from its β-tubulin binding site, phosphorylated (by activated ALK receptors) and translocated as a complex with SMAD4 to the nucleus where it promotes expression of cardiomyocyte-specific genes. Relevant to our study, neither Cx45 nor Cx40 support cardiomyocyte differentiation in this manner because these proteins do not interact with tubulin at the SMAD2/3 binding site. Thus, there may be connexin-specific differences in TGF-β activation and/or signaling, and channel function may not be necessary for these processes.
Clearly, dissecting the mechanism(s) by which Cx43- and Cx45-containing gap junctions mediate TGF-β activation and endothelial-induced mural cell differentiation will be complex, and beyond the scope of this manuscript. However, ongoing studies are designed to address these issues and should shed light on the cellular and molecular regulation of these processes that are needed for blood vessel formation. Information gained from these studies will not only further our understanding of normal blood vessel development but also provide needed insights into the regulation of neovascularization in postnatal tissues that occurs in response to tissue growth, injury or progressive pathology.
Supplementary Material
Acknowledgements
Sources of Funding: These studies were supported by NIH R01-HL077675 (KKH & JMB) and R01-HL096360 to KKH.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose in regards to these data.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Nakamura H. Electron microscopic study of the prenatal development of the thoracic aorta in the rat. Am J Anat. 1988;181:406–418. doi: 10.1002/aja.1001810409. [DOI] [PubMed] [Google Scholar]
- (2).Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol. 1996;178:375–392. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- (3).Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- (4).Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol. 1993;121:439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- (9).Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- (11).Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- (12).Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- (13).Simon AM, McWhorter AR, Dones JA, Jackson CL, Chen H. Heart and head defects in mice lacking pairs of connexins. Dev Biol. 2004;265:369–383. doi: 10.1016/j.ydbio.2003.09.036. [DOI] [PubMed] [Google Scholar]
- (14).Gilula NB, Reeves OR, Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- (15).Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Larson DM, Carson MP, Haudenschild CC. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc Res. 1987;34:184–199. doi: 10.1016/0026-2862(87)90052-5. [DOI] [PubMed] [Google Scholar]
- (17).Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- (18).Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- (19).Li X, Simard JM. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;281:H1890–H1898. doi: 10.1152/ajpheart.2001.281.5.H1890. [DOI] [PubMed] [Google Scholar]
- (20).Beyer EC, Reed KE, Westphale EM, Kanter HL, Larson DM. Molecular cloning and expression of rat connexin40, a gap junction protein expressed in vascular smooth muscle. J Membr Biol. 1992;127:69–76. doi: 10.1007/BF00232759. [DOI] [PubMed] [Google Scholar]
- (21).Moore LK, Beyer EC, Burt JM. Characterization of gap junction channels in A7r5 vascular smooth muscle cells. Am J Physiol. 1991;260:C975–C981. doi: 10.1152/ajpcell.1991.260.5.C975. [DOI] [PubMed] [Google Scholar]
- (22).Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res. 2004;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- (23).Theis M, Mas C, Doring B, Degen J, Brink C, Caille D, Charollais A, Kruger O, Plum A, Nepote V, Herrera P, Meda P, Willecke K. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294:18–29. doi: 10.1016/j.yexcr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- (24).Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- (25).Martyn KD, Kurata WE, Warn-Cramer BJ, Burt JM, TenBroek E, Lau AF. Immortalized connexin43 knockout cell lines display a subset of biological properties associated with the transformed phenotype. Cell Growth Differ. 1997;8:1015–1027. [PubMed] [Google Scholar]
- (26).el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- (27).Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, ulser DF, Willecke K, Nicholson BJ. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci. 1998;111(Pt 1):31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- (28).Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- (29).Bohnsack BL, Hirschi KK. Red light, green light: signals that control endothelial cell proliferation during embryonic vascular development. Cell Cycle. 2004;3:1506–1511. doi: 10.4161/cc.3.12.1334. [DOI] [PubMed] [Google Scholar]
- (30).Khalil N. TGF-beta: from latent to active. Microbes Infect. 1999;1:1255–1263. doi: 10.1016/s1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- (31).Tian H, Mythreye K, Golzio C, Katsanis N, Blobe GC. Endoglin mediates fibronectin/alpha5beta1 integrin and TGF-beta pathway crosstalk in endothelial cells. EMBO J. 2012;31:3885–3900. doi: 10.1038/emboj.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Peng F, Zhang B, Wu D, Ingram AJ, Gao B, Krepinsky JC. TGFbeta-induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol. 2008;295:F153–F164. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature. 1966;209:1248–1249. doi: 10.1038/2091248a0. [DOI] [PubMed] [Google Scholar]
- (34).Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979;560:1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- (35).Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2011;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- (36).Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- (37).Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Heyman NS, Burt JM. Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions. Biophys J. 2008;94:840–854. doi: 10.1529/biophysj.107.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- (40).Newby AC, Henderson AH. Stimulus-secretion coupling in vascular endothelial cells. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- (41).Rodland KD, Muldoon LL, Magun BE. Cellular mechanisms of TGF-beta action. J Invest Dermatol. 1990;94:33S–40S. doi: 10.1111/1523-1747.ep12875031. [DOI] [PubMed] [Google Scholar]
- (42).Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).van Veen TA, van Rijen HV, Jongsma HJ. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc Res. 2000;46:496–510. doi: 10.1016/s0008-6363(00)00047-x. [DOI] [PubMed] [Google Scholar]
- (44).Hertlein B, Butterweck A, Haubrich S, Willecke K, Traub O. Phosphorylated carboxy terminal serine residues stabilize the mouse gap junction protein connexin45 against degradation. J Membr Biol. 1998;162:247–257. doi: 10.1007/s002329900362. [DOI] [PubMed] [Google Scholar]
- (45).Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- (46).Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell. 2007;18:2264–2273. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.