Abstract
Background
Early highly active antiretroviral therapy (HAART) is recommended for HIV-1 infected infants. There are limited data on lipid changes during infant HAART.
Methods
Non-fasting total (TC), low density lipoprotein (LDL), and high density lipoprotein (HDL) cholesterol, and triglycerides (TG) were measured at 0, 6 and 12 months. Correlates of lipid levels and changes post-HAART were assessed using linear regression.
Results
Among 115 infants, pre-HAART median age was 3.8 months, CD4% was 19%, and weight-for-age z-score (WAZ) was −2.42. Pre-HAART median lipid levels were: TC, 108.7 mg/dl, LDL, 42.5 mg/dl, HDL, 29.4 mg/dl and TG, 186.9 mg/dl. Few infants had abnormally high TC (6.2%) or LDL (5.6%), but many had low HDL (76.5%) or high TG (69.6%). Higher pre-HAART WAZ and HAZ were each associated with higher pre-HAART TC (P=0.04 and P=0.01) and LDL (P=0.02 and P=0.008). From 0–6 months post-HAART, TC (P<0.0001), LDL (P<0.0001), and HDL (P<0.0001) increased significantly, and 23.1% (P=0.002), 14.0% (P=0.2), 31.3% (P<0.0001), and 50.8% (P=0.2) of infants had abnormally high TC, high LDL, low HDL, and high TG, respectively. Changes in TC and HDL were each associated with higher gain in WAZ (P=0.03 and P=0.01) and HAZ (P=0.01 and P=0.007). Increased change in LDL was associated with higher gain in HAZ (P=0.03). Infants on protease inhibitor (PI)-HAART had smaller HDL increase (P=0.004).
Conclusions
Infants had substantive increases in lipids, which correlated with growth. Increases in HDL were attenuated by PI-HAART. It is important to determine clinical implications of these changes.
Keywords: lipids, pediatric HIV-1, highly active antiretroviral therapy, infants, Africa
Highly active antiretroviral therapy (HAART) substantially reduces pediatric HIV-1-associated mortality (1–3). Current recommendations are to provide all HIV-1-infected infants <24 months old with antiretroviral therapy based on survival benefits (4). Antiretroviral drugs, while effective for HIV-1 treatment, have been associated with metabolic alterations (5, 6).
HIV-1 infection by itself is associated with metabolic abnormalities, including reduced total cholesterol (TC), low density lipoprotein cholesterol (LDL), and high density lipoprotein cholesterol (HDL), and increased triglycerides (TG) in adults (7, 8). In children, adolescents and adults, highly active antiretroviral therapy (HAART) has been associated with increased TC, LDL, and TG, particularly for regimens including protease inhibitors (PIs) (5–7, 9–16).
There are limited data for lipid changes in HIV-1-infected infants (17). Normal reference values for lipids in children >2 years old are currently defined based on data from the Lipid Research Clinic Prevalence Study conducted among American children in the 1970s (18, 19). Data on normal lipid profiles in children <2 years of age are lacking. Children in the US may have higher cholesterol levels than children in Africa (20). Genetic, environmental, and dietary factors likely play a role in lipid metabolism and it is unclear whether US standards are applicable for monitoring HIV- and HAART-associated dyslipidemia in African infants.
Dyslipidemia, especially elevated lipid levels, is an important risk factor for cardiovascular disease in adults (21). The consequences of early dyslipidemia during infancy are unknown; one hypothesized consequence is development of premature atherosclerotic disease (22). Several studies have shown increased risk of atherogenic dyslipidemia among children on PI-regimens (22).
Africa is home to >90% of the world’s HIV-1-infected children, and increased access to early infant diagnosis is expected to result in both earlier treatment and longer survival. It will be important to determine metabolic changes and the consequences of these changes as these infants age into early childhood and adulthood. In this study, we determined lipid levels in HIV-1-infected infants before and after HAART initiation and examined correlates of lipid levels pre-HAART and following initiation of HAART.
MATERIALS AND METHODS
HIV-1 infected subjects
HIV-1 infected infants included in this analysis were enrolled in one of two randomized clinical trials, Optimizing Pediatric HAART 03 (OPH03; NCT00428116) and Optimizing Pediatric HAART 612 (OPH04; NCT00427297), which enrolled participants from 2007–2009. For both studies, subjects were HIV-1-infected, HAART naïve, less than one year of age, and were identified at clinics for prevention of mother-to-child transmission of HIV-1 (PMTCT) and Nairobi hospital wards, as described previously (23). The OPH03 Study was a trial of continued vs. interrupted HAART among children who were successfully treated for at least 24 months, and only data from the pre-randomization phase were included in this analysis. The OPH612 Study was a trial of nevirapine-based (NVP)-HAART vs. lopinavir-boosted-ritonavir (LPV/r)-based HAART among children exposed to NVP for PMTCT, and with no evidence of non-nucleoside reverse transcriptase inhibitor resistance by population-based sequencing.
Following enrollment into both trials, infants were started on HAART and followed in the study clinic at Kenyatta National Hospital, Nairobi, Kenya. Information on the child’s socio-demographic characteristics, caregiver characteristics, WHO staging, and growth parameters were assessed pre-HAART. The child’s weight was determined using a calibrated weighing scale and length (height) using a non-extendable measuring tape. Plasma HIV-1 RNA level, CD4 lymphocyte percentage and count, and lipid levels for each child were assessed at enrollment before HAART was initiated and at 6 and 12 months following HAART. Infants enrolled in OPH03 received first-line regimens according to the Kenya National guidelines consisting of either a LPV/r-based regimen including a backbone of zidovudine and lamivudine (if NVP exposed for PMTCT) or a NVP-based regimen including the same backbone (if not NVP exposed for PMTCT). Infants enrolled in OPH612 were randomized to receive either LPV/r or NVP-based HAART. None of the children received stavudine. Ethical approval for the study was obtained from the University of Nairobi, Kenyatta National Hospital, and University of Washington Ethical Review Committees.
Growth Parameters
Weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) z-scores were calculated using the WHO child growth standards (24). Underweight was defined as having WAZ<-2, wasted was defined as WHZ<-2, and stunted was defined as HAZ<-2.
Laboratory Testing
Blood samples were drawn in the clinic and lipid levels were assessed in the Department of Paediatrics and Child Health Laboratory, University of Nairobi. Plasma lipid levels were measured using the cholesterol phenol 4-aminoantipyrine peroxidase (CHOD-PAP) method (Human Diagnostics, Wiesbaden, Germany). This is an enzymatic colorimetric test for lipids with lipid clearing factor using a Humalyzer-3000. Lipid levels were determined after enzymatic hydrolysis and oxidation had taken place. LDL was estimated from the quantitative measurements of TC, HDL and TG using the Friedewald Equation: LDL = [TC] – [HDL] - [TG/5] (where all the concentrations are given in mmol/L). Lipid levels were then converted to mg/dL for all analyses. The Paediatrics Department Laboratory participates in the Human Quality Assessment Scheme (HuQAS).
CD4 percentages and counts were measured using flow cytometry. Plasma HIV-1 RNA levels were measured using cryopreserved samples in Seattle, WA and the Gen-Probe HIV-1 viral load assay (San Diego, CA), which has been validated for detection of HIV-1 subtypes prevalent in Kenya (25).
Statistical Analysis
Because no reference levels exist for infants <2 years, National Cholesterol Education Program (NCEP ATPIII) guidelines for children >2 years were used to define abnormally high TC (>200 mg/dL), high LDL (>130 mg/dL), low HDL (<40 mg/dL), high TG (>150 mg/dL) and a high TC-HDL ratio (>4.0) (18, 19).
Lipids were described as continuous variables and as categorical variables using the NCEP ATPIII guideline cut-offs.
Linear regression was used to examine correlates of pre-HAART lipid levels. Correlates examined were gender, age (dichotomized according to the median), birth weight, breastfeeding status (ever or never), receipt of antiretrovirals to mother or infant for PMTCT. and pre-HAART characteristics (clinical WHO HIV-1 disease stage, CD4 percentage and count, plasma HIV-1 RNA level, WAZ, HAZ, and WHZ).
Pre-HAART vs. 6-month and 12-month lipid levels were compared using paired Wilcoxon sign-rank tests. McNemar’s test (a paired test of proportions) was used to compare proportions of infants with high TC, high LDL, low HDL, high TG, and high TC:HDL ratio pre- and post-HAART (6-months). Pre-HAART lipid data were summarized for all infants with available data; lipid levels and proportions of infants with abnormal lipids were similar between the overall cohort and the subset with follow-up data. Linear regression was used to examine correlates of 6- and 12-month lipid levels, and of the change in lipid level from pre-HAART to 6- and 12-months, after adjusting for pre-HAART lipid level. Correlates included each of those mentioned for analyses of pre-HAART lipid levels, use of a PI-based regimen, and change in CD4 percentage, CD4 count, plasma HIV-1 RNA level, WAZ, HAZ, and WHZ. Data for TG were skewed and thus were log10 transformed for regression analyses. For ease of interpretation, only results for analyses using untransformed data are reported. For significant cofactors, multivariate analyses adjusted for age and gender were evaluated, but were not reported due to similarity in results.
Two-tailed P-values <0.05 were considered statistically significant, and were reported in either the text or the tables. Analyses were performed using Intercooled Stata, version 10.0 (StataCorp, College Station, TX).
RESULTS
Pre-HAART (Baseline) Characteristics
Of 115 enrolled HIV-1-infected infants, the median age at baseline assessment was 3.8 months (interquartile range (IQR), 3.0, 4.4) (Table 1). At enrollment, many children in the cohort displayed some evidence of growth failure: 55.7% were underweight (WAZ<-2), 47.8% were stunted (HAZ<-2), and 31.3% were wasted (WHZ <-2). Median WAZ, HAZ, and WHZ were −2.42 (IQR, −3.79, −0.98), −1.87 (IQR, −3.25, −0.88) and −0.84 (−2.31, 0.24). The majority of infants had evidence of immunosuppression at this young age; the median CD4% was 19% (IQR, 14, 24) and 48.2% were categorized as either WHO clinical stage III or IV. The infants had strikingly high plasma HIV-1 RNA viral loads, with a median of 6.5 log10 copies/ml (IQR, 6.0, 7.0).
TABLE 1.
Pre-HAART characteristics among HIV-1-infected infants.
| Pre-HAART Characteristics | N | n (%) or median (IQR) |
|---|---|---|
| Age (months) | 115 | 3.8 (3.0, 4.4) |
| Male | 115 | 58 (50.4%) |
| Birth weight (kg) | 110 | 3.1 (2.7, 3.5) |
| Ever breastfed | 95 | 83 (87.4%) |
| PMTCT for mother or infant | 109 | 69 (63.3%) |
| Infant growth | ||
| WAZ | 115 | −2.42 (−3.79, −0.98) |
| Underweight (WAZ <-2) | 115 | 64 (55.7%) |
| HAZ | 115 | −1.87 (−3.25, −0.88) |
| Stunted (HAZ <-2) | 115 | 55 (47.8%) |
| WHZ | 115 | −0.84 (−2.31, 0.24) |
| Wasted (WHZ <-2) | 115 | 36 (31.3%) |
| Infant immune, virological and clinical status | ||
| CD4% | 113 | 19% (14, 24) |
| CD4% <15% | 113 | 31 (27.4%) |
| CD4 count (cells/ml) | 113 | 1,282 (755,1,982) |
| HIV-1 plasma viral load (log10 copies/ml) | 96 | 6.5 (6.0, 7.0) |
| WHO Stage III/IV | 112 | 54 (48.2%) |
| Baseline lipid levels | ||
| TC (mg/dL) | 113 | 108.7 (90.5, 143.9) |
| LDL (mg/dL) | 107 | 42.5 (22.4, 66.1) |
| HDL(mg/dL) | 115 | 29.4 (20.1, 39.8) |
| TG (mg/dL) | 115 | 186.9 (132.9, 251.5) |
| TC-HDL ratio | 113 | 3.69 (2.78, 5.44) |
HAART indicates highly active antiretroviral therapy; PMTCT, prevention of mother-to-child transmission; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; TC, total cholesterol, LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; and TG, triglycerides.
Levels and Correlates of Pre-HAART Lipids
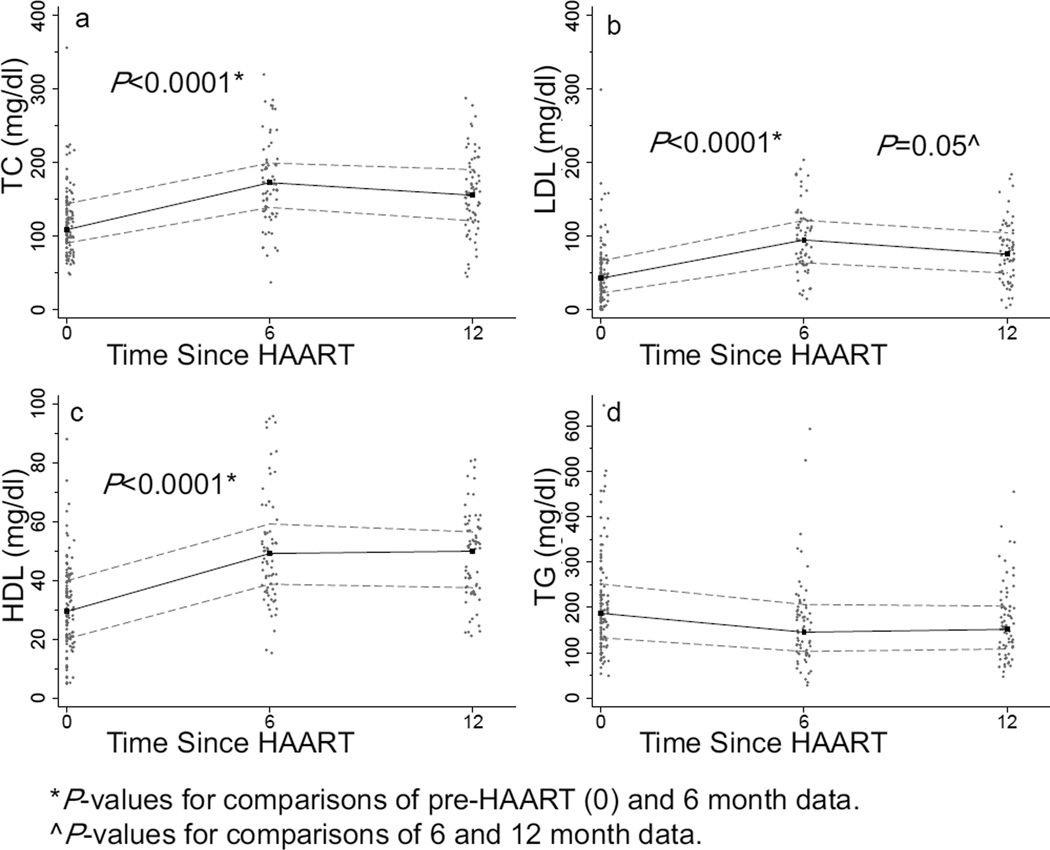
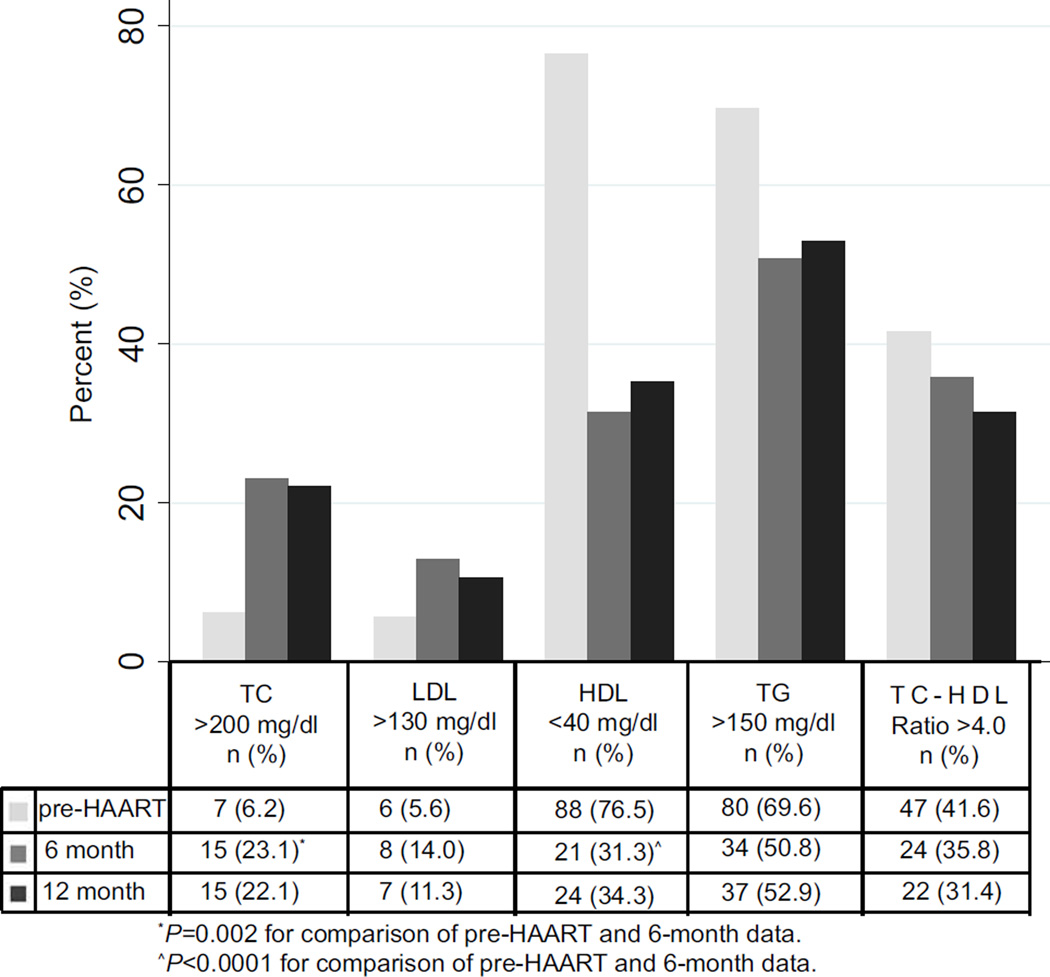
Among 115 infants with pre-HAART lipid data, median pre-HAART lipid levels were: TC, 108.7 mg/dL (IQR, 90.5, 143.9), LDL, 42.5 mg/dL (IQR, 22.4, 66.1), HDL, 29.4 mg/dL (IQR, 20.1, 39.8) and TG, 186.9 mg/dL (IQR, 132.9, 251.5) (Fig. 1). Pre-HAART, 6.2%, 5.6%, 76.5% and 69.6% of infants had high TC (>200 mg/dL), high LDL (>130 mg/dL), low HDL (<40 mg/dL), and high TG (>150 mg/dL), respectively (Fig. 2). The median TC:HDL ratio was 3.69 (IQR, 2.78, 5.44), and 41.6% had a TC:HDL ratio >4.0.
FIG. 1.
Scatterplots for a, total cholesterol (TC); b, low density lipoprotein (LDL); c, high density lipoprotein (HDL), and d, triglycerides (TG) at 0, 6, and 12 months post-highly active antiretroviral therapy (HAART). Median (black line) and 25th and 75th percentile (gray lines) lipid levels.
FIG. 2.
The proportion of children with abnormally high total cholesterol (TC), high low density lipoprotein (LDL), low high density lipoprotein (HDL), high triglycerides (TG) and high TC-HDL ratio are illustrated for pre-HAART, 6- and 12-months post- highly active antiretroviral therapy (HAART).
Correlates of pre-HAART lipid data were evaluated for the 115 infants with available data. A higher CD4% was significantly associated with a higher HDL level (regression coefficient, 0.3 mg/Ll per unit increase in CD4 percentage; P=0.03). Higher pre-HAART WAZ and HAZ were each associated with a higher pre-HAART TC (regression coefficient, 5.0 mg/dL per unit increase in WAZ; P=0.04, and regression coefficient, 6.4 mg/dL per unit increase in HAZ; P=0.01, respectively) and LDL (regression coefficient, 5.4 mg/dL per unit increase in WAZ; P=0.02, and regression coefficient, 6.2 mg/dL per unit increase in HAZ; P=0.008, respectively). There were trends for increased TC and LDL in children with ≥-2 WAZ (P=0.06 and P=0.05, respectively) and with ≥-2 HAZ (P=0.04 and P=0.04, respectively) (Table 2). Results were similar for the 70 infants who remained in follow-up at 12 months; however, in this subset, males had significantly higher HDL levels than females (means, 38.5 mg/dL vs. 27.0 mg/dL; P=0.003), and higher pre-HAART plasma HIV-1 RNA level was associated with a lower LDL level (regression coefficient, −13.07 mg/dL per unit increase in HIV-1 RNA log10 copies/mL; P=0.04)
TABLE 2.
Selected correlates of pre-HAART TC, LDL and HDL levels among all infants with pre-HAART lipid data.
| TC mg/dL N=113 |
LDL mg/dL N=107 |
HDL mg/dL N=115 |
||
|---|---|---|---|---|
| Correlate | N* | Mean^ | ||
| Overall Mean | 118.9 | 51.8 | 30.9 | |
| Females | 56 | 120.7 | 56.9 | 28.4 |
| Males | 57 | 117.1 | 46.8 | 33.4¶ |
| Age <4 months | 74 | 124.1 | 54.4 | 30.6 |
| Age ≥4 months | 39 | 109.1 | 46.7 | 31.4 |
| WHO Stage 1/2 | 57 | 124.6 | 61.9 | 30.5 |
| WHO Stage 3/4 | 53 | 112.8 | 42.8§ | 31.1 |
| WAZ ≥-2 | 50 | 128.1 | 60.5 | 30.9 |
| WAZ <-2 | 63 | 111.6¶ | 44.5§ | 30.9 |
| HAZ ≥-2 | 60 | 127.4 | 59.7 | 30.2 |
| HAZ <-2 | 53 | 109.3§ | 42.4§ | 31.6 |
Number of study participants for analyses of TC. Total N for other lipids were similar.
P-values refer to linear regression analyses used to evaluate potential correlates of lipid levels.
0.05>P<0.1.
P≤0.05.
HAART indicates highly active antiretroviral therapy; WAZ weight-for-age Z-score; HAZ, height-for-age Z-score; TC, total cholesterol, LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; and TG, triglycerides.
Increased Lipid Levels Following HAART Initiation
Among infants with follow-up data, lipid levels increased significantly during the first 6 months after HAART initiation. Median TC was 167.8 mg/dL (IQR, 134.6, 197.6), and was significantly higher than the pre-HAART level (P<0.0001), LDL increased to 88.2 mg/dL (IQR, 61.9, 115.6) (P<0.0001), and HDL increased to 46.0 mg/dL (IQR, 36.3, 59.2) (P<0.0001) (Fig. 1). TG decreased to 150.6 mg/dL (IQR, 99.2, 200.2) at 6 months, but this difference was not statistically significant (P=0.2). There was no significant difference between lipid levels at 6 vs. 12 months for TC (P=0.2), LDL decreased somewhat (P=0.05), and HDL (P=0.7) and TG (P=0.4) did not change. At 6 and 12 months, the TC-HDL ratio was lower than at pre-HAART (median, 3.68 [IQR, 2.75, 4.35]; P=0.07 and median, 3.38 [IQR, 2.58, 4.18]; P=0.007, respectively).
The proportion of children with abnormal lipid levels also significantly changed during the first 6 months after HAART initiation. Among infants with 6-month data, the proportion with high TC significantly increased to 23.1% (P=0.002), and increased, though not significantly, to 14.0% for LDL (P=0.2) (Fig. 2). The proportion of infants with abnormally low HDL significantly decreased to 31.3% (P<0.0001). There was a non-significant reduction in the proportion with high TG (50.8%; P=0.2). The proportion of children with a TC:HDL ratio >4.0 decreased slightly to 35.8% (6 months) (P=0.4) and 31.4% (12 months) (P=0.1).
Correlates of Changes in Lipid Levels after HAART initiation
In analyses adjusted for pre-HAART lipid level, pre-HAART growth, immune and clinical parameters were associated with lipid changes. Infants with a pre-HAART WAZ ≥-2 had a trend for lower increase in TC at 6 months (P=0.09), and this was significant at 12 months (adjusted mean change, 23.5 vs. 52.3 mg/dL; P=0.02) (Table 3). Higher pre-HAART CD4 count was associated with a higher 12-month increase in TG (adjusted regression coefficient, 2.5 mg/dL per 100 cells/ml increase; P=0.02). Conversely, infants with a WHO Stage 3 or 4 diagnosis prior to HAART had a higher 12-month increase in HDL (P=0.01) (Table 3). Age, gender and birth weight were also associated with gain in LDL and HDL. Infants older than 4 months at HAART had a lower 12-month gain in LDL (P=0.03) (Table 3). Male gender was associated with 12-month gain in HDL (P=0.04), and higher infant weight at birth was associated with 6-month gain in HDL (adjusted regression coefficient, 10.0 mg/dL per kg increase; P=0.05).
TABLE 3.
Selected correlates of the 12 months post-HAART level and the change, adjusted for baseline, from pre-HAART to 12 months for TC, LDL and HDL levels among infants with 12 months of follow-up.
| TC mg/dL N=68 |
LDL mg/dL N=62 |
HDL mg/dL N=70 |
|||||
|---|---|---|---|---|---|---|---|
| Correlate | N* | 12 Month Mean^ |
Adjusted Mean Change¶ |
12 Month Mean^ |
Adjusted Mean Change¶ |
12 Month Mean^ |
Adjusted Mean Change¶ |
| Overall Mean | 158.9 | 39.4 | 80.2 | 26.9 | 48.3 | 16.6 | |
| Females | 36 | 155.0 | 33.8 | 78.4 | 21.7 | 44.8 | 10.9 |
| Males | 32 | 163.3 | 45.6 | 82.3 | 29.0 | 52.2¥ | 18.9¥ |
| Age <4 months | 36 | 169.7 | 46.6 | 91.5 | 35.0 | 47.0 | 13.9 |
| Age ≥4 months | 32 | 146.8 | 25.7 | 66.5¥ | 10.0¥ | 49.7 | 17.2 |
| WHO Stage 1/2 | 38 | 148.8 | 27.7 | 74.6 | 16.7 | 44.0 | 11.2 |
| WHO Stage 3/4 | 28 | 168.1 | 47.1 | 83.6 | 28.7 | 53.2‡ | 20.2‡ |
| Pre-HAART WAZ ≥-2 | 35 | 148.1 | 23.5 | 73.3 | 14.4 | 45.3 | 12.3 |
| Pre-HAART WAZ <-2 | 33 | 170.4§ | 52.3¥ | 87.5 | 34.7§ | 51.5§ | 18.4§ |
| Pre-HAART HAZ ≥-2 | 39 | 154.9 | 31.2 | 79.2 | 20.3 | 46.3 | 14.2 |
| Pre-HAART HAZ <-2 | 29 | 164.4 | 44.8 | 81.6 | 26.5 | 50.7 | 17.8 |
| NVP-based regimen | 40 | 164.9 | 43.9 | 83.5 | 26.7 | 51.9 | 20.9 |
| LPV/r-based regimen | 28 | 150.30.3 | 028.6.3 | 075.0.4 | 15.8 | 42.9‡ | 10.7¤ |
Number of study participants for analyses of TC. Total N for other lipids were similar.
P-values refer to linear regression analyses used to evaluate potential correlates of 12-month lipid levels.
P-values refer to linear regression analyses used to evaluate potential correlates of the change in lipid level from pre-HAART to 12 months, after adjusting for pre-HAART lipid level; mean change was derived from these models.
0.05>P<0.1.
0.01>P<0.05.
0.005>P≤0.01.
P<0.005.
HAART indicates highly active antiretroviral therapy; WAZ weight-for-age Z-score; HAZ, height-for-age Z-score; TC, total cholesterol, LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; TG, triglycerides; NVP, nevirapine; and LPV/r, lopinavir-boosted ritonavir.
Use of LPV/r-based HAART was associated with a lower 6-month increase in HDL (P=0.003), and this difference persisted at 12 months. The pre-HAART to 12-month adjusted mean change in HDL was 10.7 mg/dL for LPV/r- vs. 20.9 mg/dL NVP-based HAART (P=0.004) (Table 3).
There were significantly higher increases in lipids in children who had higher gains in growth and immune parameters. For each unit increase in the pre-HAART to 12-month gain in WAZ, there was an 8.7 mg/dL higher increase in TC, adjusted for pre-HAART TC level (P=0.03) (Table 4). Similarly, 12-month gain in HAZ was associated with a higher 12-month increase in TC (P=0.01) and LDL (P=0.03). Twelve-month gains in WAZ and HAZ were each also associated with 12-month increase in HDL (P=0.01 and P=0.007). These associations remained, after adjusting for use of LPV/r-based HAART. The 6-month post-HAART increases in TC and LDL were each associated with better CD4 count reconstitution (increase from pre-HAART to 6 months) (adjusted regression coefficient, 0.8 mg/dL per 100 cells/ml increase; P=0.03, and adjusted regression coefficient, 0.7 mg/dL per 100 cells/ml increase; P=0.02, respectively); however, these associations were not sustained at 12 months.
TABLE 4.
Relation between pre-HAART to 12-month post-HAART change in immune and growth parameters and the pre-HAART to 12-month change, adjusted for baseline, in TC, LDL and HDL levels among infants with 12 months of follow-up.
| TC N=68 | LDL N=62 | HDL N=70 | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient | Regression Coefficient | Regression Coefficient | ||||
| Pre-HAART to 12-month change |
12-Month Level^ (P-value) |
Adjusted 12-Month Change¶ (P-value) |
12-Month Level^ (P-value) |
Adjusted 12-Month Change¶ (P-value) |
12-Month Level^ (P-value) |
Adjusted 12-Month Change¶ (P-value) |
| CD4 count per 100 cells/ml* |
0.4 (0.4) |
0.4 (0.9) |
0.1 (0.9) |
0.2 (0.8) |
0.3 (0.1) |
0.3 (0.1) |
| WAZ | 6.7 (0.09) |
8.7 (0.03) |
4.4 (0.2) |
6.0 (0.06) |
2.7 (0.01) |
2.7§ (0.01) |
| HAZ | 12.5 (0.03) |
14.1 (0.01) |
8.5 (0.06) |
10.1 (0.03) |
4.1 (0.007) |
4.1¥ (0.007) |
N=67, TC; N=61, LDL; N=69, HDL.
P-values refer to linear regression analyses used to evaluate potential correlates of lipid levels at 12 months.
P-values refer to linear regression analyses used to evaluate potential correlates of the change in lipid level from pre-HAART to 12-months, after adjusting for pre-HAART lipid level.
In a model adjusted for LPV/r-use, regression coefficient=2.2 mg/dL per unit increase in pre-HAART to 12-month change in WAZ; P=0.04.
In a model adjusted for LPV/r-use, regression coefficient=3.3 mg/dL per unit increase in pre-HAART to 12-month change in HAZ; P=0.03.
HAART indicates highly active antiretroviral therapy; WAZ weight-for-age Z-score; HAZ, height-for-age Z-score; TC, total cholesterol, LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; and TG, triglycerides
DISCUSSION
In this study, we determined lipid levels among HIV-1-infected infants less than a year of age before and after HAART initiation. Six months after HAART initiation, we observed significant increases in TC, LDL and HDL levels, and a slight but non-significant decline in TG levels. These changes persisted over the 12-month period after HAART initiation. Growth and immune reconstitution were associated with many of the changes we observed in lipid levels during the first year of HAART. It is possible that the combination of HAART and rapid growth reconstitution in infants with severe growth compromise prior to treatment led to more marked changes in lipids following initiation of HAART.
In our study, a small proportion of infants had evidence of TC or LDL elevation (<10%), and the majority had low HDL (77%) and high TG (70%) prior to initiation of HAART. This lipid profile is similar to pre-HAART data for adults (8), and South African HIV-1-infected infants (median age, 9.3 months) (17). Among South African infants, none had high TC or LDL levels, 93% had low HDL, and 63% had high TG (17). In untreated Latin American children aged 12–23 months, pre-HAART TC levels were comparable to TC levels in our infant cohort, whereas TG levels were considerably lower than in infants (26). In addition, we observed lower levels of pre-HAART TC and LDL, but higher levels of TG than has been reported in untreated older children from the US and the United Kingdom (27, 28). Differences in lipid levels in HIV-1-infected infants compared with children may be due to demographics, age, or the rapid course of early pediatric HIV-1 in infants.
Malnutrition has been associated with hypocholesterolemia and low LDL (29). Approximately half of infants in the cohort were malnourished (WAZ <-2 and HAZ <-2) at HIV-1 diagnosis (enrollment), which may also partly explain the lower TC and LDL levels we observed in this cohort pre-HAART. Higher pre-HAART TC and LDL levels were associated with higher WAZ and HAZ, suggesting better nourished children had higher TC and LDL. We also observed an association between higher pre-HAART HDL levels and higher pre-HAART CD4%, suggesting a relationship between HIV-1 control and HDL level. A similar relationship was also reported in South African HIV-1-infected infants (17).
Six months after HAART initiation, the median increase in TC was 59 mg/dL (corresponding to a 54% increase), with 23% of infants having abnormally high TC levels by 6 months. The magnitude of increase was substantially higher than has been reported in US/EU cohorts with over one year of HAART exposure, in which the mean increase in TC corresponded to a 19–20% change in children and adults (28, 30). In our study, median levels of HDL also increased by 17 mg/dL, which represented a 58% increase. This increase in HDL is also higher than what has been reported over a similar timeframe in children (~20%) (28) and in adults, in whom the magnitude of change in HDL has been variable (2–22%) (14, 30).
Increases in TC and LDL after HAART initiation were associated with higher gain in CD4 count at 6 months in this study, suggesting that improved immune reconstitution may be associated with an increase in lipid levels following HAART initiation. Similarly, Lapphra et al, noted a relationship between CD4% gain and TC increase in children (12). Chantry et al. found an association between increased HDL and increased CD4% at 6 months (31). Higher CD4 counts have been associated with high TC and LDL in adults (7, 32).
Changes in TC, LDL, and HDL from pre-HAART to 6 or 12 months were associated with increased growth reconstitution. This finding is consistent with a previous US/EU study which noted that increased LDL and HDL were associated with increased weight (28). Infants in our study who were malnourished pre-HAART had larger changes in TC than infants who were not malnourished pre-HAART. Their growth reconstitution could have resulted from viral control and immune reconstitution following initiation of HAART, which enabled redirection of nutrients from fighting infection to growth.
In our study, use of a PI-based regimen was significantly associated with a smaller increase in HDL levels following start of HAART (P=0.004). Previous studies have shown an association between the use of PIs and the development of dyslipidemia in children (5, 6, 13). Infants switched from LPV/r- to NVP-based HAART had higher HDL, and lower TG and a TC:HDL ratio than infants who maintained their PI-based regimen (17). Lower weight gain was recently observed in South African infants randomized to LPV/r- vs. NVP-based HAART (33); however, it is not likely that any negative impact of LPV/r-based HAART on growth can account for lower gain in HDL in our study, because this association remained, even after adjusting for gain in either weight or height. It is noteworthy that use of LPV/r-based HAART mitigated some potentially beneficial changes in lipid profiles following initiation of HAART.
The implications of early serum lipid abnormalities pre- and post-HAART initiation in infants are undefined. Following initiation of HAART, we found an increase in TC, LDL, and HDL consistent with other studies (17, 28, 31). Interestingly, the proportion of infants with abnormally low HDL pre-HAART (77%) dramatically decreased by 6 months (31%). In addition, the prevalence of infants with an abnormal TC/HDL ratio decreased from 42% pre-HAART to 31.4% at month 12. This pattern contrasts with data for adults, that demonstrated the TC:HDL ratio increased after HAART initiation and remained higher than the pre-HAART level (14), but is consistent with some pediatric studies (17, 28). In addition, we observed post-HAART reductions in both the proportion of infants with low HDL, and high TG, consistent with data for South African HIV-infected infants on HAART (17). It is noteworthy that 6- and 12-month post-HAART levels of TC were comparable to those in HIV-1-exposed uninfected children aged 12–23 months, whereas post-HAART TG levels remained high compared with these uninfected children (26).
There are several limitations to our study. First, we did not obtain fasting lipid levels, because this is not feasible during the first year of life. However, in a comparison of fasting and non-fasting lipid levels in children (34), there was no difference in group means of TC and HDL. In contrast, TG is affected by fasting, and hence the non-fasting levels of TG may represent overestimates and calculated levels of LDL may be underestimates. Second, we defined dyslipidemia using reference values for >2 year olds in the US (18, 19), which may not be directly applicable to infants in our study who differ with regard to diet, socioeconomic status, and race. There are no current guidelines for abnormal lipid levels in children <2 years in either developing or developed countries, making it difficult to determine whether HAART may have negatively impacted the rate of lipid change in this age group.
In conclusion, this study shows marked increases in lipid levels shortly after HAART initiation in HIV-1-infected infants, which correlated with growth and immune reconstitution. These changes may be explained by both direct effects of HAART and indirect effects, including nutritional repletion and immune reconstitution following reduction of viral burden. The longer term implications of dyslipidemia and rapid lipid changes in infancy in HIV-1-infected children are unknown. As increasing numbers of infants are started on treatment, it will be important to characterize lipid changes over time and to determine potential clinical implications of these changes on increased disease morbidity.
ACKNOWLEDGEMENTS
The Optimizing HIV-1 Therapy Study was supported by the National Institute of Child Health and Human Development (NICHD) grant 2 R01 HD023412. Field site support was provided by the University of Washington Center for AIDS Research International Core, an NIH funded program (P30 AI027757) which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM). AL was supported by the NIH Research Grant # D43 TW000007, funded by the Fogarty International Center. SBN was supported by 2 R01 HD023412. KT was supported by the NIH grants P30 AI027757 and 2 R01 HD023412. GJS was supported by NIH grant K24 HD054314.
The authors are grateful to the research personnel, laboratory staff, and data management teams in Nairobi, Kenya and Seattle, Washington; the Nairobi City Council Clinics for their contribution and cooperation; the Divisions of Obstetrics and Gynaecology and Paediatrics at Kenyatta National Hospital for providing facilities for laboratory and data analysis; Jennifer Slyker for helpful discussions and scientific input, and the members of the Kizazi Working Group and the Kenya Research Program for comments and insights during data analysis and manuscript preparation. We are indebted to the infants and their caregivers who participated in this research study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Source of Funding: All authors declare that they have no conflicts of interest.
Author Contributions
AL conceived the study, led clinical study management and data collection, analyzed and interpreted data and wrote the manuscript. SBN, DW, KT, BAR, and GJS also contributed to study design, data analysis and interpretation and writing. EN led clinical study implementation and data collection. LD contributed data analysis and interpretation. AM provided critique of intellectual content and interpretation of data. All authors participated in critical review and final approval of the manuscript.
Data presented previously at the 18th Conference on Retroviruses and Opportunistic Infections, Abstract #703, Boston, MA, March, 2011.
REFERENCES
- 1.Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 2.Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–731. doi: 10.1086/423178. [DOI] [PubMed] [Google Scholar]
- 3.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 7.Anastos K, Lu D, Shi Q, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr. 2007;45:34–42. doi: 10.1097/QAI.0b013e318042d5fe. [DOI] [PubMed] [Google Scholar]
- 8.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 9.Aldamiz-Echevarria L, Pocheville I, Sanjurjo P, et al. Abnormalities in plasma fatty acid composition in human immunodeficiency virus-infected children treated with protease inhibitors. Acta Paediatr. 2005;94:672–677. doi: 10.1111/j.1651-2227.2005.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 10.Beregszaszi M, Dollfus C, Levine M, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 11.Melvin AJ, Lennon S, Mohan KM, Purnell JQ. Metabolic abnormalities in HIV type 1-infected children treated and not treated with protease inhibitors. AIDS Res Hum Retroviruses. 2001;17:1117–1123. doi: 10.1089/088922201316912727. [DOI] [PubMed] [Google Scholar]
- 12.Lapphra K, Vanprapar N, Phongsamart W, Chearskul P, Chokephaibulkit K. Dyslipidemia and lipodystrophy in HIV-infected Thai children on highly active antiretroviral therapy (HAART) J Med Assoc Thai. 2005;88:956–966. [PubMed] [Google Scholar]
- 13.Tassiopoulos K, Williams PL, Seage GR, 3rd, et al. Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47:607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddler SA, Li X, Chu H, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med. 2007;8:280–287. doi: 10.1111/j.1468-1293.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 15.Jevtovic DJ, Dragovic G, Salemovic D, Ranin J, Djurkovic-Djakovic O. The metabolic syndrome, an epidemic among HIV-infected patients on HAART. Biomed Pharmacother. 2009;63:337–342. doi: 10.1016/j.biopha.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 17.Strehlau R, Coovadia A, Abrams EJ, et al. Lipid Profiles in Young HIV-infected Children Initiating and Changing Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2011;60:369–376. doi: 10.1097/QAI.0b013e318243760b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics. 2007;120:e215–e219. doi: 10.1542/peds.2006-1812. [DOI] [PubMed] [Google Scholar]
- 19.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 20.Akuyam SA, Isah HS, Ogala WN. Evaluation of serum lipid profile of under-five Nigerian children. Ann Afr Med. 2007;6:119–123. doi: 10.4103/1596-3519.55722. [DOI] [PubMed] [Google Scholar]
- 21.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(Suppl 2):S79–S85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 22.Leonard EG, McComsey GA. Metabolic complications of antiretroviral therapy in children. Pediatr Infect Dis J. 2003;22:77–84. doi: 10.1097/00006454-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Wamalwa D, Benki-Nugent S, Langat A, et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J. 31:729–731. doi: 10.1097/INF.0b013e3182587796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. p. 312. [Google Scholar]
- 25.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazra R, Cohen RA, Gonin R, et al. Lipid levels in the second year of life among HIV-infected and HIV-exposed uninfected Latin American children. Aids. 26:235–240. doi: 10.1097/QAD.0b013e32834dc5fc. [DOI] [PubMed] [Google Scholar]
- 27.Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 28.Rhoads MP, Smith CJ, Tudor-Williams G, et al. Effects of highly active antiretroviral therapy on paediatric metabolite levels. HIV Med. 2006;7:16–24. doi: 10.1111/j.1468-1293.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Akuyam A, Isah HS, Ogala WN. Serum lipid profile in malnourished Nigerian children in Zaria. Niger Postgrad Med J. 2008;15:192–196. [PubMed] [Google Scholar]
- 30.Levy AR, McCandless L, Harrigan PR, et al. Changes in lipids over twelve months after initiating protease inhibitor therapy among persons treated for HIV/AIDS. Lipids Health Dis. 2005;4:4. doi: 10.1186/1476-511X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chantry CJ, Hughes MD, Alvero C, et al. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–e138. doi: 10.1542/peds.2007-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floris-Moore M, Howard AA, Lo Y, et al. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med. 2006;7:421–430. doi: 10.1111/j.1468-1293.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 33.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner MJ, Skinner AC, Perrin EM. Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study. Pediatrics. 2011;128:463–470. doi: 10.1542/peds.2011-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]