Abstract
Ayurveda, which is one of the traditional systems of medicine of India, reports the seeds of Abrus precatorius (family: Fabaceae) can be used therapeutically after shodhana process, which removes the toxin. The main objective was to scientifically study the shodhana process by evaluating the safety and efficacy of A. precatorius seeds. Aqueous extract (A1) and detoxified extract (A2) of the seeds were prepared by a process described in Ayurvedic pharmacopoeia. Thin-layer chromatography (TLC) method was developed for the two extracts using different solvent systems. Identical spots were obtained in A1 with reference values (Rf) 0.27, 0.47, and 0.79, whereas A2 showed the absence of spot having Rf value 0.47, which could possibly be the toxin found in the intact seed. A1 and A2 were evaluated for their safety and efficacy. The acute toxicity studies for A1 and A2 revealed that A1 was toxic, whereas A2 was safe at the dose of 2 g/kg. Absence of toxicity in the detoxified extract suggests removal of toxic material in processed seeds. The results obtained for hair growth activity of both the extracts were comparable to that of the standard. However, A2 showed better results in comparison to A1. Thus, the shodhana process described in Ayurveda helps in removing the toxin, while retaining the efficacy at the same time. The statistical analysis was done using one-way analysis of variance.
Keywords: Abrin, acute toxicity, alopecia, Gunja seeds
INTRODUCTION
Abrus precatorius (family: Fabaceae), also known as Gunja, is a slender, perennial climber, best known for its seeds, which are used as beads and are toxic because of the presence of abrin. The roots, seeds, and leaves of this plant have been used traditionally for their purgative, emetic, tonic, aphrodisiac, and hair growth-promoting properties.[1,2,3,4,5,6,7]
Ayurvedic pharmacopoeia describes Shodhana of Gunja seeds, which refers to its purification by various processes.[4,5] This article deals with the detoxification process of the seeds, evaluation of the toxicity, and efficacy of the extracts.
MATERIALS AND METHODS
The seeds of A. Precatorius were purchased from the local market in Vashi, India and authenticated (no authentication number) at Piramal Life Sciences, Mumbai. Minoxidil topical solution USP 2% was used as the reference standard for promoting hair growth. Cow's milk supplied by Aarey Dairy (Mumbai, India) was purchased from the local market.
Male Swiss albino mice weighing 20-30 g were used for the acute toxicity studies. Male Wistar albino rats, weighing 200-250 g, were used for hair growth studies. They were placed in cages and kept in (23 ± 5°C, 60 ± 5% relative humidity) standard environmental conditions, fed with standard diet, and allowed free access to drinking water during the period of acclimatization. All animal experiments were carried out in accordance with the guidelines of Committee for the Purpose and Supervision of Experiments on Animals (CPCSEA), and the study was approved by the Institutional Animal Ethics committee (IAEC).
A total of 250 g of seeds were powdered using a mixer, and powdered seeds were soaked in chloroform: Water (5:95) (2500 mL) overnight for maceration. The extract was filtered, and the filtrate was dried in the hot air oven at 60°C for 3-5 h, weighed, stored, and labeled as A1. Then, 250 g of powdered seeds were tied in a muslin cloth, immersed in cow's milk, and boiled for 6 h. The seeds were cleaned, and the husk was separated.[6] Following this, the seeds were extracted as described earlier, and the extract was labeled as A2.
Thin layer chromatography (TLC) development
TLC system was developed, modifying the mobile phases already reported, for A1 and A2 using Propan-1-ol: Ethyl Acetate: Water (7:1:2)[8,9,10] as the mobile phase, silica gel G254F as the stationary phase, and vanillin sulfuric acid as the spraying reagent.
Acute oral toxicity studies
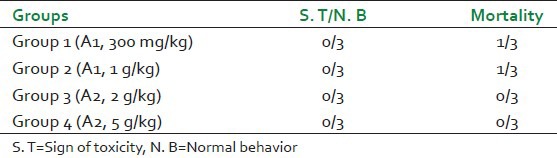
Acute oral toxicity of the extracts was carried out as per the OECD guidelines 423. The animals were divided into three groups of 3 animals each. Each of the group received a dose of 300 mg/kg and 2 g/kg of A1 and 2 g/kg of A2. The observations for sign of toxicity were recorded within a period of 48 h. The food intake and body weight were monitored for a period of 14 days.[11]
Efficacy studies
Rats were divided into two groups of 6 animals each to study the primary irritation of the extracts.[12] The study of hair growth initiation was carried out on rats that were divided into four groups of 6 animals each. Hair clippers were used to remove the hair from dorsal portion of all the test animals. Commercially available depilatory cream (Anne French) was used to ensure complete removal of hairs from denuded area, which was of nearly 4 cm2 area. Test sites were cleaned with surgical spirit. The reported dose is 60-170 mg of powdered seeds.[6] One ml quantity of both A1, A2 in a concentration of 2.5% w/v was applied over the respective test sites on one side of the spine to observe for any irritation. The test sites were observed for erythema and edema for 48 h after application.
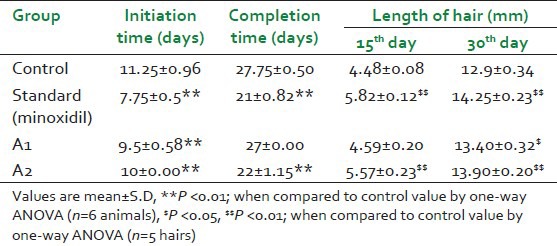
For the hair initiation test, group I served as control, wherein the animals were topically applied with the water and group II was treated with Minoxidil topical solution USP 2% and served as positive control, the animals of remaining groups were given application of A1 and A2 respectively, once a day. This treatment was continued for 30 days, during which the course the hair growth initiation pattern was observed and reported.
Parameters studied for hair growth activity were hair growth initiation time (i.e., minimum time to initiate hair growth on denuded skin region), hair growth completion time (i.e., minimum time to completely cover the denuded skin region with new hair), and hair length. Hair was plucked randomly from the test area of selected rats from each group on 15th and 30th day of the treatment and length and diameter of five hairs was measured. The length was measured using calibrated vernier calipers.[13,14] The results obtained were statistically analyzed using one-way analysis of variance (ANOVA).
RESULTS
Thin layer chromatography development
A1 shows three spots, whereas A2 shows two spots (0.27 and 0.79). It is observed that the spot with a reference value of (Rf) 0.47 in A1 is not seen in A2.
Acute oral toxicity studies
Acute toxicity studies were performed to check the effects produced by administration of both the extracts A1 and A2. As seen in Table 2, for A2, which is the detoxified extract, no mortality was seen at a dose of 2 g/kg, whereas the extract A1showed mortality at all dose levels. The animals receiving either A1 or A2 showed no signs of toxicity. Thus, it can be said that LD50 of A1 is in 2000-5000 g/kg and A2 has an LD50 of more than 5000 g/kg.[11]
Table 2.
Effect of extracts on hair growth initiation time and hair length in albino rats
None of the prepared formulations showed any erythema and/or edema, indicating that prepared formulations were not irritant on the skin of rats. None of the animals in any of the groups used during the entire study showed any signs of irritation on completion of the study.
Efficacy studies
In control group animals, initiation of hair growth in denuded area was observed in the second week. Hair growth initiation was noted on day 8 in rats of Minoxidil-treated standard group. The formulations A1 and A2 exhibited hair growth initiation on day 10. Complete hair growth with Minoxidil and control group was observed on days 21 and 28, respectively. The extract A1 showed complete hair growth after 27 days and A2 after 23 days [Table 1]. The experiment thus clearly demonstrated hair growth-promoting activity of the extracts.
Table 1.
Acute toxicity profile of the extracts
The length of the hair began to increase until the end of the treatment course [Table 2]. The extract A2 produced a comparable effect on the length of hair as the standard and had greater effect than any other groups, being 13.9 mm at the end of the course as compared to 13.4 mm in the A1, 14.25 mm in the standard, and 12.9 mm in the control group.
DISCUSSION
The process of detoxification involves boiling the seeds in cow's milk, which claims to remove the toxin. The toxins reported to be present in Abrus seeds include a toxic lectin, abrin, a fat-splitting enzyme, a glucoside abrussic acid, urease, alkaloids-abrine, abarnin, trigonelline, choline, and hypaphorine and steroidal oil that have abortive effects.[15,16,17,18,19] The contents found in cow's milk include lactose, fatty acid, milk proteins, casein, milk enzymes-plasmin, lipoprotein lipase, acid phosphatase, xanthin oxidase, lacto peroxidase, peptides, and salts of calcium, phosphates, and citrates.[20]
When Abrus seeds and cow's milk are mixed and heated, the above mentioned toxins might be removed by one of the mechanisms: (1) The toxic protein abrin is a type II ribosome inactivating protein (RIP), consisting of an A chain, which is a single polypeptide chain and the toxic principle binding to cytosol and a B chain, which is a lectin linked through a disulfide bond. A chain is non toxic to intact cells and requires B chain for its action. The enzymes in the milk might cleave the bonds and thereby deactivate the proteins. (2) The heat given for the treatment might denature the proteins. (3) The alkaloids in the seeds might form a complex with one of the constituents of the milk and hence get removed. (4) The steroidal oil might get dissolved in the fatty acid part of the milk, which itself is an emulsion.
The spot having Rf value 0.47 could not be detected in the A2. This spot obtained a brown color after being treated with vanillin sulphuric acid, which might refer to the spot of an oil. Thus, we can say that the spot might be that of the steroidal oil responsible for the abortifacient effect.
The acute toxicity tests did not show significant change in the body weight and the feed intake of mice. However, mortality was reported in mice treated with A1. Thus, it can be inferred that A1 is toxic, whereas A2 is safe in dose of 2 g/kg. The extracts were further found to be non-irritant when applied on the skin. The exact toxin that is removed and the mechanism for the reaction remain to be investigated. From the results of the efficacy studies, it can be seen that, when compared with the control, A2 showed significant increase in hair growth both on day 15 and day 30. However, the standard (Minoxidil) used showed results that were comparable to that of both the extracts.
These findings suggest that the said procedure of shodhana as described in Ayurveda is capable of removing the toxin and retaining the efficacy at the same time.
Footnotes
Source of Support: SVKM's Narsee Monjee Institute of Management Studies.
Conflict of Interest: None declared.
REFERENCES
- 1.Ross I. Totowa: Human Press; 2003. Medicinal Plants of the World: Chemical Constituents, Traditional and Medicinal Uses; pp. 15–25. [Google Scholar]
- 2.Nadkarni KM. Mumbai: Bombay Popular Prakashan; 1976. Indian Materia Medica; pp. 5–7. [Google Scholar]
- 3.Warrier PK, Nambiar VP, Ramankutty C. Chennai: Orient Longman Pvt. Ltd; 1993. Indian medicinal plants; pp. 10–5. [Google Scholar]
- 4.Ranade S. Delhi: Motilal Banarsidass Publishers Private Limited; 1994. Natural Healing Through Ayurveda; pp. 115–7. [Google Scholar]
- 5.Anonymous. Ayurvedic Pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy. (1st ed) 2001;3:44. part 1. [Google Scholar]
- 6.Sastry JL. Varanasi: Chaukhambha Orientalia; 2010. Drvayaguna Vijnana; pp. 691–2. [Google Scholar]
- 7.Vaidya AD, Raut AA, Vaidya RA. Abrus precatorius, Gaertin: A ayurvedic potent phytomedicine. J Assoc Physicians India. 2005;53:739–40. [PubMed] [Google Scholar]
- 8.Mukherjee P. New Delhi: Buisness Horizons; 2002. Quality Control of Herbal Drugs; pp. 246–370. [Google Scholar]
- 9.Stahl E. Berlin: Springer; 2005. Thin Layer Chromatography; pp. 208–47. (423-5, 854-909). [Google Scholar]
- 10.Wagner H, Bladt S. Berlin: Springer; 2004. Plant Drug Analysis A Thin Layer Chromatography Atlas; p. 14. (349-52). 73, 126, 151, 247, 263, 291. [Google Scholar]
- 11.OECD guideline for testing of chemicals (423)-Acute oral toxicity-Acute toxic class method [Google Scholar]
- 12.Rieger MM. Manhattan: Chemical Publishing Company; 2000. Harry's Cosmeticology; p. 768. [Google Scholar]
- 13.Adhirajan N, Ravi Kumar T, Shanmugasundaram N, Babu M. In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn. J Ethnopharmacol. 2003;88:235–9. doi: 10.1016/s0378-8741(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 14.Bregar RR, Gordon M, Whitney EN. Hair root diameter measurement as an indicator of protein deficiency in nonhospitalized alcoholics. Am J Clin Nutr. 1978;31:230–6. doi: 10.1093/ajcn/31.2.230. [DOI] [PubMed] [Google Scholar]
- 15.Panda H. New Delhi: Asia Pacific Buisness Press; 2004. Handbook of Medicinal Herbs with Uses; pp. 6–7. [Google Scholar]
- 16.Van Damme JM. West Sussex: John Wiley and Sons; 1998. Handbook of Plant Lectins: Properties and Biomedical Applications; pp. 77–9. [Google Scholar]
- 17.Chauhan NS. New Delhi: Indus Publication Company; 1999. Medicinal and Aromatic Plants of Himachal Pradesh; pp. 49–52. [Google Scholar]
- 18.Dimetry NZ, Gengaihi SE, Reda AS, Amer SA. Biological effect of some isolated Abrus precatorius L. alkaloids towards Tetranychus urticae Koch. Anzeiger fur Schadlingskunde. 1992;65:99–101. [Google Scholar]
- 19.Desai RV, Sirsi M. Chemical and pharmacological investigation of Abrus precatorius. Indian J Pharm. 1966;29:235–7. [Google Scholar]
- 20.Smit G. Cambridge: Woodhead Publishing; 2003. Dairy Processing: Improving quality; pp. 5–38. [Google Scholar]


