Abstract
Objective:
Evaluation of antidiabetic potential of the hydroalcoholic extract of Withania coagulans Dunal dried fruit (WCDF) alone and in combination with glipizide, in streptozotocin-induced diabetes, and evaluation of possible antihyperlipidemic activity of the same extract in high-cholesterol diet-induced hyperlipidemia, in albino rats.
Materials and Methods:
Experimental diabetes was induced in 30 albino rats with intraperitoneal injection of streptozotocin (55 mg/kg). The rats were divided into five groups receiving the following treatments orally for 4 weeks: Vehicle, glipizide (2.5 mg/kg), WCDF extract (1000 mg/kg), WCDF extract (1000 mg/kg) plus glipizide (1 mg/kg) and WCDF extract (1000 mg/kg) plus glipizide (2.5 mg/kg). Fasting and postprandial blood glucose levels were measured every week for 4 weeks. Endocrine pancreas histopathology was done at the end. In a separate set of experiment, five groups of six albino rats each, received orally for 4 weeks, vehicle, cholesterol (25 mg/kg/day), cholesterol (25 mg/kg/day) plus atorvastatin (7.2 mg/kg/day), cholesterol (25 mg/kg/day) plus WCDF extract (1000 mg/kg/day) and no treatment, respectively. Estimation of serum lipid profile and liver histopathology was done at the end of 4 weeks.
Statistical Analysis:
Between-group and within-group comparisons were respectively done by analysis of variance (ANOVA) and repeated measures ANOVA, followed by post hoc Tukey's test, with a significance level of P < 0.05.
Results and Conclusions:
The 4-week treatment with WCDF extract significantly reversed hyperglycemia in streptozotocin-induced diabetes that was comparable to glipizide. When combined with glipizide (2.5 mg/kg), WCDF extract produced a synergistic antihyperglycemic effect as well as improvement in pancreatic histopathology. Moreover, hydroalcoholic extract of WCDF was effective and comparable to atorvastatin in controlling the high-cholesterol diet-induced hyperlipidemia in rats.
Keywords: Antidiabetic, antihyperlipidemic, high-cholesterol diet, streptozotocin, Withania coagulans Dunal
INTRODUCTION
Diabetes mellitus (DM) and its attendant acute and long-term complications are a major health hazard globally. Its prevalence has now reached pandemic proportions in India. The mention of the disease in ancient Indian texts as “madhumeha” suggests it's presence in India even before 2500 BC. It could have been quite common in ancient India too.[1]
Characterized by a state of chronic hyperglycemia resulting from a diversity of etiologies, diabetes is often associated with dyslipidemia, and dyslipidemia is a major cause of cardiovascular (CV) morbidity and mortality in diabetic patients,[2] who require additional lipid-lowering agents along with their antidiabetic medications. While the currently available antidiabetic agents do not exert a favorable effect on lipid abnormalities vis-à-vis CV disease,[3] the most commonly used hypolipidemic agents, the statins, adversely influence glycemic control.[4,5]
There has been a growing emphasis on therapy of dyslipidemia associated with diabetes and on the need to develop newer antidiabetic agents with potential antihyperlipidemic effects. Dry fruits of Withania coagulans Dunal (Family: Solanaceae) have shown antidiabetic potential.[6] The plant, known by different names in India for example, Rishyagandha (Sanskrit), Panirdodi (Urdu), Panir ka phool (Hindi), Panirband (Hindi), Paneer phool (Bengali), has the property of coagulating milk and has been used for preparing a vegetable rennet ferment for making cheese. It's antidiabetic property is mentioned in ancient Ayurvedic literature, Charaka Samhita under Brihaniya Mahakashaya and Madhur skandha dravya.[7] In northern India, traditional healers use dried fruits of Withania coagulans in the treatment of diabetes and this plant is well-known for it's ethnopharmacological applications.[8] It is also used in parts of West Bengal, Chattisgrah, Bihar, and Madhya Pradesh for management of diabetes as home remedies.
The aqueous extract of Withania coagulans Dunal dried fruits (WCDFs) has been shown to possess an effective antidiabetic activity at a dose of 1 g/kg body weight in streptozotocin-induced diabetic rats without any discernible toxic effect.[9] Additionally, WCDF aqueous extract (1 g/kg; per oral) has also been reported to have hypolipidemic activity in triton-induced hypercholesterolemia in albino rats.[10]
We contemplated the present study to assess the antidiabetic and antihyperlipidemic efficacy of the hydroalcoholic extract of WCDF in albino rat models, compared to standard treatment with glipizide and atorvastatin, respectively. We conducted two successive experiments for this purpose in suitable experimental rat models.
MATERIALS AND METHODS
Materials
Chemicals
Streptozotocin (STZ) bearing batch no T835796, manufactured by Sisco Laboratories Private Limited (SRL), Citrate buffer, Glipizide (2.5 mg tablet, Manufacturer-USV Limited, Batch no: 13004115), Atorvastatin (10 mg tablet, Manufacturer-Dr. Reddy's, Batch no-E200392), WCDF extract, carboxymethyl cellulose (CMC), cholesterol powder (Merck India Limited), coconut oil.
Plant material
Dried fruits of Withania coagulans Dunal, (family: Solanaceae) were procured from a recognized supplier of herbs in Kolkata (M/s Dutta and Co, Kolkata). The identification and authentication was done at Department of Pharmacognosy, National Research Institute of Ayurveda for Drug Development, Kolkata.
The whole fruits (1 kg) were mechanically crushed (mesh size 20) and extracted with a water-alcohol mixture (60% ethyl alcohol) through cold percolation at room temperature up to 72 h.[11] The extract was filtered and concentrated in rotary evaporator under reduced pressure to obtain semisolid material, which was then lyophilized to get a powder (yield: 22.2% w/w). The lyophilized powder was dissolved in distilled water and used.
Experimental animals
Male albino Wistar rats of same age group, and body weight 150-200 g, procured from a recognized laboratory-animal breeder were employed in the study. Rats were housed in polypropylene cages at an ambient temperature of 25-30°C and 45-55% relative humidity with a 12 h each of dark and light cycle. All the rats were given a 14-day period of acclimatization before starting the experiment. Rats were fed pellet diet and water ad libitum. Animal housing, care and the conduct of experimental procedures were done in accordance with Committee for the Purpose of Control and Supervision on Experiments on Animals guidelines in India. The study was initiated after getting ethics approval.
Methods
Methods to evaluate the antidiabetic potential of WCDF extract
Induction of diabetes mellitus
DM was induced by a single intraperitonial injection of freshly prepared STZ 55 mg/kg in 0.1 M citrate buffer (pH: 4.6) to all groups of overnight fasted rats.[9] After a gap of 5 days following STZ administration, fasting blood glucose levels were measured, and rats with fasting blood glucose above 150 mg/dL were selected for the study.
Animal grouping
A total of 30 rats with STZ induced hyperglycemia were divided randomly into five groups, each containing six animals. The rats were fed orally with the respective regimens for 4 weeks using a feeding cannula:
Group 1: Negative control receiving vehicle (2% CMC solution).
Group 2: Positive control receiving glipizide at a dose of 2.5 mg/kg as a standard drug.
Group 3: Receiving WCDF extract at a dose of 1000 mg/kg.
Group 4: Receiving WCDF extract at a dose of 1000 mg/kg plus glipizide at a dose of 2.5 mg/kg.
Group 5: Receiving WCDF extract at a dose of 1000 mg/kg plus glipizide at a dose of 1 mg/kg.
Assessment parameters
Fasting and postprandial blood glucose
Animals were deprived of food overnight and for at least 16 h but allowed free access to drinking water. Fasting blood glucose was estimated in such state. The same rats were then given an oral glucose load (2 g/kg). Postprandial blood glucose levels were estimated 2 h after such oral glucose challenge. Blood was drawn from tip of the tail with the help of disposable lancet. Blood glucose levels were estimated in each group before and after STZ administration and then weekly up to 4 weeks with the help of strip and glucometer (Glucochek, Major Biosystem Corp., Taiwan.).
Histopathological examination
At the end of the experiment, one rat from each group having the most prominent change in blood glucose levels was sacrificed for histopathological examination of pancreas. Standard histopathological procedure was followed.[12] Pancreas specimens were fixed in formal saline. Then tissue dehydration was done using ascending grades of alcohol (ethanol), followed by tissue clearing using xylene. The tissues were transferred to molten paraffin for impregnation and embedded in paraffin blocks. After fine sectioning, staining was done using hematoxylin and eosin (H and E) stain and examined under a microscope.
Evaluation of antihyperlipidemic activity of aqueous extract of WCDF
Induction of hyperlipidemia
For inducing hyperlipidemia, the rats of Groups II, III, and IV were fed through feeding cannula with cholesterol-rich diet containing cholesterol 25 mg/kg/day dissolved in coconut oil.[13] Group I and V did not receive high-cholesterol diet.
Animal grouping
Five groups of six rats in each were used in the experiment. They received different regimens orally for 4 weeks.
Group I: Served as vehicle control receiving only vehicle (2% CMC solution) and receiving no other treatment.
Group II: Served as the hyperlipidemia group receiving no treatment.
Group III: Served as the standard treatment group having received atorvastatin (7.2 mg/kg/day) (corresponding to the maximum recommended human dose, i.e., 80 mg/day).[3]
Group IV: Served as the investigational treatment group having received WCDF extract (1000 mg/kg/day).[9]
Group V: Served as untreated control receiving no treatment.
Assessment parameters
Lipid profile
Total cholesterol, serum triglycerides, and high-density lipoprotein (HDL) cholesterol were estimated using enzymatic commercial kits marketed by M/s Transasia Bio-Medicals Ltd. Very low-density lipoprotein (VLDL) cholesterol was calculated as triglycerides/5.[14] Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula: LDL cholesterol = Total cholesterol – (HDL + VLDL).[15] All estimations were done using auto analyzer (Merck, Germany).
Histopathological examinations
One albino rat from each group having the most significant changes in serum lipid profile level was sacrificed for histopathological examinations of liver. Standard histopathological procedure was followed.[12] Liver specimens were fixed in formal saline. The rest of the procedure was same as before.
Statistical analysis
Analysis was done with the help of standard statistical software, namely Microsoft Excel, SPSS version 17 and Graph pad prism version 5. Data was expressed as mean ± standard error of the mean. Different groups were compared with analysis of variance (ANOVA) and same group in different time points with repeated measures ANOVA followed by post hoc Tukey's test in both occasions. A two-tailed P < 0.05 was considered as statistically significant.
RESULTS
Evaluation of the antidiabetic potential of WCDF
Effect on fasting blood glucose (FBG) level
The base line value in all the five groups of rats showed that the FBG levels were within normal levels. After single dose (55 mg/kg) of STZ administration in all the groups, diabetes was effectively induced, as evidenced by statistically significant (P < 0.001) increase in FBG level from the baseline value 5 days following STZ administration. The WCDF extract treatment for just a week in Group 3, resulted in statistically significant reduction in FBG level. With increase in duration of WCDF treatment, the reduction in blood glucose levels was more and more pronounced. [Figure 1]
Figure 1.
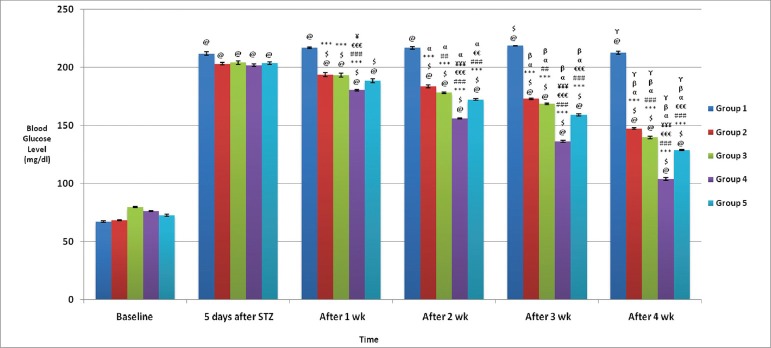
Comparison of postprandial blood glucose levels (mg/dl) in different groups: Values are expressed in Mean ± SEM, n = 6 in each group @P < 0.05 as compared to baseline, $P < 0.05 as compared to 5 days after STZ, ***P < 0.001. **P < 0.01, *P < 0.05 as compared to Gr 1, ###P < 0.001, ##P < 0.01, #P < 0.05 as compared to Gr 2, €€€P < 0.001, €€P < 0.01, €P < 0.05 as compared to Gr 3, ¥¥¥P < 0.001, ¥¥P < 0.01, ¥P < 0.05 as compared to Gr 5, α P < 0.05, β P < 0.05, γ P < 0.05 as compared to 1 wk, 2 wk and 3 wk values respectively
Similarly, a qualitatively equivalent trend in blood glucose lowering effect was seen with glipizide treatment in Group 2 rats. In both the Groups 4 and 5, the statistically significant impact of the combination treatments was apparent with just 1 week treatment that became more and more pronounced with increasing duration of treatment. When the data sets were compared between groups after 4 weeks of treatment, the most pronounced impact was seen with Group 4 (P < 0.001) that received the optimum standard dose of glipizide plus 1000 mg/kg/day WCDF extract. A qualitatively similar effects were also seen with Group 5 that received suboptimal dose of glipizide plus 1000 mg/kg/day WCDF extract; there were significant lowering of fasting blood glucose compared to Group 2 (P < 0.001) or Group 3 (P < 0.001), respectively. Thus, it points to a synergistic effect exerted by the combination regimen [Figure 1].
Effect on postprandial glucose (PPG) level
Both glipizide and WCDF extract treatment when used alone, that is, in Groups 2 and 3 respectively, significantly (P < 0.05) lowered PPG level, after 2-week treatment in the former and even with 1 week of treatment in case of latter group. However, the reduction was more significant with the increased duration in treatment in either group [Figure 2].
Figure 2.
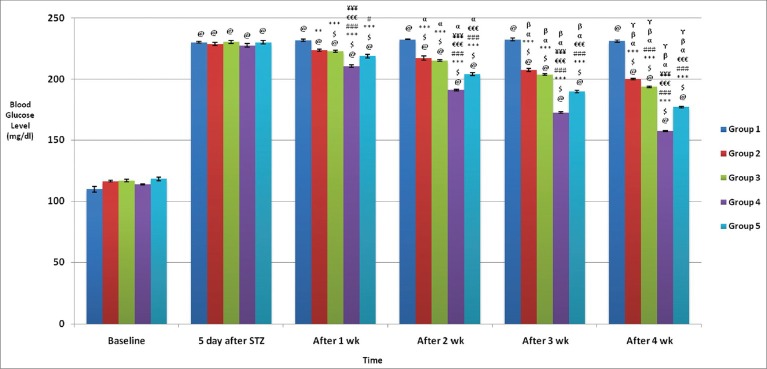
Comparison of postprandial blood glucose levels (mg/dl) in different groups: Values are expressed in Mean ± SEM, n = 6 in each group @P < 0.05 as compared to baseline, $P < 0.05 as compared to 5 days after STZ, ***P < 0.001, **P < 0.01, *P < 0.05 as compared to Gr 1, ###P < 0.001, ##P < 0.01, #P < 0.05 as compared to Gr 2, €€€P < 0.001, €€P < 0.01, €P < 0.05 as compared to Gr 3, ¥¥¥P < 0.001, ¥¥P < 0.01, ¥P < 0.05 as compared to Gr 5, α P < 0.05, β P < 0.05, γ P < 0.05 as compared to 1 wk, 2 wk and 3 wk values respectively
In Group 4, where glipizide and WCDF extract were combined (WCDF extract 1000 mg/kg plus glipizide 2.5 mg/kg) statistically significant (P < 0.05) synergistic impact on PPG lowering was demonstrated even with just 1-week treatment. Such impact was more pronounced with longer duration of treatment [Figure 2].
An analysis of observations on PPG values in Group 5 (WCDF extract 1000 mg/kg plus glipizide 1 mg/kg) demonstrated that although there was a general trend of synergism, this was much less as compared to Group 4 (WCDF extract 1000 mg/kg plus glipizide 2.5 mg/kg) findings [Figure 2].
Histopathological findings
The impact of different treatment on the structure of the beta cells of pancreas was studied as compared to the vehicle-treated diabetic (STZ-treated) control and the representatives from each group were photographed as shown in Figures 3-7. Although all four treatment groups showed improvement in pancreatic islets cell histopathology, Group 4 and Group 5 showed a far better evidence of recovery. However, due to healing and fibrosis, islets cell morphology was changed leading to cell shrinkage and deviation from normal histological appearance.
Figure 3.
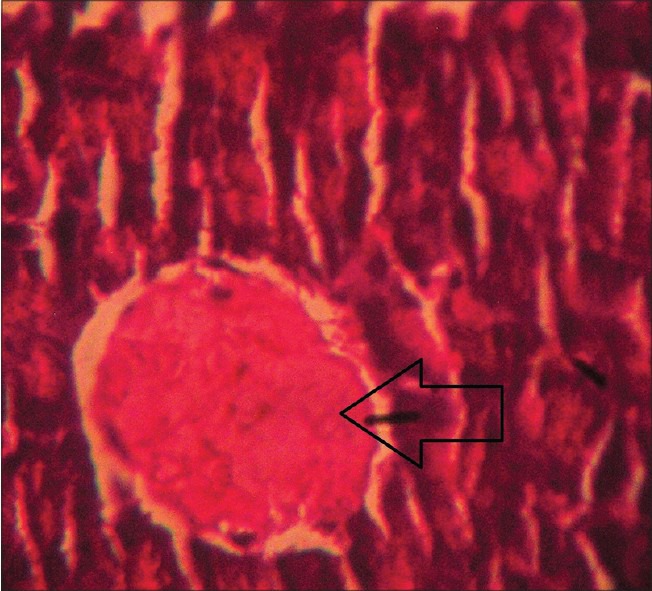
Histopathological picture of pancreas of STZ-induced diabetic rat after receiving vehicle (2% CMC) for 4 weeks, showing beta cell destruction as indicated by arrow (Group 1) (H and E, ×40)
Figure 7.
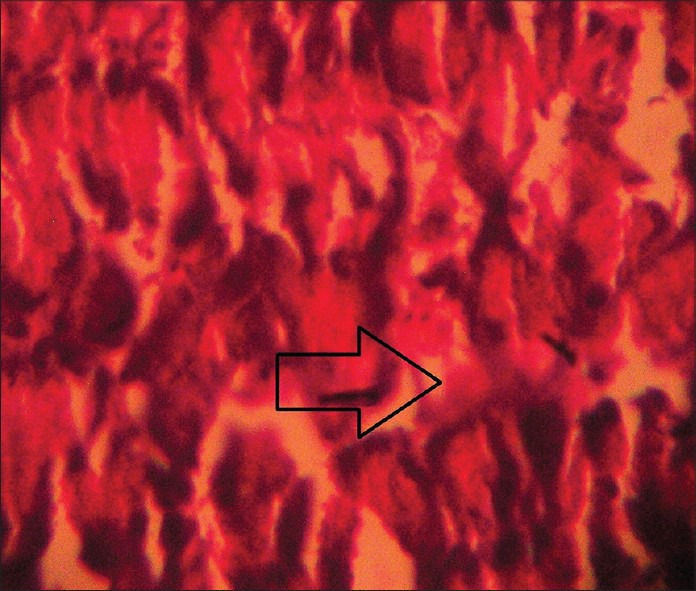
Histopathological picture of pancreas of STZ-induced diabetic rat after 4 weeks treatment with WCDF extract (1000 mg/kg) + glipizide (2.5 mg/kg) showing even a far better trend of recovery of pancreatic beta cell destruction as indicated by arrow (Group 4) (H and E, ×40)
Figure 4.
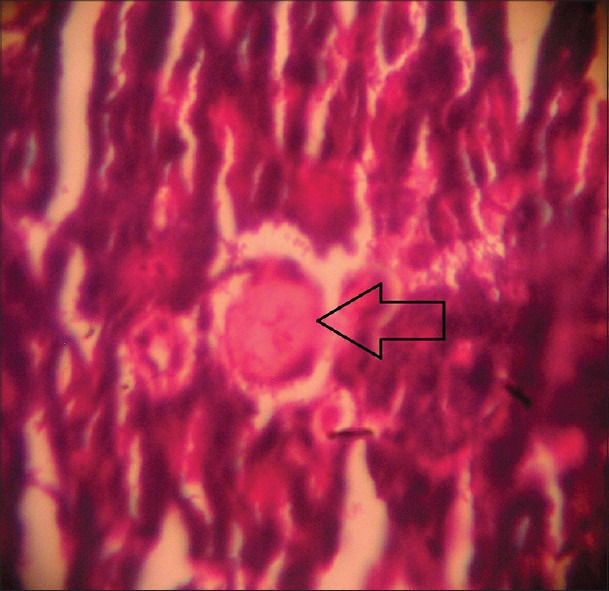
Histopathological picture of pancreas of STZ-induced diabetic rat after 4 weeks treatment with glipizide (2.5 mg/kg) showing a trend of recovery of pancreatic beta cell destruction as indicated by arrow (Group 2) (H and E, ×40)
Figure 5.
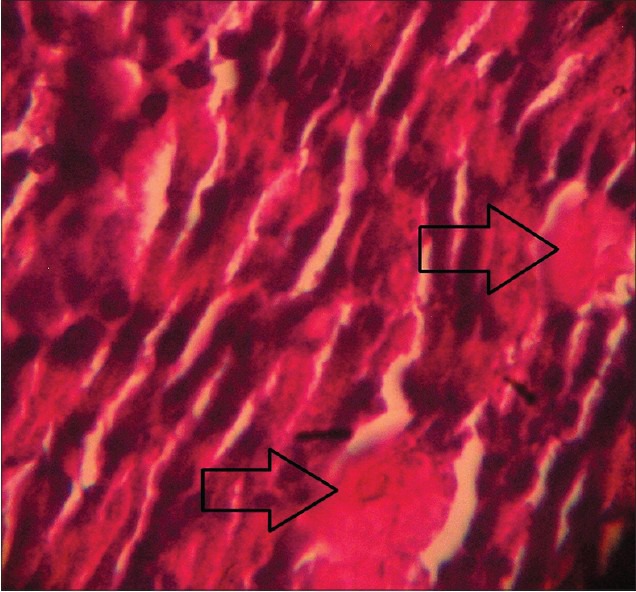
Histopathological picture of pancreas of STZ-induced diabetic rat after 4 weeks treatment with WCDF extract (1000 mg/kg) showing a trend of recovery of pancreatic beta cell destruction as indicated by arrow (Group 3) (H and E, ×40)
Figure 6.
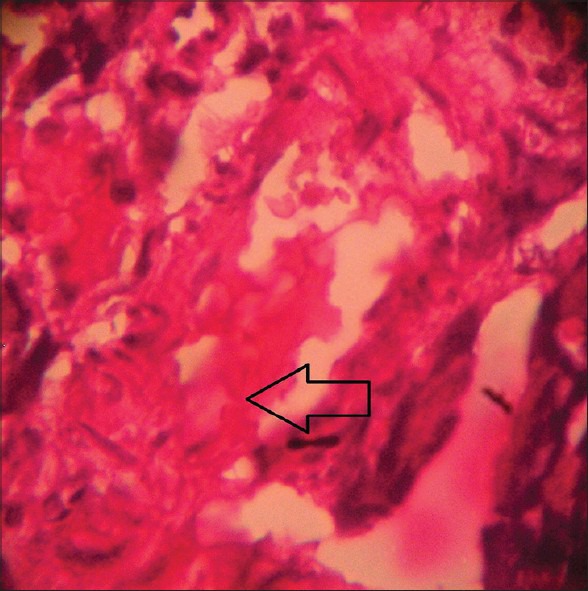
Histopathological picture of pancreas of STZ-induced diabetic rat after 4 weeks treatment with WCDF extract (1000 mg/kg) + glipizide (1 mg/kg) showing a better trend of recovery of pancreatic beta cell destruction as indicated by arrow (Group 5) (H and E, ×40)
Evaluation of antihyperlipidemic activity of WCDF extract
Effects on lipid profile:
Total serum cholesterol, serum triglyceride, serum LDL and VLDL values were significantly decreased and serum HDL significantly increased in Groups I, III, IV, and V in comparison to respective value of Group II (P < 0.001). Total serum cholesterol and serum LDL in Groups III and IV were significantly increased in comparison to respective value of Group V (P < 0.05). Total serum cholesterol in Group IV and serum LDL in Groups III and IV values were significantly increased in comparison to respective value of Group I (P < 0.05) [Figure 8].
Figure 8.
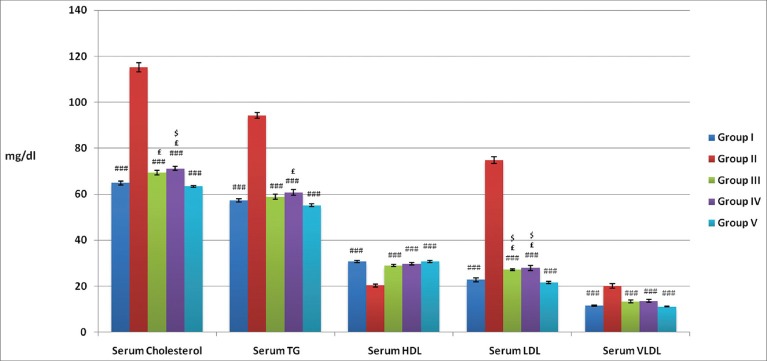
Effects of atorvastatin and WCDF extract on lipid profile (mg/dl) in albino rats: Values are expressed in Mean ± SEM, n = 6 in each group ###P < 0.001 £P < 0.05 and $P < 0.05 as compared Gr II, Gr V and Gr II values respectively
Histopathological findings
Histopathological picture of liver in none the five groups revealed any abnormalities.
DISCUSSION
DM, a disorder of glucose metabolism, is often associated with hyperlipidemias. The conventional hypoglycaemic drugs like glipizide[9] cannot benefit dyslipidemia. This warrants coadministration of lipid lowering agents, for example, statins which have many limiting adverse effects. Products of herbal origin like WCDF extract have been shown to possess both antidiabetic as well as antihyperlipidemic properties in earlier studies. In the present study, we investigated WCDF hydroalcoholic extract further for its potential antidiabetic and antihyperlipidemic effects in experimental models. Besides, attempts were made to assess if WCDF extract treatment could have synergistic effect with standard oral hypoglycemic and lipid lowering agents like glipizide and atorvastatin, respectively.
The combination of WCDF extract plus glipizide showed a greater glucose-lowering potential than either drug alone. And, this trend was gradually more and more pronounced with longer duration of treatment namely 2, 3, or 4 weeks.
However, when comparison was done between the findings of Groups 4 and 5 it was evident that glipizide at a higher dose level (2.5 mg/kg) showed appreciable synergism with WCDF extract (1000 mg/kg), when the dose of glipizide was reduced as 1 mg/kg, the blood glucose lowering effect was not to the same tune but was significantly more in comparison to either WCDF extract (1000 mg/kg) or glipizide (2.5 mg/kg) used alone.
WCDF extract in the dose used significantly reversed the hyperglycemia in STZ-induced diabetes and a 4-week treatment with WCDF extract was as good as that with glipizide. Combined with an optimum dose of glipizide (2.5 mg/kg), WCDF extract produced a synergistic effect. The effect of WCDF extract treatment was sufficiently pronounced to have impact on pancreatic beta cell histology which was even comparable with glipizide-induced changes. The combination therapy was shown to produce a better and synergistic impact on histological findings. The blood glucose lowering effect of hydralocoholic extract of WCDF has been attributed to increased glucose utilization in the peripheral tissues as evidenced through isolated rat hemidiaphragm experiment.[16]
Statins offer effective choice for treating dyslipidemia, having an overall cardioprotective benefits. These drugs competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, enzyme which catalyzes an early, rate-limiting step in hepatic cholesterol biosynthesis, thereby interfering with cholesterol synthesis. Higher doses of statins also can reduce triglyceride levels and raise HDL-C levels. Statins also reduce LDL levels through a mevalonic acid-like moiety that competitively inhibits HMG-CoA reductase and also by enhancing the removal of LDL precursors (VLDL and IDL) and by decreasing hepatic VLDL production. Elevated triglyceride levels are also substantially reduced by statins.[3] However, recently there are concerns against high-dose statin therapy due to their adverse impact on blood sugar level. In a meta-analysis study, statin therapy was found to be associated with a 9% increased risk for incident diabetes.[4] In another study, a pooled analysis of data from five statin trials, intensive-dose statin therapy was associated with an increased risk of new-onset diabetes compared with moderate dose statin therapy.[5]
In the present study, WCDF extract treatment caused improved lipid profile in high-fat diet-induced hyperlipidemic rats, a finding that is in consonance with an earlier study.[10] Antihyperlipidemic and antiatherosclerotic action of WCDF extract has also been reported in streptozotocin-induced diabetic rats.[17] Also the drug has shown hypolipidemic effects in triton-induced hypercholesterolemia.[10] The hypolipidemic activity may be due to its interference in synthesis, metabolism, and excretion of lipids. Studies are going on to identify the active principles responsible for this action. WCDF extracts contain many withanolides and lactones which have been reported to show CV benefits in dyslipidemia.[18,19,20,21]
The present study also revealed that the antihyperlipidemic activity of WCDF extract and atorvastatin in high-cholesterol diet-induced hyperlipidemia in albino rats was comparable.
At the end of the study, the rate of reduction of total serum cholesterol, serum triglyceride, LDL and VLDL levels, and elevation of HDL levels by atorvastatin compared to the vehicle-treated control and untreated control corresponded with that of WCDF extract. In comparison to the vehicle-treated control, atorvastain had more beneficial effects on lowering serum cholesterol than WCDF extract but effects on other parameters were same with both the drugs. Again, in comparison to the untreated control, effects on lipid profile were same with both the drugs. The values attained at the end of the experiment were still higher than normal untreated control. The short duration of the present study might be a limiting factor. However, considering the mean values, atorvastatin had better effect than WCDF extract. But in diabetic hyperlipidemic patients WCDF extract may offer advantage over atorvastatin due to blood glucose increasing potential of the latter and dual protection offered by the former.
CONCLUSION
Finally, it may be concluded that the WCDF extract can be considered as an adjuvant in the treatment of type 2 DM which can possibly lower the dose requirement of standard oral hypoglycemic agents like glipizide. The hydroalcoholic WCDF extract was effective and comparable to atorvastatin in controlling lipid profile in high-cholesterol diet-induced hyperlipidemia in albino rats. Further studies with reduced dose of WCDF extract and human studies are needed to prove the safety and efficacy of long-term administration of this drug as potential antihyperlipidemic agent in routine clinical practice. Moreover, options of combining WCDF extract with moderate or low dose of atovastatin can also be explored.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Weaver LJ, Narayan KM. Reconsidering the history of type 2 diabetes in India: Emerging or re-emerging disease? Natl Med J India. 2008;21:288–91. [PubMed] [Google Scholar]
- 2.Tang WH, Maroo A, Young JB. Ischemic heart disease and congestive heart failure in diabetic patients. Med Clin North Am. 2004;88:1037–61. doi: 10.1016/j.mcna.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Fisman EZ, Tenenbaum A, Motro M, Adler Y. Oral antidiabetic therapy in patients with heart disease. A cardiologic standpoint. Herz. 2004;29:290–8. doi: 10.1007/s00059-004-2476-5. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 5.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 6.Budhiraja RD, Garg KN, Sudhir S, Arora B. Protective effect of 3-beta-hydroxy-2,3-dihydrowithanolide F against CCl4-induced hepatotoxicity. Planta Med. 1986;1:28–9. doi: 10.1055/s-2007-969059. [DOI] [PubMed] [Google Scholar]
- 7.Sadavirechaniya Shatashritiya Adhayaya. In: Charaka Samhita. 2nd ed. vol. 1. Sutrasthan 4. Nag Kj BC., editor. Kolkata: Nabapatra Prakashan; 1994. pp. 29–42. [Google Scholar]
- 8.Kirtikar KR, Basu BD. Indian Medicinal Plants. In: Basu CM, editor. Vol. 2. Allahabad: 1933. pp. 1777–81. [Google Scholar]
- 9.Jaiswal D, Rai PK, Watal G. Antidiabetic effect of Withania coagulans in experimental rats. Indian J Clin Biochem. 2009;24:88–93. doi: 10.1007/s12291-009-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemalatha S, Wahi AK, Singh PN, Chansouria JP. Hypolipidemic activity of aqueous extract of Withania coagulans Dunal in albino rats. Phytother Res. 2006;20:614–7. doi: 10.1002/ptr.1916. [DOI] [PubMed] [Google Scholar]
- 11.Handa SS, Khanuja SP, Longo G, Rakesh DD. Extraction techniques for Medicinal and Aromatic plants. Trieste: International Center for Science and High Technology. 2008:22–33. [Google Scholar]
- 12.Chakraborty P, Chakraborty G. Vol. 33. Kolkata: New Central Book Agency (P) Ltd; 2003. Practical Pathology; pp. 293–300. [Google Scholar]
- 13.Ochani PC, D’Mello P. Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa Linn. Leaves and calyces extracts in rats. Indian J Exp Biol. 2009;47:276–82. [PubMed] [Google Scholar]
- 14.Which cholesterol test should you get? Harvard Health Publications. Harvard medical school first printed in the 2004 Nov issue of the Harvard Health Letter. [Last cited on 2011 Sep 12]. Available from: http://www.health.harvard.edu/newsweek/Which_cholesterol_test_should_you_get.htm .
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Hemalatha S, Sachdeva N, Wahi AK, Singh PN, Chansouria JP. Effect of aqueous extract of fruits of Withania coagulans on glucose utilization by rat hemidiaphragm. Indian J Nat Prod. 2005;21:20–1. [Google Scholar]
- 17.Hoda Q, Ahmad S, Akhtar M, Najmi AK, Pillai KK, Ahmad SJ. Antihyperglycaemic and antihyperlipidaemic effect of poly-constituents, in aqueous and chloroform extracts, of Withania coagulans Dunal in experimental type 2 diabetes mellitus in rats. Hum Exp Toxicol. 2010;29:653–8. doi: 10.1177/0960327109359638. [DOI] [PubMed] [Google Scholar]
- 18.ur-Rahman A, Dur-e-Shahwar, Naz A, Choudhary MI. Withanolides from Withania coagulans. Phytochemistry. 2003;63:387–90. doi: 10.1016/s0031-9422(02)00727-6. [DOI] [PubMed] [Google Scholar]
- 19.ur-Rahman A, Shabbir M, Yousaf M, Qureshi S, Dur-e-Shahwar, Naz A, et al. Three withanolides from Withania coagulans. Phytochemistry. 1999;52:1361–4. doi: 10.1016/s0031-9422(02)00727-6. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian SS, Sethi PD, Glotter E, Kirson I, Lavie D. 5,20α (R)-dihydroxy-6α,7α-epoxy-1-oxo-(5α) witha-2,24-dienolide, a new steroidal lactone from Withania coagulans. Phytochemistry. 1971;10:685–8. [Google Scholar]
- 21.Budhiraja RD, Sudir S, Garg KN. Cardiovascular effects of a withanolide from Withania coagulans, dunal fruits. Indian J Physiol Pharmacol. 1983;27:129–34. [PubMed] [Google Scholar]


