Abstract
Objective
To describe the genotypes, phenotypes, immunophenotypes, and treatments of PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne), a rare autoinflammatory disease, in 5 patients.
Methods
Clinical information was gathered from medical records and through interviews with 5 patients from 4 kindreds. PSTPIP1 (CD2BP1) exon 10 and exon 11 sequencing was performed in each patient. Neutrophil granule content and cytokine levels were determined in plasma and stimulated peripheral blood mononuclear cells (PBMCs) from patients and controls.
Results
We identified 2 previously described PAPA syndrome–associated PSTPIP1 mutations, A230T and E250Q, and a novel change, E250K. Disease penetrance was incomplete, with variable expressivity. The cutaneous manifestations included pathergy, cystic acne, and pyoderma gangrenosum. Interleukin-1 (IL-1β) and circulating neutrophil granule enzyme levels were markedly elevated in patients compared to those in controls. PBMC stimulation studies demonstrated impaired production of IL-10 and enhanced production of granulocyte–macrophage colony-stimulating factor. Good resolution of pyoderma gangrenosum was achieved in 3 patients with tumor necrosis factor (TNFα) blockade treatment.
Conclusion
This analysis of 5 patients demonstrates that mutations in PSTPIP1 are incompletely penetrant and variably expressed in the PAPA syndrome. Neutrophil granule proteins are markedly elevated ex vivo and in the plasma, and elevated levels might be compatible with a diagnosis of PAPA syndrome. TNFα blockade appears to be effective in treating the cutaneous manifestations of PAPA syndrome.
INTRODUCTION
PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum [PG], and acne) (OMIM ID #604410) is a rare autosomal-dominant autoinflammatory disease caused by mutations in PSTPIP1 (also known as CD2BP1), the gene for proline/serine/threonine phosphatase–interacting protein 1 (PSTPIP-1). PSTPIP-1 is a cytoskeleton-associated adaptor protein expressed predominantly in hematopoietic cells, and it modulates T cell activation (1), cytoskeletal organization, and interleukin-1β (IL-1β) release (2). The only 2 mutations described, A230T and E250Q, have been found in 7 kindreds (3–9) and in several sporadic cases (10,11). The mutations are thought to disrupt the binding of PSTPIP-1 with protein tyrosine phosphatase–PEST, a regulatory phosphatase, and increase its avidity for pyrin in the cytosol, thereby causing dysregulation of IL-1β production (12).
PAPA syndrome typically presents with recurrent sterile, erosive arthritis in childhood, occurring spontaneously or after minor trauma, occasionally resulting in significant joint destruction. By puberty, joint symptoms tend to subside and cutaneous symptoms increase. Cutaneous manifestations include pathergy, frequently with abscesses at the sites of injections, severe cystic acne, and recurrent nonhealing sterile ulcers, often diagnosed as PG (3).
Over the last 15 years, we have identified PSTPIP1 mutations in 11 individuals from 4 kindreds; only 5 of the individuals have the classic triad of pyogenic arthritis, PG, and cystic acne. Their clinical presentations, immunologic features, and medical management are reported herein.
PATIENTS AND METHODS
Patients
Patient 1. The patient, a 46-year-old white man, was described as having “streaking leukocyte factor disease” (13). Symptoms started in infancy with pathergy at the site of vaccination and pustules along the gingiva. Recurrent sterile arthritis started at age 2 years, with improvement in the second decade of life. PG lesions started at age 8 years, typically following minor trauma. Severe cystic acne appeared at age 10 years. Minimally effective PG therapies included plasmapheresis, thalidomide, dapsone, and anakinra. Treatment with dapsone caused acute severe colonic inflammation requiring partial colectomy. PG remitted briefly after the patient was treated with high-dose steroids. Tacrolimus treatment was initiated (13), but was discontinued due to hypertension. Monthly infliximab was initiated, resulting in sustained resolution of the symptoms (Table 1).
Table 1.
Genotype and Clinical Features of the 5 Patients with PAPA syndrome*
| Patient | Mutation | Characteristic clinical Features |
Other clinical features | Poor response to therapy |
Good response to therapy |
|---|---|---|---|---|---|
| 1 | A230T | Sterile skin abscesses Sterile arthritis PG Cystic acne |
Severe colonic inflammation with dapsone |
Plasmapheresis Thalidomide Dapsone† Anakinra |
Tacrolimus† Infliximab |
| 2 | E250Q | Sterile skin abscesses Sterile arthritis Sterile osteomyelitis PG |
Recurrent otitis | Etanercept | Corticosteroids† Anakinra† Infliximab† Adalimumab |
| 3 | A230T | Sterile abscesses Sterile arthritis Cystic acne PG |
Recurrent otitis | IVIG Colchicine Infliximab |
Combination methotrexate, prednisone and adalimumab |
| 4 | A230T | Sterile arthritis PG |
Isotretinoin Anakinra |
||
| 5 | E250K | Sterile arthritis PG |
Lymphadenopathy, splenomegaly, thrombocytopenia, hemolytic anemia, pharyngeal papillomatosis, T cell LGL |
Anakinra | Cyclosporine† Tacrolimus (not sustained) |
PAPA Syndrome = pyogenic sterile arthirits, pyoderma gangrenosum, and acne; PG = pyoderma gangrenosum; IVIG = intravenous immunoglobulin; LGL = large granular lymphocytosis.
Discontinued due to adverse effects
Patient 2. The patient, a 10-year-old Hispanic boy, presented at age 4 months with pathergy following vaccination, and sterile arthritis of the left knee. At age 1 year he was diagnosed as having osteomyelitis of the left distal femur and right elbow. Over the next several years, recurrent sterile skin abscesses and PG developed. He had moderate response to high-dose corticosteroids, complicated, however, by growth retardation and Cushingoid habitus. He had minimal response to etanercept therapy. Anakinra was initiated, but was discontinued due to multiple infections. Infliximab was then started, but also had to be discontinued due to an anaphylactic reaction. He is now being treated successfully with subcutaneous adalimumab.
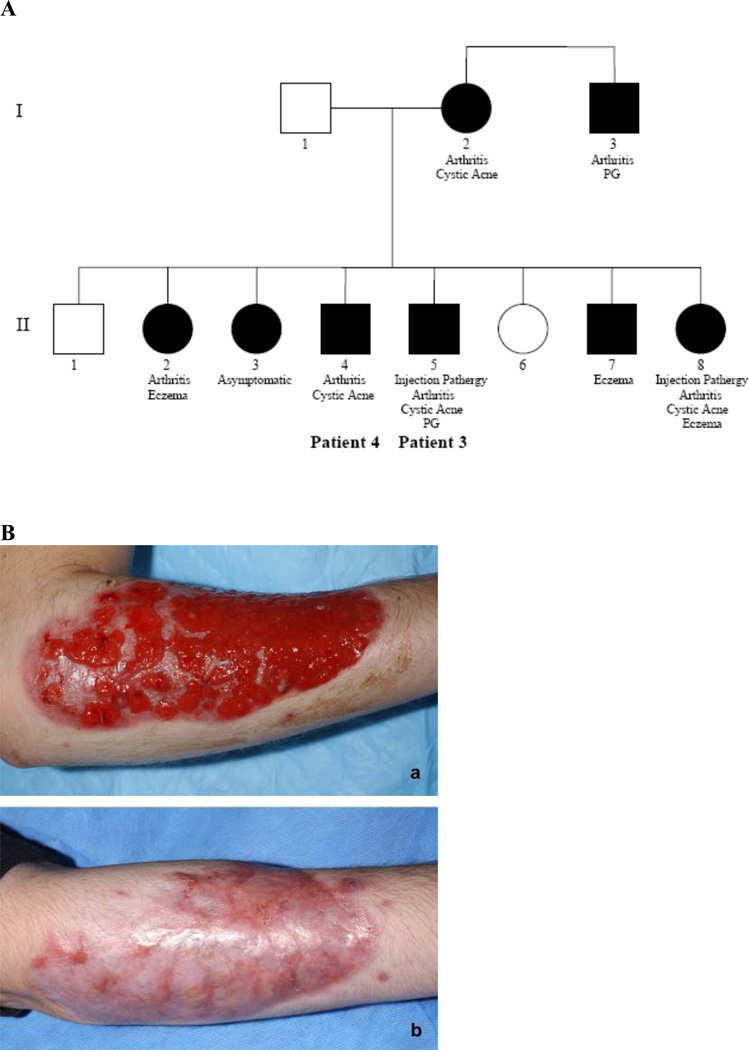
Patient 3. The patient, a 20-year-old white man, presented in infancy with sterile abscesses at vaccination sites. He subsequently developed recurrent sterile arthritis. Severe cystic acne developed at age 11 years, followed by PG at age 15 years. Chronic PG of the arm, severe acne and extensive scarring on the face and back, and pathergy have persisted. Intravenous immunoglobulin, colchicine, and infliximab were ineffective. He has had a good response to methotrexate and prednisone with biweekly adalimumab (Figure 1B).
Figure 1.
A, Pedigree of the family of Patients 3 and 4. Black symbols = positive for a mutation; white symbols = negative for a mutation. B, Pyoderma gangrenosum of the right forearm in Patient 3 before treatment (a) and after 15-month treatment with adalimumab (b).
Five of the patient’s 7 siblings, his mother, and his maternal uncle have confirmed mutations in PSTPIP1 (Figure 1A). PG has occurred only in the proband’s uncle; 3 siblings had eczema of the neck, arms, and palms during adolescence. Arthritis occurred in 6 of the family members with PSTPIP1 mutations. One sibling with the PSTPIP1 mutation has remained asymptomatic to age 16 years despite substantial trauma with lacerations in a motor vehicle accident (family member II3 in Figure 1A).
Patient 4. The patient, the 18-year-old brother of patient 3, developed recurrent arthritis of the right ankle following a fracture at age 8 years. At age 12 years he developed painful cystic acne on the face and back, which responded to topical isotretinoin but relapsed after its cessation. His disease has been well controlled on a regimen of anakinra. He has not had PG or pathergy.
Patient 5. Patient 5, a white girl, developed a maculopapular rash at age 1 month. Cervical lymphadenopathy and splenomegaly, with thrombocytopenia and hemolytic anemia, developed at age 6 months. Although her platelet count normalized, bleeding diathesis with epistaxis and easy bruisability continued throughout childhood. This was attributed to abnormal protease activity in the peripheral blood, leading to cleavage of high molecular weight von Willebrand factor (Gralnick H, Rick M: unpublished observations). Recurrent sterile arthritis developed in the large joints at age 8 years and PG started at age 18 years. Cystic acne did not develop. Recurrent upper respiratory infections and pneumonias, and episodic lymphangitis and cellulitis, continued into adulthood. Saddlenose deformity followed spontaneous septal perforation; pharyngeal papillomatosis developed in her 20s. There was no relevant family history. Flow cytometry of the peripheral blood showed increased clonal CD3+CD8+CD57+ cells, indicating large granular lymphocytosis of the T cells; bone marrow biopsy showed no lymphocyte infiltration, myelodysplasia, or leukemia.
The patient was started on a regimen of corticosteroids for PG in early childhood. Cyclosporine improved the PG but was poorly tolerated. Efficacy of tacrolimus was short-lived. Granulocyte colony-stimulating factor, initiated because of neutropenia, rapidly worsened the PG. Treatment with anakinra did not improve the PG or her extensive cutaneous ulcers. She died at age 36 years of sepsis-associated multiorgan failure.
Genotype analysis
Exon 10 and exon 11 of PSTPIP1 were amplified from genomic DNA and screened for mutations by fluorescent sequencing with BigDye Terminator version 3.1 chemistry (Applied Biosystems) on an ABI 3700 automated sequencer analyzed with Sequencher 4.7 (Gene Codes).
Analysis of cytokines and neutrophil proteins in plasma
Freshly drawn heparinized blood was centrifuged at 450g and the plasma was harvested. A second centrifugation at 450g removed residual platelets and cells. Enzyme-linked immunoassays were performed to determine levels of lactoferrin, myeloperoxidase (Oxis International), neutrophil gelatinase (R&D Systems), α-defensins (human neutrophil proteins [HNPs] 1–3), neutrophil elastase, and lipopolysaccharide binding protein (Cell Sciences). Plasma levels of IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, interferon-γ (IFNγ), and tumor necrosis factor α (TNFα) were measured by multiplex cytokine analysis (Pro-Inflammatory Ultra-Sensitive 9-Plex; Meso Scale Discovery) using a Sector Imager 6000 reader according to the instructions of the manufacturer (Meso Scale Discovery). Levels in the patients were compared with those in stored plasma from 59 healthy control subjects.
Stimulation of peripheral blood mononuclear cells (PBMCs) for determination of cytokine production
PBMCs were isolated from heparinized blood by standard discontinuous gradient centrifugation using Ficoll-Paque Premium (GE Healthcare), washed twice with Hanks’ balanced salt solution without divalent cations, and resuspended at a density of 2 × 106/ml in RPMI 1640 medium (Lonza) containing 10% fetal bovine serum (HyClone). Cell suspensions (100 µl/well) were cultured in a 96-well flat-bottomed tissue culture plate (Costar #3596; Corning). Agonists added to selected wells were as follows: phytohemagglutinin (PHA) (1%; Invitrogen), IL-12 (10 ng/ml; PeproTech), PHA plus IL-12, IFNγ (1,000 units/ml; Biogen), lipopolysaccharide (LPS) (200 ng/ml, ultrapure from Escherichia coli O111:B4; InvivoGen), LPS plus IFNγ, IL-1β (100 ng/ml; PeproTech), phorbol myristate acetate (100 ng/ml) plus ionomycin (1 µM; Sigma Chemical), Staphylococcus aureus Cowan 1 (SAC) (0.01%; EMD Chemicals), muramyl dipeptide (10 µg/ml), palmitoyl-3-cysteine-serine-lysine-4 (Pam3CSK4) (1 µg/ml), heat-killed Listeria monocytogenes (108 cells/ml), poly(I-C) (25 µg/ml), flagellin (1 µg/ml), Pam2CGDPKHPKSF (fibroblast-stimulating lipopeptide 1; 0.1 µg/ml), imiquimod (1 µg/ml), single-stranded RNA40 (10 µg/ml), and oligodeoxynucleotide 2006 (5 µM; InvivoGen). Plates were incubated at 37°C with 5% CO2 for 48 hours, then centrifuged at 300g for 10 minutes. Harvested supernatants were analyzed for IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IFNγ, granulocyte–macrophage colony-stimulating factor, and TNFα by multiplex cytokine analysis (Pro-Inflammatory Tissue Culture 9-Plex) using a Sector Imager 6000 reader.
Statistical analysis
Levels of cytokines and other analytes in plasma and culture supernatants were compared to those in control subjects by Student’s unpaired t-test (Graph-Pad Prism). Data were log-transformed prior to analysis to satisfy homogeneity of variance requirements.
RESULTS
Mutational analysis
Patients 1, 3, and 4 had the previously reported heterozygous mutation c.688G > A in exon 10 leading to A230T (14). Patient 2 had the previously reported heterozygous mutation c.748G > C in exon 11 leading to E250Q (14). Patient 5 was heterozygous for c.748G > A leading to E250K, a novel mutation in exon 11.
Elevated plasma neutrophil enzyme and acute-phase reactant levels in PAPA syndrome
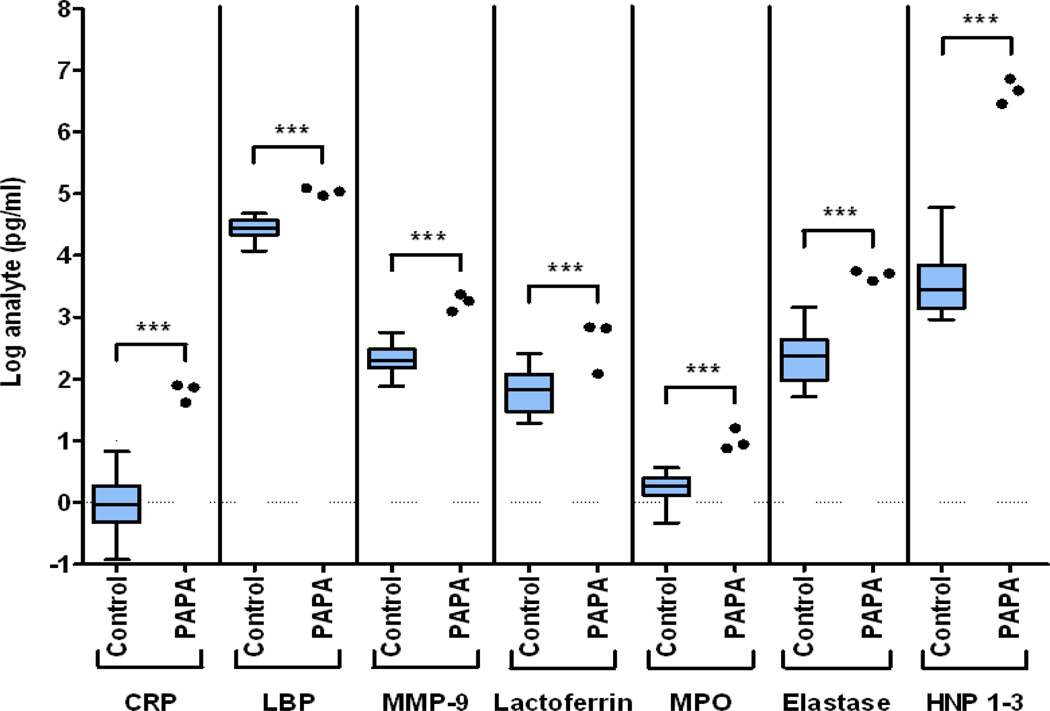
Histopathologically, PAPA syndrome is predominantly characterized by neutrophil dysfunction whether symptoms develop in the joints or the skin, but the syndrome typically occurs without systemic leukocytosis. Thus, we measured plasma levels of neutrophil enzymes from patients 1, 2, and 5. Levels of the neutrophil granule proteins myeloperoxidase, elastase, HNPs 1–3, and lactoferrin were significantly elevated (P < 0.005), regardless of whether the protein lodged in the primary or secondary neutrophil granules. Levels of the acute-phase reactants C-reactive protein (CRP) and lipopolysaccharide binding protein and of the enzyme matrix metalloproteinase 9 were also significantly elevated compared to those in controls (Figure 2).
Figure 2.
Plasma levels of neutrophil granule enzymes and acute phase reactants in patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) and control subjects. Data from 59 control subjects are shown as box plots. Each box represents the 25th and 75th percentiles, and lines inside the boxes represent the median. Whiskers represent the minimum and maximum values. Circles represent values in patients 1, 2, and 5. Samples were drawn once for each control subject and each patient. Levels of all analytes were significantly elevated in the patients. CRP = C-reactive protein; LBP = lipopolysaccharide binding protein; MMP-9 = matrix metalloproteinase 9 (neutrophil gelatinase); MPO = myeloperoxidase; HNP = human neutrophil α-defensins. *** = P < 0.005.
Plasma cytokine levels
In view of the elevated circulating levels of neutrophil granule proteins, we used the same samples to investigate circulating levels of cytokines that regulate neutrophil activation. Plasma concentrations of IL-1Β were marginally elevated in PAPA syndrome patients (P < 0.01), while levels of TNFα, IL-2, IL-10, IL-12, and IFNγ were not significantly different from those in controls.
Cytokine production in stimulated PBMCs
To attempt to understand cytokine dysregulation in PAPA syndrome, we measured cytokine levels in stimulated PBMCs. Cytokine levels were normal in both unstimulated and phorbol myristate acetate/ionomycin–stimulated PBMCs. However, in response to stimulation with IL-1β, LPS, PHA, poly(I-C), flagellin, and SAC, IL-10 production was diminished (for all P < 0.05). No agonist caused increased production of IL-1β or TNFα in the PBMCs of patients with PAPA syndrome compared to controls. Increased secretion of granulocyte–macrophage colony-stimulating factor was seen after stimulation with several Toll-like receptor and cytokine agonists including PAM3CSK4, heat-killed Listeria monocytogenes, LPS, IFNγ, LPS plus IFNγ, and SAC (for all P < 0.05).
Immunophenotyping
Serum immunoglobulin levels were normal in patients 1, 2, and 4; patient 3 had slightly low levels of IgA (55 mg/dl [normal 91–499]) and IgG (421 mg/dl [normal 642–1,730]). Levels of complement factors 3 and 4 were normal. Patient 5 had polyclonal IgA elevation (between 600 and 1,000 mg/dl) at age 10 years. Antinuclear antibody, anti–doublestranded DNA, anti–extractable nuclear antigen, anticardiolipin antibody, and rheumatoid factor were negative in all patients.
Patients 1, 3, and 4 had normal percentages of CD4+ cells (39.7%, 45.5%, and 54.2%, respectively [normal 29.0–57.3]), CD8+ cells (30.4%, 40.1%, and 29.8% [normal 25.2–50.8]), double-negative (CD3+CD4−CD8−) α/β T cell receptor–positive lymphocytes (1.0%, 0.5%, and 0.5% [normal 0.1–0.9]), and CD3+CD8+CD57+ cells (5.5%, 1.2%, and 1.8% [normal 0–15.8]). Absolute levels of CD4+ and CD8+ cells were decreased in patient 1 and increased in patient 3 (CD4+ 240/µl and 1,590/µl, respectively [normal 362–1,275]; CD8+ 184/µl and 1,401/µl [normal 344–911]). Patients 1, 3, 4, and 5 exhibited decreased absolute quantities of natural killer cells (CD16+CD3− or CD56+CD3−) (68/µl, 70/µl, 31/µl, and 16/µl, respectively [normal 87–505]). Patient 5 also had decreased levels of CD4+ cells (325/µl; 26.8%), B cells (23/µl [normal 88–330]; 1.4% [normal 4.8–15.9]), and markedly increased CD8+ lymphocyte levels (1,142/µl; 70.3%), CD3+/HLA–DR+ cells (903/µl [normal 0–291]; 55.6% [normal 0–15.1]), and CD3+CD8+CD57+ cells (344/µl [normal 0–239]; 38.2% [normal 0–15.8]), consistent with T cell large granular lymphocytosis. Lymphocyte phenotyping was not performed on patient 2.
DISCUSSION
PAPA syndrome is a rare autosomal-dominant disease caused by heterozygous mutations in PSTPIP1, and it is typically characterized by aseptic inflammation of the joints and skin. Of the 5 patients reported herein, 4 (including 3 probands) had previously described PAPA syndrome–associated mutations and were phenotypically similar to other reported patients. Their disease courses involved childhood arthritis provoked by minimal trauma, cystic acne, and PG. Patient 5 had a novel mutation, E250K, and presented with a distinct phenotype. Her childhood lymphadenopathy and splenomegaly were associated with hypergammaglobulinemia, hemolytic anemia, and thrombocytopenia, which regressed as her typical PAPA syndrome symptoms increased. The patient also had neutropenia and large granular lymphocytosis, which have not been previously reported in PAPA syndrome. It is currently unclear whether her entire phenotype can be directly ascribed to this novel E250K mutation. Previously reported patients with the E250Q mutation, affecting the same amino acid as E250K, had more classic PAPA syndrome presentations.
Genotype analysis of the family of patients 3 and 4 showed variable penetrance and expression, including family members without symptoms and others who had typical PAPA syndrome manifestations. PAPA syndrome with minimal skin involvement, such as psoriasis and rosacea (7), has been reported previously. This variable expressivity and incomplete penetrance suggest that the manifestations of PAPA syndrome may be broader than previously recognized, and therefore, the prevalence of this disease may be greater than previously thought.
The PAPA syndrome–causing mutations in PSTPIP1 all fall within the coiled-coil region of the molecule and disrupt PSTPIP-1–PEST phosphatase binding and regulation, resulting in hyperphosphorylation of the mutant protein and increased avidity for pyrin (12). This binding may release pyrin from its autoinhibition, triggering increased ASC oligomerization and caspase 1 activation (15), thereby resulting in overproduction of the activated proinflammatory cytokine IL-1β (2). The increased PSTPIP-1–pyrin avidity also results in altered PSTPIP-1 cellular distribution, with greater recruitment to the ASC inflammasome (12).
Although we found elevated levels of IL-1β and TNFα in patient plasma, stimulation of PBMCs did not cause up-regulation of these cytokines, contrary to previous reports (3,4,9). Interestingly, stimulation with IL-1β markedly impaired IL-10 induction. IL-10 is a critical regulator of inflammation, and defective signaling is associated with other inflammatory conditions, namely, severe inflammatory bowel disease (16). While colitis was noted in only 1 PAPA syndrome patient in our series, there were other prominent proinflammatory manifestations.
Plasma levels of CRP and HNPs 1–3 were 1–3 logs higher in PAPA syndrome patients than in normal controls; all other neutrophil granule products were significantly elevated as well. The degree of elevation did not correlate with the site of granule protein storage or synthesis, and therefore, likely reflects global neutrophil activation. We must still determine whether the degree of neutrophil granule protein elevation var ies with disease activity, as these markers might be useful surrogate measures for therapeutic response.
Medications directed at the antagonism of IL-1β and TNFα have been effective in the treatment of PAPA syndrome, despite the lack of in vitro abnormalities in cytokine levels upon PBMC stimulation. The most consistent responses have been to TNFα inhibitors; however, responses varied among our patients. Infliximab, which was previously reported to be successful (6), showed efficacy in 2 of the 3 patients who received it, but was discontinued in 1 due to an adverse reaction. Treatment with adalimumab was successful in the 2 patients who received it, as expected. Response to anakinra varied. Of the 4 patients who received anakinra, 2 patients had inadequate responses to the treatment, and 1 discontinued the therapy due to adverse effects. The fourth patient exhibited a good response to anakinra and has continued the treatment. Since PSTPIP-1 is involved in numerous leukocyte functions, some of the new immunosuppressive medications may also be effective in the treatment of PAPA syndrome.
Our series adds to the genetic, phenotypic, and immunologic characterization of this rare syndrome and expands its treatment possibilities. Whether the mechanisms that are involved in PAPA syndrome are relevant to other conditions involving neutrophilic inflammation, such as Sweet syndrome, Felty’s syndrome, or Behcet’s disease, remains to be determined. Given the rarity of PAPA syndrome, yet its broad variation in penetrance and expressivity, development of a screening test could be valuable for case finding and potentially for monitoring of therapy. The influence of plasma CRP and HNP 1–3 levels on disease activity warrants further investigation.
Acknowledgements
The content of this publicaton does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, and with funds from the National Cancer Institute, NIH, under contract HHSN-261200800001E. Dr. Demidowich was a Clinical Research Training Program fellow at the NIH. His fellowship was supported by a joint partnership between the Pfizer Foundation and the Foundation for the NIH.
REFERENCES
- 1.Yang H, Reinherz E. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatase (PTP)-PEST. J Immunol. 2006;176:5898–5907. doi: 10.4049/jimmunol.176.10.5898. [DOI] [PubMed] [Google Scholar]
- 2.Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum and acne: PAPA syndrome. Mayo Clin Proc. 1997;72:611–615. doi: 10.1016/S0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 4.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D’Urbano LE, et al. Abnormal production of the tumor necrosis factor α and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome. J Pediatr. 2004;145:851–855. doi: 10.1016/j.jpeds.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 2005;44:406–408. doi: 10.1093/rheumatology/keh479. [DOI] [PubMed] [Google Scholar]
- 6.Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma gangrenosum with infliximab in a patient with PAPA syndrome. Pediatr Dermatol. 2005;22:262–265. doi: 10.1111/j.1525-1470.2005.22320.x. [DOI] [PubMed] [Google Scholar]
- 7.Tallon B, Corkill M. Peculiarities of PAPA syndrome. Rheumatology (Oxford) 2006;45:1140–1143. doi: 10.1093/rheumatology/kei178. [DOI] [PubMed] [Google Scholar]
- 8.Renn CN, Helmer A, Megahed M. Hautarzt. Vol. 58. German: 2007. Pyogenic arthritis, pyoderma gangrenosum and acne syndrome (PAPA syndrome) pp. 383–384. [DOI] [PubMed] [Google Scholar]
- 9.Schellevis MA, Stoffels M, Hoppenreijs EP, Bodar E, Simon A, van der Meer JW. Variable expression and treatment of PAPA syndrome. Ann Rheum Dis. 2011;70:1168–1170. doi: 10.1136/ard.2009.126185. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol. 2009;161:1199–1201. doi: 10.1111/j.1365-2133.2009.09404.x. [DOI] [PubMed] [Google Scholar]
- 11.Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. J Clin Rheumatol. 2010;16:244–245. doi: 10.1097/RHU.0b013e3181e969b9. [DOI] [PubMed] [Google Scholar]
- 12.Waite AL, Schaner P, Richards N, Balci-Peynircioglu B, Masters SL, Brydges SD, et al. Pyrin modulates the intracellular distribution of PSTPIP1. PLoS One. 2009;4:e6147. doi: 10.1371/journal.pone.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Elmagd K, van Thiel DH, Jegasothy BV, Jacobs JC, Carroll P, Rodriquez-Rilo H, et al. Resolution of severe pyoderma gangrenosum in a patient with streaking leukocyte factor disease after treatment with tacrolimus (FK 506) Ann Intern Med. 1993;119:595–598. doi: 10.7326/0003-4819-119-7_part_1-199310010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP-PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 15.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]