Abstract
Early childhood stress is a risk factor for the development of substance-abuse disorders. A nonhuman primate model of early life stress, social impoverishment through nursery-rearing rather than mother-rearing, has been shown to produce increased impulsive and anxiety-like behaviors, cognitive and motor deficits, and increased alcohol consumption. These behavioral changes have been linked to changes in cerebrospinal fluid (CSF) levels of 5-hydroxyindoleacetic acid (5-HIAA), a serotonin (5-HT) metabolite. The effects of different rearing conditions on ethanol drinking and three measures of 5-HT function in the central nervous system were evaluated, including CSF 5-HIAA levels and tissue levels of 5-HT and 5-HIAA in brain samples. Brain samples were taken from the dorsal caudate, putamen, substantia nigra (SN) pars reticulata, SN pars compacta and hippocampus. There was a clear effect of rearing condition on the 5-HT system. Overall 5-HIAA and 5-HIAA/5-HT ratio measures of 5-HT turnover were significantly lower in nursery reared compared to mother-reared animals. In addition, there was a strong within-subject correlation between CSF and brain tissue 5-HIAA levels. Ethanol drinking was greater in nursery reared monkeys, consistent with previous results. These findings show that CSF 5-HIAA measurements can be used to predict brain 5-HT activity that may be involved in behavioral outcomes such as anxiety and alcohol consumption. Thus, CSF sampling may provide a minimally invasive test for neurochemical risk factors related to alcohol abuse.
Keywords: Serotonin, Metabolite, Stress, Tissue content, Putamen, Caudate, Substantia nigra, Hippocampus
Introduction
Childhood trauma consistently emerges as an important risk factor for developing affective disorders, substance-abuse disorders, and alcoholism (Bremner, Southwick, Johnson, Yehuda, & Charney, 1993; Widom, Ireland, & Glynn, 1995; for review see De Bellis, 2002; Gordon, 2002). Little is known, however, about whether there are biochemical markers that are associated with increased risk that might suggest prevention targets. Furthermore, there are few studies that have disentangled the contribution of early adversity to neurobiological and behavioral changes that might underlie increased risk of loss of control over alcohol and drug consumption.
Extended infancy and the complex interplay between social and affective processes are central to the strength of nonhuman primate models in alcohol research focused on investigation of the role of early experiences and stress as risk factors for excessive alcohol consumption. The disruption of the maternal–infant relationship, as well as various forms of social impoverishment during infancy, forms the basis of an experimental model for studying the outcome of early adverse experience in nonhuman primates (Harlow & Harlow, 1965; Harlow & Zimmermann, 1959; Seay & Harlow, 1965; see Hofer, 1996 for review). Nearly fifty years of work using this model has clearly shown the deleterious outcomes of separating monkeys from their mothers at birth and rearing them in varying degrees of social impoverishment (nursery-rearing). Relative to their mother-reared counterparts, nursery reared monkeys show alterations in an array of physiological and behavioral processes (Chamove, Rosenblum, & Harlow, 1973; Champoux et al., 2002; Gluck & Sackett, 1974; Lewis, Gluck, Beauchamp, Keresztury, & Mailman, 1990; Martin, Spicer, Lewis, Gluck, & Cork, 1991; Meyer & Bowman, 1972; Ruppenthal, Arling, Harlow, Sackett, & Suomi, 1976; Sanchez, Ladd, & Plotsky, 2001; Suomi & Ripp, 1983).
Early life stress, including altered rearing conditions, appear to be an important factor in shaping the development of the serotonin (5-HT) system in the central nervous system (CNS). Previous studies have demonstrated that monkeys reared in a nursery with age-matched peers exhibit low cerebrospinal fluid (CSF) 5-hydroxyindolacetic acid (5-HIAA) concentrations from late infancy into adolescence and continuing into adulthood, compared to mother-reared subjects (Higley et al., 1993, Higley, Suomi, & Linnoila 1996a, 1996b; Mehlman, Higley, & Faucher, 1994; but see Clarke et al., 1996).
Early life stress appears to play a role in the later development of alcohol use disorders. Alcohol dependence and abuse are generally considered to be a result of environmental and genetic factors (Shannon et al., 2005). Stressful environmental factors create emotional and physiological changes in animal and human behavior (Higley, Suomi, & Linnoila, 1992). As compared to mother-reared monkeys, peer-reared monkeys show an increase in anxiety-related behaviors (including clinging to one another, decreases in playing, and increases in self-directed behaviors) which correspond to increased plasma corticotrophin, cortisol, and altered CSF 5-HIAA (Clarke, Kraemer, & Kupfer, 1998; Higley, Hasert, Suomi, & Linnoila, 1991). CSF 5-HIAA levels in mother- and peer-reared animals have been shown to be inversely correlated with alcohol preference in a drinking study (Vivian, Higley, Linnoila, & Woods, 1999). Thus, this model provides a way to test the effects of early life stress on individual responsiveness to stress and stress-related alcohol consumption (Higley et al., 1991).
While some animal and human studies have shown a correlation between the initiation and continuation of drinking and exposure to stress, and other studies have linked stress to relapses in drinking (Heilig & Koob, 2007; Higley et al., 1993, 1996a, 2011; Shannon et al., 2005), the neurochemical and neurobiological mechanisms behind stress leading to drinking problems remains unknown, though the serotonin system has been implicated in behavioral and genetic studies (Heinz, Beck, Meyer-Lindenberg, Sterzer, & Heinz, 2011; Higley et al., 1996b; Roy, Hu, Janal, & Goldman, 2007). Stress affects numerous neurotransmitter and neuromodulator systems in the brain, especially monoamines, amino acids, neurosteroids, and neurotrophic factors. The response to an environmental stressor activates the hypothalamic-pituitary-adrenocortical (HPA) axis, a part of the endocrine system responsible for stress responses, which is composed of direct and indirect feedback from connections between the hypothalamus, pituitary gland and adrenal cortex. Activation of the HPA axis leads to changes in a variety of neurochemical systems including dopamine (DA), norepinephrine (NE), 5-HT, corticotrophin releasing factor, and corticosterone.
To our knowledge, this is the first study to quantify 5-HIAA and 5-HT levels in brain tissue from mother-reared and peer-reared monkeys. The goal of this study was to assess changes in brain tissue 5-HT and metabolite content to compare with CSF 5-HIAA levels in order to validate CSF samples as accurate correlates of brain 5-HT system activity that can be obtained in a minimally invasive manner. These results expand on the body of work implicating the 5-HT system as playing a critical role in the development of alcohol related disorders.
Materials and methods
Animals
Male rhesus monkeys were born at the Laboratory of Comparative Ethology at the National Institute of Child Health and Human Development and transferred to Wake Forest University School of Medicine at roughly three years of age. The animals were initially socially-housed and then moved to individual housing (76 × 60 × 70 cm3) approximately 1.5 years prior to this study to participate in a long-term voluntary ethanol drinking study. The home cage for each animal was equipped with an operant panel that supplied food, water, and ethanol for each animal. Monkeys were trained to operate the drinking panel to self-administer water or ethanol (4% w/v in water, see Grant et al., 2008 for more details). During the final 12 months of the experiment, animals were allowed free access to water and ethanol for 22 h/day. Monkeys were fed a diet of Primate Food pellets (Research Diets Inc., New Brunswick, N.J.) and fresh fruit. Water was available ad libitum. Experiments were performed when the monkeys were six years of age. This study was conducted in accordance with the Guidelines of the Committee on the Care and Use of Laboratory Animals (NRC, 1996) and approved by the Wake Forest University Animal Care and Use Committee. Upon completion of the drinking study, necropsies were performed and samples collected for the appropriate assays.
Rearing
Mother-reared animals (n = 9; MR) were reared by adult females (in a social group comprised of mother-infant dyads) as well as two adult males. Mother-reared subjects lived from birth to approximately seven months of age in social groups housed in indoor–outdoor cages. Over the course of the first seven months the diet of the mother-reared subjects consisted of breast milk and weaning to chow and water.
Nursery reared animals (n = 9; NR) were separated from their mothers within 24-hr of birth, moved to a neonatal nursery, and reared under surrogate-peer-reared conditions using procedures based on those developed at the University of Wisconsin Harlow Primate Laboratory. Briefly, infants were housed in an incubator containing an inanimate surrogate equipped with a spring to allow rocking motion, covered with a heating pad, and encased in thick fleece. Infants were fed formula (50:50 Similac:Primilac) and weaned to solid foods and water over the course of 6 months. At 14 days infants were moved to one quadrant of a cage within a room of the nursery and within visual, auditory, and olfactory contact with other monkeys. At approximately 37 days of age, daily 1 h socialization periods were given in which four infants were united in a play cage. Animals remained in these social groupings until the time of the ethanol consumption study when they were individually housed.
Necropsy
Necropsies of adult animals (6 years of age) took place at 9 am. The animals were anesthetized with ketamine (10 mg/kg, i.m.) and maintained at a deep surgical plane (determined by lack of reflexes including palpebral, coreneal, and pain withdrawal) of anesthesia with pentobarbital (15–20 mg/kg, 15–20 min following ketamine administration). CSF samples were collected from the foramen magnum following the administration of ketamine, but prior to the administration of pentoparbital. Animals were under anesthesia for approximately 1 h prior to tissue collection. The monkeys were transcardially perfused with cold oxygenated Ringer’s solution (125 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM D-glucose) for 2 min. Nuclei of interest (caudate, putamen, substania nigra pars compacta (SNpc), substantia nigra pars reticulata (SNpr) and hippocampus) were microdissected by hand (10–50 mg/sample) and flash frozen in liquid nitrogen. Tissue was stored at −80 °C until the time of analysis. Following this drinking study, other regions of interest were used for different analyses – mRNA, protein levels of several neurotransmitters, receptors and subunits.
Neurotransmitter analysis
Brain samples (10–30 mg/sample wet weight) were homogenized in 250 μL of 0.1 M HClO4 and analyzed for protein concentration by the BCA method (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Inc., Rockford, IL). Extracts were centrifuged and the supernatants removed and analyzed for 5-HT and its metabolite 5-HIAA using high performance liquid chromatography (HPLC) coupled to electrochemical detection at +220 mV (ESA Inc., Chelmsford, MA). Neurotransmitters and their metabolites were separated on a Luna 100 × 3.0 mm C18 3 μm HPLC column (Phenomenex, Torrance, CA). The mobile phase consisted of 50 mM citric acid, 90 mM sodium dihydrogen phosphate, 1.7–2.0 mM 1-octanesulfonic acid, 50 μM ethylenediaminetetracetic acid, 10–12% acetonitrile and 0.3% triethylamine in a volume of 1 L (pH 3.0). Analytes were quantified using PowerChrom software (eDAQ Inc, Colorado Spring, CO) and neurotransmitter and metabolite concentrations measured using a calibration curve.
Chemicals and drugs
Components of the mobile phase and neurotransmitter standards were of HPLC grade or the highest quality obtainable from Sigma–Aldrich (St. Louis, Missouri).
Statistical analyses
One-way ANOVA with Tukey’s post-hoc analyses and one-tailed t-tests were used to assess differences due to rearing condition. A Spearman’s correlation analysis was used to determine the relationship between CSF and tissue 5-HIAA levels.
Results
Tissue levels of 5-HIAA were measured to determine any differences between rearing conditions. No significant differences were found when comparing tissue content measures of 5-HT and 5-HIAA ethanol drinking and control (naïve) subjects within rearing condition (Table 1, 5-HIAA:F(3,78)2.271, p = 0.0868; 5-HT:F(3,73)0.1149, p = 0.9511). Therefore, groups were collapsed to examine rearing condition alone for subsequent analyses. Levels of 5-HIAA were averaged by treatment, across brain regions (Fig. 1A) and then by individual brain region (Fig. 1B). 5-HIAA levels were significantly lower in nursery reared compared to mother-reared animals (Fig. 1A, t = 2.453, p < 0.01). Subsequent regional analysis (Fig. 1B, F(8,32)=10.58, p < 0.0001) revealed significantly lower 5-HIAA concentrations in the putamen of nursery reared animals compared to mother-reared animals (t = 2.165, P = 0.0229), but no significant differences in the caudate, substantia nigra or hippocampus. Analysis of ethanol intake (Table 1) indicates significantly greater ethanol intake in NR subjects as compared to MR subjects. The average daily intake in the MR group was 2.18 ± 0.03 g/kg and the NR group intake was 2.87 ± 0.03 g/kg, p < 0.05. This difference translates to an average daily intake of 8–9 drinks per day in the MR group and 11–12 drinks per day in the NR group.
Table 1.
Early life stress produces serotonin system alterations that are not influenced by a history of ethanol self-administration.
| MR – Naïve | MR – EtOH | NR – Naïve | NR – EtOH | |
|---|---|---|---|---|
| (Tissue content in mean ± SEM in ng/mg wet weight) | ||||
| 5-HIAA | 110.4 ± 13.37 | 143.6 ± 18.93 | 86.04 ± 11.45 | 103.5 ± 13.24 |
| 5-HT | 55.35 ± 7.055 | 60.21 ± 8.905 | 54.05 ± 9.388 | 58.94 ± 8.993 |
| (Ethanol intake in avg daily g/kg) | ||||
| Ethanol intake | 2.18 ± 0.03 | 2.87 ± 0.03* | ||
No significant differences in monoamine tissue levels were found as a result of ethanol treatment condition in MR and NR animals, p > 0.05 for all comparisons. Drinking data (average daily g/kg, * represents p < 0.05) are presented for the ethanol drinking groups. Data were collapsed within rearing condition for all subsequent analyses.
Fig. 1.
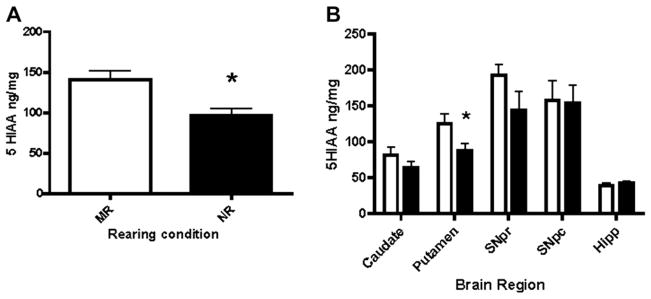
A. Nursery reared animals have lower overall brain tissue levels of 5-hydroxyindoleacetic acid (5-HIAA) than mother-reared subjects. Animals that were subjected to maternal separation in the nursery-rearing condition exhibited significantly lower tissue concentrations of 5-HIAA (P = 0.0041). B. Regional analysis (SNpr = substantia nigra pars reticulate, SNpc = substantia nigra pars compacta, Hipp = hippocampus) indicates a trend toward lower 5-HIAA concentrations in striatal regions, with a significant difference in the putamen (p = 0.0184). Open bars represent MR and closed bars represent NR animals.
Similarly, tissue concentrations of 5-HT were measured in samples taken from animals raised under both nursery reared and mother-reared conditions. There were no significant differences in 5-HT concentrations, averaged across brain regions, between nursery and mother-reared animals (Fig. 2A, F1,71 = 0.04117, P = 0.8398). Regional data revealed a trend toward greater 5-HT in hippocampal tissue in NR animals as compared to MR animals (Fig. 2).
Fig. 2.
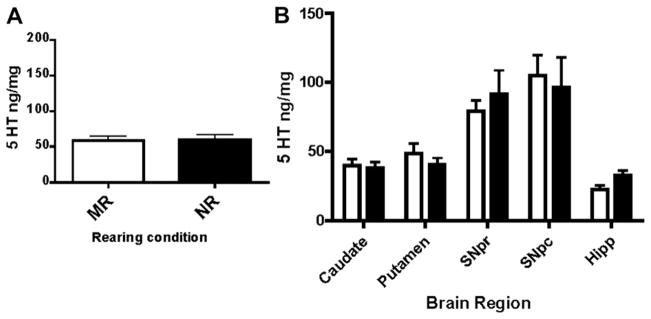
Tissue levels of serotonin (5-HT) are not different in mother-reared (MR) and peer-reared subjects (NR). A. Analysis of tissue concentrations of 5-HT revealed no overall differences between mother-reared and nursery reared animals. B. Regional analysis (SNpr = Substantia nigra pars reticulata, SNpc = Substantia nigra pars compacta, Hipp = hippocampus) indicates a weak trend toward lower 5-HT levels in nursery reared animals. Open bars represent MR and closed bars represent NR animals.
To assess potential alterations in 5-HT system activity, 5-HIAA/5-HT turnover ratios were calculated and evaluated as a function of rearing condition. Results showed a reduction in averaged 5-HT turnover in peer-reared animals (NR, Fig. 3A, F1,56 = 5.311, P = 0.0029). Analysis of individual regions revealed a significant reduction in 5-HT turnover within the hippocampus of nursery reared monkeys (Fig. 3B, P = 0.0406). Although the findings were not significant in regions other than the hippocampus, all regions in NR reared animals exhibited a downward trend in 5-HT turnover.
Fig. 3.
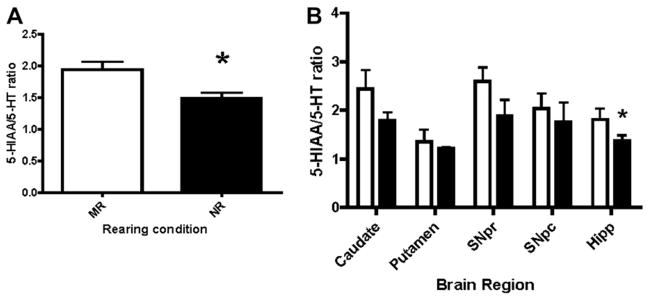
A. Activity of the 5-HT system (5-HIAA/5-HT ratio) is reduced in nursery reared animals. Nursery reared animals have lower turnover of the 5-HT system as compared to mother reared animals (P = 0.0029). B. A more detailed analysis of regions (SNpr = Substantia nigra pars reticulata, SNpc = Substantia nigra pars compacta, Hipp = Hippocampus) indicates that only the hippocampal turnover is significantly altered (P = 0.0406), though each region trends toward lower activity in nursery reared animals. Open bars represent MR and closed bars represent NR animals.
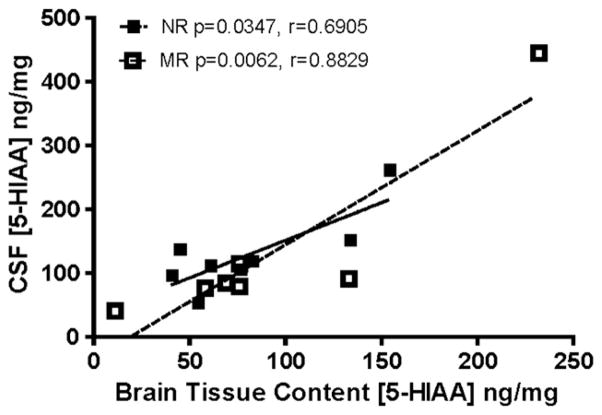
Given the reduction in CSF 5-HIAA found in previous studies (Bennett et al., 2002; Higley et al., 1992; Kraemer, Ebert, Schmidt, & McKinney, 1989; Shannon et al., 2005), CSF 5-HIAA concentrations and tissue levels of 5-HIAA for all of the animals for which we had matched data (two CSF samples were unavailable) were analyzed for a correlative relationship. We used 7 mother-reared and 8 nursery reared animals in this analysis. There was no significant difference in averaged CSF 5-HIAA levels between mother and nursery-reared animals with such a small sample size, however tissue content data averaged across brain regions revealed a positive correlation between 5-HIAA concentrations in tissue and CSF in a comparison using paired data of CSF and tissue content samples (not shown, Spearman’s two-tailed correlation r(13) = 0.6613, P = 0.0036). Separate correlation analyses (Fig. 4, NR, r = 0.6905, p = 0.0347; MR, r = 0.8829, P = 0.0062) for each rearing condition showed significant within-animal correlations between CSF 5-HIAA and brain tissue content 5-HIAA, but the correlations were not significantly different from each other based on rearing condition.
Fig. 4.
Both NR (closed squares, solid line) and MR (open squares, dashed line) groups yielded significant correlations between 5-HIAA concentrations in cerebrospinal fluid (CSF) and 5-HIAA concentrations in brain tissue when analyzed separately. Solid lines are linear regressions of the data points by rearing condition. There was also an overall correlation across all subjects (r = 0.6613, p = 0.0036). N = 15 within-subject paired observations, subjects lacking paired CSF and tissue content samples were excluded (one per treatment).
Discussion
To our knowledge, these data provide the first study to directly assess differences in brain tissue 5-HIAA concentrations between nursery- and mother-reared monkeys. Our tissue content findings are consistent with previous in vivo studies that have demonstrated decreased CSF 5-HIAA concentrations in NR monkeys compared to their MR counterparts (Higley et al., 1992; Shannon et al., 2005 but see Bennett et al., 2002; Kraemer et al., 1989). These data not only provide further evidence that alterations in the serotonergic system follow early life stress, but also demonstrate that these effects are long-lasting, with consequences that extend into adulthood. In fact, the nursery reared animals that were exposed to an ethanol drinking paradigm, drank significantly more than the mother-reared group, a finding that parallels the decreased 5-HIAA measures in nursery reared animals. Furthermore, this study provides conclusive evidence that there is a strong correlation between 5-HIAA levels in the CSF and in the brain.
Early life stress and trauma have been shown to produce many physiological and behavioral abnormalities (Clarke et al., 1998; Vivian et al., 1999), including excessive alcohol consumption (>1.4 g of alcohol per kg of body weight) (Falkhe et al., 2000; Higley et al., 1991). In addition to the increased ethanol drinking phenotype of NR monkeys, these animals have been shown to exhibit deficits in affective and motor behaviors which may be related to the function of the basal ganglia and associated structures (Champoux et al., 2002). The five brain regions used in these studies, the caudate, putamen, SNpr, SNpc and hippocampus, were chosen primarily based on 5-HT innervation as well as tissue availability (Parent, Wallman, Gagnon, & Parent, 2011). These animals, whose brain tissue and CSF were used, were a part of behavioral studies of alcohol drinking, anxiety-like and impulsivity-related behaviors, and numerous receptor binding and electrophysiological studies (manuscripts in preparation). Therefore, certain brain regions, including the brainstem raphe nuclei where many 5-HT cell bodies are located, were unavailable for these studies. Nevertheless, as previously noted, there were significant overall decreases in 5-HIAA in NR compared to MR animals in an overall brain tissue content comparison. A more detailed examination of the individual brain regions indicated that the putamen showed the largest and only significant difference in 5-HIAA, with caudate and SNpr showing trends toward decreased levels. Regional differences are not surprising given the functional differences between these brain areas. Animals from the same model of early life stress have also been shown to have decreased 5-HT transporter binding in several areas, including putamen, in NR monkeys, further suggesting a functional change in the serotonin system following early life stress (Ichise et al., 2002).
Although rearing condition had no overall effect on tissue content levels of 5-HT in these brain regions, it does appear that there may be a trend toward an increase in 5-HT in the hippocampus of nursery reared animals. Previously, it has been noted that alterations in 5-HT activity leads to reciprocal changes in tissue content of metabolites and monoamines, wherein decreased activity results in increased monoamine levels, presumably due to reduced utilization of neurotransmitter stores, and decreased metabolite levels, presumably due to reduced release of the neurotransmitter (Sharp, Zetterström, & Ungerstedt,1986). Analysis of metabolite:monoamine ratios is a widely used measure of neurotransmitter turnover and overall activity of a system. Thus a trend toward increased hippocampal 5-HT may be consistent with reduced 5-HT synaptic signaling in NR animals. Such a mechanism has been suggested in the hippocampus, where serotonergic responses to chronic stress are linked to dendritic atrophy, which is associated with animal models of depression (Müller, Wegener, & Elfving, 2011).
Reuptake of 5-HT through the 5-HT transporter (SERT) is the primary method of clearance from the synapse; metabolism occurs in the cytosol of the presynaptic terminals. The first step in 5-HT metabolism is deamination by monoamine oxidase (MAO), followed by metabolism by aldehyde dehydrogenase, to create the metabolite 5-HIAA. There are two varieties of monoamine oxidase. MAO-A has the highest affinity for the monoamine transmitters and is found in catecholinergic neurons. MAO-B is concentrated in serotonergic and histaminergic neurons and glia (Cooper, Bloom, & Roth, 2003). Thus, some metabolism of 5-HT occurs within the serotonergic terminal following release and reuptake by SERT, but 5-HT release and diffusion may result in metabolism within glia by MAO-B. 5-HIAA is the end product of the MAO route of 5-HT metabolism and is ultimately excreted as a waste product. The measurement of 5-HIAA:5-HT ratios gives a good indication of the overall activity of the 5-HT system.
The current results indicate that CSF 5-HIAA concentrations can be used to predict brain tissue levels of 5-HIAA, shown by a significant positive correlation between these two measures. These findings are the first definitive evidence that CSF 5-HIAA concentrations in nonhuman primates are correlated with serotonergic brain function and bolster the historical argument that CSF monoamines are related to brain function. CSF sampling is a routine procedure in many laboratories that can be repeatedly performed in the same animals over time. This measurement can be extrapolated to understanding general alterations in serotonergic brain activity without the need for terminal studies. Using such a measure is expected to be particularly useful in further elucidating the impact of early life stress (as modeled by rearing condition) on adulthood patterns of drinking or changes in overall consumption and susceptibility to increased alcohol intake. Thus, data reported here demonstrate that CSF 5-HIAA sampling can be used as a marker of brain 5-HT activity that may be predictive of stress-related risk factors for alcohol abuse and alcoholism. Changes in the serotonin system were persistent, regardless of exposure to ethanol, suggesting that rearing condition-related alterations in the 5-HT system are not changed by adulthood ethanol consumption. The long-lasting effects of differential early rearing experiences on aspects of neurochemistry in monkeys underscores the parallel between this animal model and observations in human populations where early childhood adversity increases lifelong risk of deleterious health outcomes, including alcoholism and other neuropsychiatric disorders.
Acknowledgments
This study was supported by NIAAA grants U01AA014091 (SRJ), P01AA17506 (SRJ, AJB, DPF) and T32AA007565 (KNH). We thank Jim Daunais, April Davenport and Joanne Konstantopoulos for expert technical assistance.
References
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Rosenblum LA, Harlow HF. Monkeys (Macaca mulatta) raised only with peers. A pilot study. Animal Behaviour. 1973;21:316–325. doi: 10.1016/s0003-3472(73)80073-9. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett AJ, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Hedeker DR, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. Rearing experience and biogenic amine activity in infant rhesus monkeys. Biological Psychiatry. 1996;40:338–352. doi: 10.1016/0006-3223(95)00663-X. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Kraemer GW, Kupfer DJ. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Research. 1998;79:91–104. doi: 10.1016/s0165-1781(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. 8. Oxford University Press; 2003. [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributaory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- Falkhe C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism: Clinical & Experimental Research. 2000;24:644–650. [PubMed] [Google Scholar]
- Gluck JP, Sackett GP. Frustration and self-aggression in social isolate rhesus monkeys. Journal of Abnormal Psychology. 1974;83:331–334. doi: 10.1037/h0036584. [DOI] [PubMed] [Google Scholar]
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology. 2002;27:115–126. doi: 10.1016/s0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcoholism: Clinical & Experimental Research. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The effect of rearing conditions on behavior. International Journal of Psychiatry. 1965;1:43–51. [PubMed] [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotrophin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nature Reviews Neuroscience. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1: low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcoholism: Clinical & Experimental Research. 1996a;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism? Part 2: diminished social cpmetence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism: Clinical & Experimental Research. 1996b;20:643–650. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Thompson WT, Champoux M, Goldmand D, Hasert MF, Kraemer GW, et al. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta) Archives of General Psychiatry. 1993;50:615–623. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- Hofer MA. On the nature and consequences of early loss. Psychosomatic Medicine. 1996;58:570–581. doi: 10.1097/00006842-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, et al. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience. 2002;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of difference social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Gluck JP, Beauchamp AJ, Keresztury MF, Mailman RB. Long-term effects of early social isolation in Macaca mulatta: changes in dopamine receptor function following apomorphine challenge. Brain Research. 1990;513:67–73. doi: 10.1016/0006-8993(90)91089-y. [DOI] [PubMed] [Google Scholar]
- Müller HK, Wegener G, Elfving B. Chronic restrain stress affects serotonin transporter uptake kinetics but not binding sites in the rat hippocampus. Synapse. 2011 doi: 10.1002/syn.20986. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. Journal of Neuroscience. 1991;11:3344–3358. doi: 10.1523/JNEUROSCI.11-11-03344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. American Journal of Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Bowman RE. Rearing experience, stress and adrenocorticosteroids n the rhesus monkey. Physiology & Behavior. 1972;8:339–343. doi: 10.1016/0031-9384(72)90382-4. [DOI] [PubMed] [Google Scholar]
- Parent M, Wallman MJ, Gagnon D, Parent A. Serotonin innervations of basal ganglia in monkeys and humans. Journal of Chemical Neuroanatomy. 2011;41:256–265. doi: 10.1016/j.jchemneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, Suomi SJ. A 10-year perspective of motherless-mother monkey behavior. Journal of Abnormal Psychology. 1976;85:341–349. doi: 10.1037//0021-843x.85.4.341. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Seay B, Harlow HF. Maternal separation in the rhesus monkey. Journal of Nervous & Mental Disease. 1965;140:434–441. doi: 10.1097/00005053-196506000-00006. [DOI] [PubMed] [Google Scholar]
- Shannon C, Schwandt JL, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, et al. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. American Journal of Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterström T, Ungerstedt U. An in vivo study of dopamine release and metabolism in rat brain regions using intracerebral dialysis. Journal of Neurochemistry. 1986;47:113–122. doi: 10.1111/j.1471-4159.1986.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Ripp C. A history of motherless mother monkey mothering at the University of Wisconsin Primate Laboratory. In: Reite M, Caine N, editors. Child abuse: The nonhuman primate data. New York: Alan R. Liss; 1983. pp. 49–78. [Google Scholar]
- Vivian JA, Higley JD, Linnoila M, Woods JH. Oral ethanol self-administration in rhesus monkeys: behavioral and neurochemical correlates. Alcoholism: Clinical & Experimental Research. 1999;23:1352–1361. [PubMed] [Google Scholar]
- Widom CS, Ireland T, Glynn PJ. Alcohol abuse in abused and neglected children followed-up: are they at increased risk? Journal of Studies on Alcohol. 1995;56:207–217. doi: 10.15288/jsa.1995.56.207. [DOI] [PubMed] [Google Scholar]