Abstract
All laboratory animals shall be provided some form of environmental enrichment (EE) in the nearest future (Directive 2010/63/EU). Displacing standard housing with EE entails the possibility that data obtained under traditional housing may be reconsidered. Specifically, while EE often contrasts the abnormalities of consolidated disease models, it also indirectly demonstrates that their validity depends on housing conditions. We mimicked a situation in which the consequences of a novel pharmacological compound were addressed before and after the adoption of the Directive. We sub-chronically exposed standard- or EE-reared adolescent CD1 mice (postnatal days 23-33) to the synthetic compound JWH-018, and evaluated its short- and long-term potential cannabinoid properties on: weight gain, locomotion, analgesia, motor coordination, body temperature, brain metabolism (1H MRI/MRS), anxiety- and depressive-related behaviours. While several parameters are modulated by JWH-018 independently of housing, other effects are environmentally mediated. The transition from standard housing to EE shall be carefully monitored.
“All animals shall be provided with space of sufficient complexity […]. They shall be given a degree of control and choice over their environment […]. Establishments shall have appropriate enrichment techniques in place, to extend the range of activities […] including physical exercise, foraging, manipulative and cognitive activities, as appropriate to the species. Environmental enrichment in animal enclosures shall be adapted to the species and individual needs of the animals concerned” (Directive 2010/63/EU, hereafter Directive, Annex III). This is the section regarding the provision of environmental enrichment to laboratory animals as reported in the Directive. Notwithstanding some variation in the specific timing of its adoption by Member States, this requirement entails that each facility operating within the EU shall guarantee a sufficient degree of environmental enrichment (EE) to laboratory animals. The Directive represents a welcome advancement in fundamental research as it will beget substantial improvements in the quality of life of experimental subjects1. Yet, a widespread adoption of EE triggers a series of considerations that warrant systematic examination. From a theoretical and methodological perspective, the variation in housing conditions will most likely alter baseline levels of relevant parameters ranging from behavioural2, neuroendocrine3, neurochemical4, and immune5 outcomes, to name a few. The fact that significant variations in housing conditions will modify the baseline levels across a wide range of domains rests upon a large body of experimental evidence demonstrating that substantial6, minor7, or even theoretically-absent8 variations in housing conditions result in large phenotypic differences (see9 for a discussion). Beside these observations, the simple consideration that a given phenotype is the result of a constant interaction between a genotype and its environment10, shall allow the assumption that baseline data will be modified by such radical variation in rearing standards.
Although EE has been shown not to affect the internal reproducibility of experimental findings2, robust experimental evidence indicate that it may remarkably influence the effects of genetic manipulations11, drug treatments12 and brain lesions13 on behavioural14, cognitive15, endocrine, and neurobiological16 parameters. Ultimately, EE has been demonstrated capable of influencing gene expression17 and neuronal plasticity18. Traditionally13, EE has been considered a tool capable of mitigating or contrasting the aberrant phenotype exhibited by disease models (see19 for a discussion). This theoretical approach fostered a multitude of studies demonstrating that the pathological phenotype exhibited by animal disease models under traditional housing was largely mitigated by EE. For example, EE has been shown to “normalize” the phenotype exhibited by animal models of Parkinson's, Alzheimer's, Huntington's disease, and drug addiction19. Importantly, these models have been validated under classical standard conditions. Under a scenario in which “classical” EE strategies shall become the novel standard, the validity of the aforementioned disease models would fail to pass experimental testing. Specifically, in the absence of phenotypic differences between EE disease models and EE controls, the most conservative conclusion would be that a given disease-provoking treatment does not induce significant variations compared to controls. Thus, not only does EE adjust individual phenotype, but also it may skew the conclusions drawn from experiments conducted in animal models consolidated under standard conditions. Paradoxically, a valid model of Parkinson's disease, developed in 200313 may no longer be considered as such in 2013. Ultimately, the transition from traditional to novel standards (EE) may also alter the conclusions drawn from classical reports.
While these considerations pertain to a vast number of studies, we decided to systematically address them within the field of behavioural pharmacology. Specifically, pharmacological research largely rests upon preclinical studies investigating the consequences of novel compounds. Such consequences may range from drug safety and efficacy to potential psychotropic activity of unknown substances. With respect to the latter, JWH-018 has recently emerged as a potential drug of abuse due to its in vitro cannabimimetic properties20. Specifically, JWH-018 has been detected in commercial products, officially merchandised as incense, and often mixed in joints with recreational purposes21. JWH-018 belongs to the category of those psychoactive substances originally known as “smart drugs”, and now referred to as “legal highs”. Notwithstanding the aforementioned in vitro studies, only few reports addressed whether this substance has in vivo cannabimimetic properties22,23.
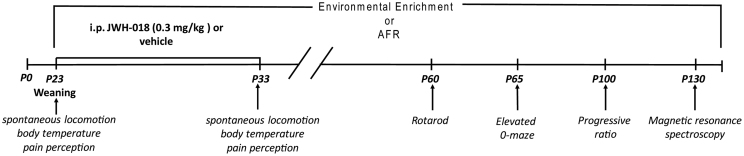
Since this substance has been as yet poorly investigated, it appears particularly suited to mimic a scenario in which the psychotropic potential of a relatively unknown compound can be addressed before and after the adoption of the Directive. This study thus intends to highlight the potential implications of the transition from current housing standards (shoebox-sized cages provided with sawdust, food and water) to EE (i.e. EE cages provided with shelters, toys and a running wheel). To this aim, we decided to investigate the cannabimimetic effects of JWH-018 under two different environmental conditions: traditional animal facility rearing (AFR) and EE, which may represent one of the standards to be used upon the official adoption of the Directive by EU Member States. Under both conditions were subjects housed in groups (see figure 1 for a general timeline of the study).
Figure 1. Experimental timeline (see Methods for details).
We investigated the short- and long-term consequences of a repeated administration of JWH-018 during adolescence on the following domains: general locomotion, body temperature, analgesic response, motor coordination, anxiety, and anhedonia. These domains have been selected as they either constitute the core aspects affected by the administration of cannabimimetic drugs24, or have been shown to be sensitive to synthetic cannabinoid agonists in our previous experiments22,25.
Furthermore, to evaluate the long-term consequences of JWH-018 on the functional state of brain areas sensitive to adolescent cannabinoid administration26, we conducted a 1H magnetic resonance imaging (MRI) guided spectroscopy (MRS) examination in adult mice. MRS constitutes a powerful, non-invasive tool for monitoring neurobiological adjustments at both clinical and preclinical levels27,28. We therefore performed a quantitative 1H MRS examination in adult mice in hippocampus and prefrontal cortex, as these areas exhibit an elevated expression of CB1 receptors29 and are involved in individual responses to the administration and consumption of cannabinoids30. For the analysis of brain metabolites, compared to creatine-based ratio measurements, we favoured the use of a quantitative approach31 as the former may be sensitive to between group differences in creatine. The latter have been observed in studies entailing the administration of psychoactive compounds28.
Results
Body weight
In line with literature observations, EE resulted in a remarkable reduction in body weight across development compared to AFR (housing: F(1,33) = 14.6; p = 0.001). Such difference was not modified by JWH-018 administration (drug: F(1,33) = 0.1; p = 0.758).
Body temperature
Predictably, acute JWH-018 administration on P23 resulted in a significant reduction in body temperature (drug: F(1,24) = 27.8; p = 0.001); specifically, body temperature significantly dropped in JWH-treated subjects 60 minutes after the injection (drug × time: F(2,24) = 13.1; p = 0.001, see fig. 2a). Repeated JWH-018 administration also resulted in a significant reduction in body temperature 60 minutes after the injection (drug × time: F(2,62) = 16.3; p = 0.001), indicating that mice did not show a tolerance profile in response to repeated administrations.
Figure 2.
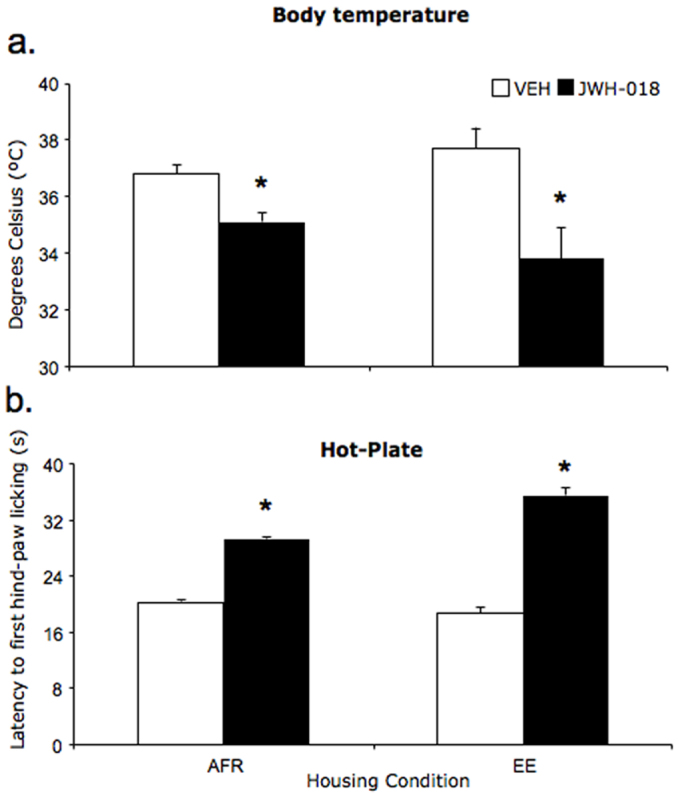
(a) Body temperature (°C, mean ± SEM) in adult mice 60 minutes after a single injection of vehicle VEH or JWH-018 (0.3 mg/kg). *p < 0.05 significantly different from baseline conditions; (b) pain perception and drug effects measured as the latency (mean ± SEM) to the first hind-paw licking on the first day of drug administration in the hot-plate test. *p < 0.05 significantly different from VEH in post-hoc tests. (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018).
Hot-plate
As expected, acute administration of JWH-018 on P23 resulted in an analgesic profile: specifically, JWH-018 treated subjects showed increased hind-paw licking latency compared to VEH-treated subjects (drug: F(1,32) = 14.1; p = 0.001). The effects of JWH-018 were similar in AFR and EE subjects (housing × drug: F(1,32) = 1.2; p = 0.270, see fig. 2b). Fore-paw licking latency on P23 was not modified by JWH-018 administration (drug: F(1,32) = 2.3; p = 0.140). Repeated administration of JWH-018 resulted in a habituation profile, whereby on P33, drug- and VEH-treated subjects failed to show any significant difference with respect to both hind-paw (F(1,31) = 0.1; p = 0.921) and fore-paw (F(1,31) = 2.3; p = 0.139) licking latency (data not shown).
General locomotion
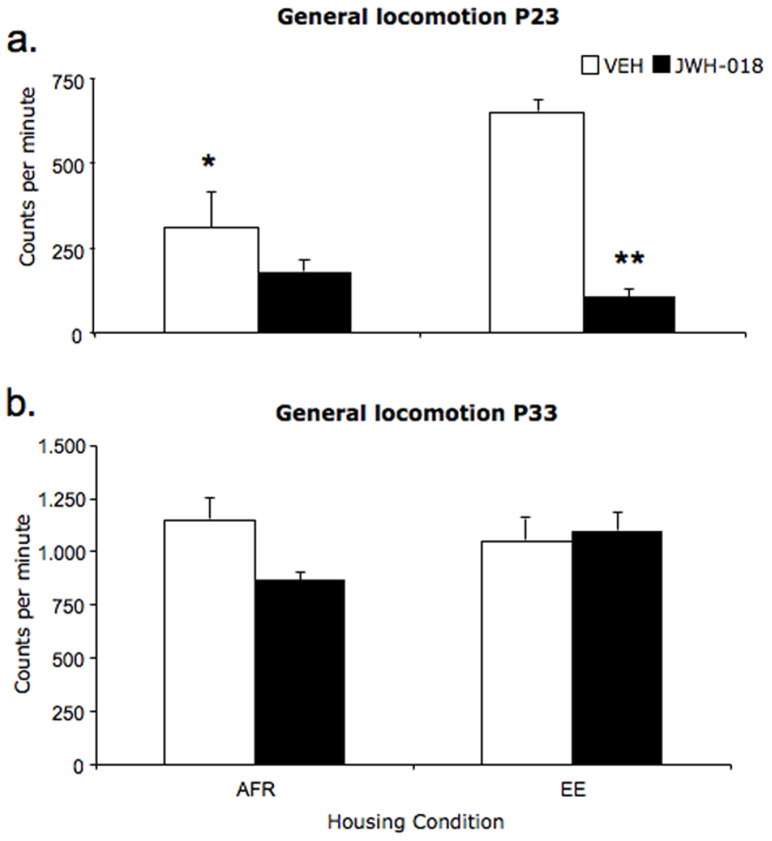
Exposure to EE had an immediate effect on general locomotion, whereby EE subjects showed on the first day of access to enriched cages (P23) increased locomotor activity (measured starting 2.5 hours following allocation to post-weaning conditions) compared to AFR individuals (housing: F(1,32) = 4.7; p = 0.037). Acute JWH-018 administration on P23 had differential effects in AFR and EE subjects (housing × drug: F(1,32) = 5,24; p = 0.029). Specifically, while AFR subjects were apparently insensitive to the effects of JWH-018, EE individuals showed reduced locomotion in response to synthetic cannabinoid administration (p < 0.05 in post-hoc tests, see fig. 3). Repeated JWH-018 administration apparently resulted in a habituation profile, whereby on P33, JWH-018 and VEH-treated subjects showed indistinguishable levels of locomotion (drug: F(1,32) = 0.14; p = 0.710; housing × drug: F(1,32) = 0.99; p = 0.326).
Figure 3. Spontaneous locomotion in adolescent mice measured in the home-cage, expressed in counts per minute (mean ± SEM), during a single 2-hr session starting 30 minutes after i.p injection with either vehicle (VEH) or JWH-018 (0.3 mg/kg), during the first (P23) (panel a) and last (P33) (panel b) administration day.
*p < 0.05 and **p < 0.01 significantly different from EE-VEH. Data are expressed as average ± SEM. (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018).
Motor coordination (rotarod test)
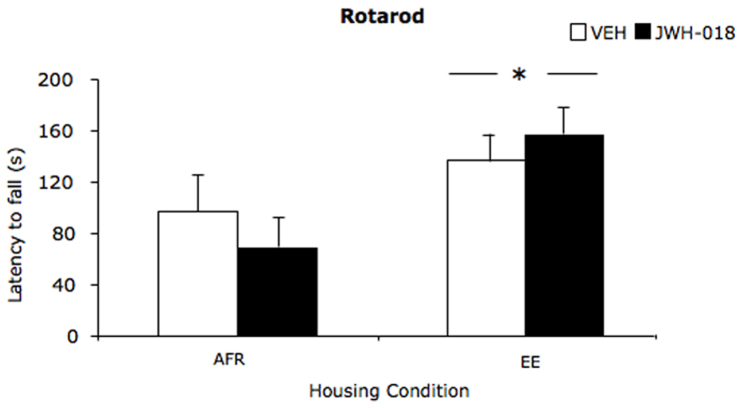
Approximately one month after the last JWH-018 administration, we evaluated individual motor coordination on the rotarod test. In the absence of significant effects of repeated JWH-018 administration (drug: F(1,36) = 0.02; p = 0.888), we observed that EE resulted in increased motor coordination, as shown by the longer latency attained to fall from the rotating rod in EE subjects compared to AFR (housing: F(1,36) = 5.2; p = 0.029, see fig. 4).
Figure 4. Motor coordination measured in drug-free state one month after JWH-018 administration through a rotating rod.
Falling latency (mean ± SEM) was measured during a single 4-min test session. *p < 0.05 significantly different from AFR. (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018).
Anxiety-related response
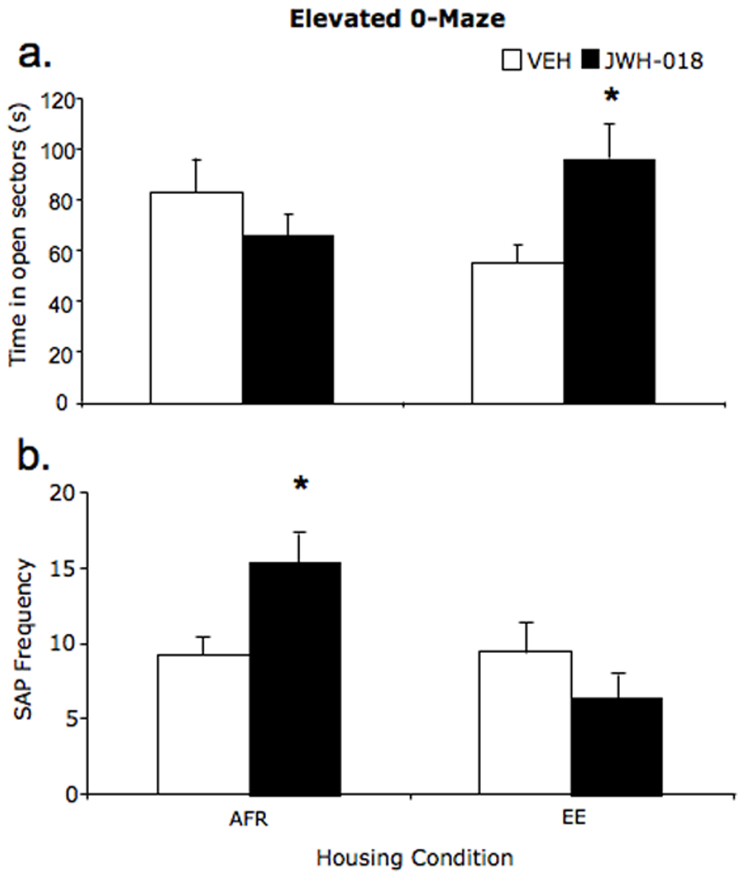
On average, subjects reared in different housing conditions spent analogous amounts of time in the open sectors of the elevated zero maze (housing: F(1,32) = 0.02; p = 0.885). Additionally, the seemingly negligible long-term effects of adolescent JWH-018 exposure on anxiety-related responses (drug: F(1,32) = 1.0; p = 0.320), were due to the fact that cannabinoid administration had differential effects in AFR and EE individuals (housing × drug: F(1,32) = 6.49; p = 0.016). Specifically, while JWH-018 did not modify the time spent in open sectors in AFR subjects, it exerted long-term anxiolytic effects in EE subjects. Thus, compared to VEH-treated EE, JWH-EE subjects spent increased amounts of time in the open sectors of the apparatus (see fig. 5a). While JWH-018 resulted in a long-term anxiolytic profile in EE subjects, it exerted opposite effects in AFR individuals. Specifically JWH-018 treated AFR subjects showed an increased frequency of SAP compared to their respective controls (housing × drug: F(1,32) = 4.53; p = 0.041, see fig. 5b). Additionally, EE subjects showed a reduced number of SAP compared to AFR mice (housing: F(1,31) = 6.26; p = 0.017).
Figure 5. Anxiety-related responses: (a) Time spent in the open sectors (s) and (b) SAP (stretched attend posture) frequency (mean ± SEM) in the elevated 0-maze.
*p < 0.05 significantly different from corresponding VEH. (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018).
Anhedonia
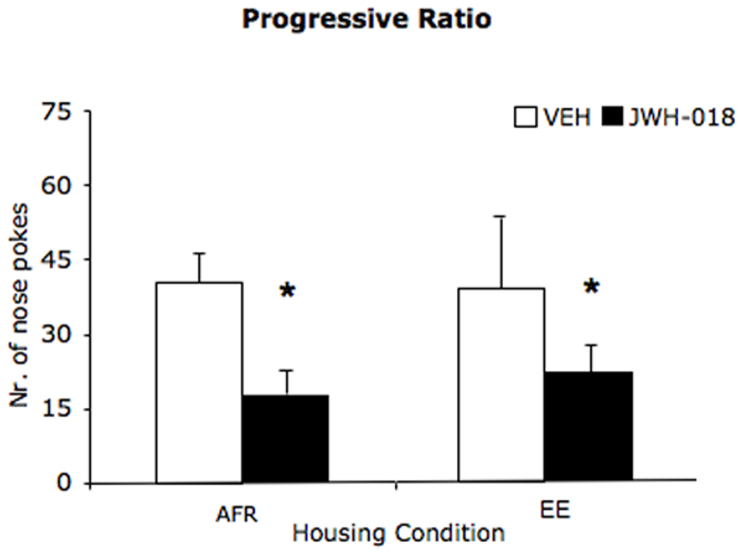
In line with our expectations, we observed that repeated JWH-018 administration during adolescence resulted in a long-term reduction in the number of operant responses performed to obtain palatable rewards. Specifically, in the FR3 stage of the test, adult subjects previously exposed to JWH-018, showed in drug-free state a reduced number of nose pokes and obtained a reduced number of rewards compared to VEH-treated subjects (drug: F(1,28) = 4.59; p = 0.041, see fig. 6). This effect was not affected by exposure to different housing conditions (housing × drug: F(1,28) = 0.19; p = 0.664).
Figure 6. Depressive-like responses: Number of nose pokes (mean ± SEM) in reinforced hole in the progressive ratio operant procedure, during the FR3 stage, conducted in drug-free state in adult mice that received either VEH or JWH-018 for 11 days during adolescence.
*p < 0.05 significantly different from VEH in post-hoc tests (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018).
Magnetic resonance imaging and spectroscopy
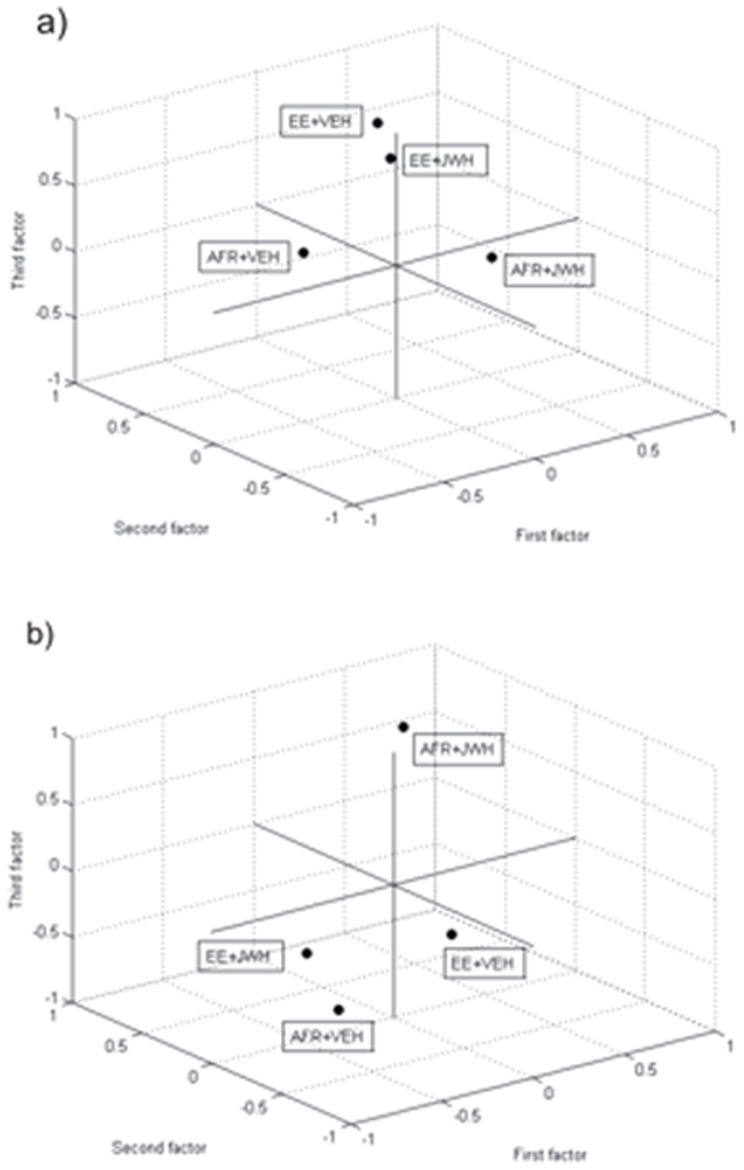
Metabolite quantification for the different brain areas is summarised in Table 1. Water T2 analyses confirmed that no changes occurred in the T2s within the groups in both regions (housing × drug: F(1,16) = 0.900; p = 0.36; F(1,24) = 0.79; p = 0.53 data not shown). PCA extracted three orthogonal factors in both regions analysed. The cumulative variance explained by the extracted factors was 67.7% in hippocampus and 77.3% in prefrontal cortex. Concerning the hippocampus, the correlation matrix revealed that the different factors were explained by the following metabolites or sum of metabolites: NAA, NAA + NAAG, Glu and Glu + Gln (factor 1); PCr, Ins, and Tau (factor 2); Gln and GPC + PCh (factor 3). Housing conditions did not affect absolute values of factors 1, 2, and 3 (housing: F(1,24) = 0.85; p = 0.366; F(1,24) = 0.33; p = 0.569; F(1,24) = 0.26; p = 0.613, respectively). As mentioned above, we used the sum of metabolites in situations in which resonance of metabolites overlapped to a remarkable extent. JWH-018 administration during adolescence had significant long-term effects on absolute levels of factors 1 and 3 (drug: F(1,24) = 4.52; p = 0.044; F(1,24) = 6.15; p = 0.022). With respect to factor 1, JWH-018 administration resulted in increased levels of factor 1 in AFR but not in EE individuals (see fig. 7). Concerning factor 3, JWH-018 administration resulted in significant reductions both in AFR and in EE individuals.
Table 1. Levels of metabolites in selected brain areas.
| Prefrontal cortex | Hippocampus | |||||||
|---|---|---|---|---|---|---|---|---|
| AFR | EE | AFR | EE | |||||
| VEH | JWH-018 | VEH | JWH-018 | VEH | JWH-018 | VEH | JWH-018 | |
| NAA | 6.25 ± 0.2 | 7.82 ± 0.5 | 7.64 ± 0.5 | 6.83 ± 0.2 | 8.15 ± 0.3 | 8.72 ± 0.1 | 8.26 ± 0.4 | 8.21 ± 0.2 |
| Cr + PCr | 6.61 ± 0.5 | 7.92 ± 0.3 | 7.9 ± 0.2 | 7.23 ± 0.4 | 9.09 ± 0.2 | 9.63 ± 0.2 | 9.04 ± 0.4 | 8.89 ± 0.2 |
| Tau | 9.58 ± 1 | 9.77 ± 0.4 | 9.96 ± 0.4 | 10.2 ± 0.5 | 10.8 ± 0.5 | 11.4 ± 0.5 | 10.1 ± 0.4 | 10.7 ± 0.4 |
| Glu | 10.9 ± 0.3 | 12.8 ± 0.6* | 10.4 ± 0.3 | 11.2 ± 0.9 | 9.24 ± 0.6 | 10.0 ± 0.2 | 8.16 ± 0.4$ | 9.12 ± 0.3* |
| Ins | 4.44 ± 0.2 | 5.0 ± 0.3 | 5.85 ± 0.2$ | 5.10 ± 0.4 | 6.23 ± 0.5 | 6.59 ± 0.3 | 6.15 ± 0.3$ | 6.30 ± 0.4 |
| GPC + PCh | 1.39 ± 0.1 | 1.84 ± 0.1 | 1.76 ± 0.1 | 1.50 ± 0.1* | 1.64 ± 0.1 | 1.55 ± 0.1 | 1.50 ± 0.1 | 1.54 ± 0.1 |
| NAA + NAAG | 7.01 ± 0.2 | 7.79 ± 0.5 | 8.22 ± 0.4 | 7.11 ± 0.3 | 8.35 ± 0.3 | 9.28 ± 0.1 | 8.64 ± 0.2 | 8.51 ± 0.2 |
| Glu + Gln | 15.3 ± 0.7 | 19.1 ± 0.7 | 13.8 ± 1.2 | 14.6 ± 1.2 | 13.8 ± 0.9 | 15.0 ± 0.5 | 13.8 ± 1.0 | 14.3 ± 0.3 |
| Gln | - | - | - | - | 5.90 ± 0.1 | 5.27 ± 0.4 | 5.80 ± 0.8 | 5.15 ± 0.3 |
Levels of metabolites (mM units; mean ± SEM) in prefrontal cortex and hippocampus, measured through 1H-MRS in drug free state.
$p < 0.05 significantly different from AFR.
*p < 0.05 significantly different from respective VEH in post-hoc tests.
AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH-018 = JWH-018 administration (P23–33).
Figure 7. Principal Component Analysis (PCA) of metabolite levels in hippocampus (a) and prefrontal cortex (b).
Factor 1 in hippocampus was composed of NAA, NAA + NAAG,Glu,Glu + Gln; Factor 2: PCr, Ins, and Tau; Factor 3: Gln, GPC + PCh. In PFC, Factor 1 was composed of: NAA, GPC + PCh, NAA + NAAG, Cr + PCr; Factor 2: Ins and Tau; Factor 3: Glu, Glu + Gln (AFR = animal facility rearing; EE = environmental enrichment; VEH = vehicle; JWH = JWH-018).
Concerning the PFC, the factors were composed by the following metabolites or sum of metabolites: NAA, GPC + PCh, NAA + NAAG, and Cr + PCr (factor 1); Ins and Tau (factor 2); Glu and Glu + Gln (factor 3). With respect to factor 1, the long-term consequences of JWH-018 treatment during adolescence were opposite in AFR and EE individuals (housing × drug: F(1,17) = 7.89; p = 0.012). Thus, while JWH-018 administration resulted in increased factor 1 values in AFR subjects, it exerted opposite effects in EE (see fig. 7). Factor 3 values were also significantly affected by housing conditions (housing: F(1,16) = 6.45; p = 0.022) and JWH-018 administration (drug: F(1,16) = 6.45; p = 0.022). Post-hoc comparisons revealed that there were no major differences in VEH-treated subjects and that JWH-018 administration significantly increased factor 3 values in AFR but not in EE individuals. Ultimately, we did not observe significant differences in factor two levels.
Discussion
Regardless of housing conditions (statistical main effect of drug), JWH-018 administration exerted remarkable effects on the entire experimental population. In line with previous findings22 and with analogous studies adopting different cannabinoid agonists, the administration of JWH-018 resulted in short-term reductions in body temperature and pain response32, and in a long-term increment in anhedonia25. Additionally, in the present study, we show that JWH-018 administration during adolescence exerts long-term effects at the level of brain metabolites. In contrast with our predictions, treatment with JWH-018 did not result in variations in general locomotion. Body temperature, pain perception and general locomotion are the core domains generally affected by cannabinoid administration24,33. Therefore, the observation that the first two domains are affected by JWH-018 constitutes evidence that the latter exert cannabinoid agonist properties. The discrepancy between the locomotion data obtained herein and those obtained in previous literature findings may be due to the fact that in the present study we tested post-weaning subjects instead of adult mice. Conversely, the absence of effects of repeated JWH-018 administration on general locomotion at the end of subchronic treatment is in accordance with previous findings25,34. Ultimately, in line with cohort studies reporting that heavy cannabis during adolescence may relate to increased indices of depression35, we observed that subchronic JWH-018 exposure during adolescence had persistent effects on anhedonia in adulthood. This result is also in line with our previous report indicating that exposure to the indirect cannabinoid agonist URB597 during adolescence resulted in increased depressive-like behaviour in adult mice25. In contrast with our previous observations22, JWH-018 administration failed to modulate anxiety-related responses in the long-term. However, as also discussed below, this apparent lack of effect is due to drug × housing interactions (see below for further details and discussion).
With respect to brain metabolites, we observed that JWH-018 administration during adolescence exerted robust long-term consequences in the hippocampus on factors 1 and 3. Factor 1 reflects a combination of NAA, NAA + NAAG, Glu and Glu + Gln. This association has already been described in previous literature suggesting that these metabolites constitute a functional unit providing an energy substrate to neurons36. Specifically, NAA and Glu contribute to the synthesis of NAAG within the neuron; NAAG is, in turn, released in the extracellular fluid to target the astrocytes wherein it is hydrolysed into Glu. Glu is then converted in Gln and recycled into neurons. Thus, variations in this functional unit may reflect generalized long-term effects of JWH-018 on neuronal metabolic and structural integrity. Since glutamate-related metabolites represent the intracellular pool contained in pyramidal glutamatergic neurons and glia, particularly in astrocyctes, alteration in factor 1 can also relate to glial altered metabolism. Variations in factor 3 (Gln and GPC + PCh) may relate to alterations in central nervous system metabolism. Specifically, Gln is the aminoacid responsible for the synthesis of glutamic acid, which, in turn serves an excitatory activity within the neuron37. Additionally, the choline-containing compounds resonance (PCho + GPC) is considered a potential biomarker for the status of membrane metabolism.
Regardless of cannabinoid agonists administration, access to EE from weaning onwards exerted immediate and delayed effects on several phenotypes. As also observed in numerous studies, EE resulted in remarkable variations in body weight in all subjects38. Whilst literature data concerning the effects of EE on body weight are inconsistent39, we believe that the presence of a running wheel may have considerably affected energy expenditure, ultimately reducing body weight gain in EE individuals. Thus, several reports indicate that voluntary exercise (as that stimulated by the presence of a running wheel in the home cage) in mice significantly reduces body weight40,41. This interpretation is also in line with the observation that EE individuals exhibited increased levels of general locomotion compared to AFR. In accordance with previous literature, EE also resulted in improved motor coordination42. Specifically, Munn and colleagues (2011) observed that EE increased motor coordination in an accelerating rotarod in a study involving eight different mouse strains. The behavioural effects of environmental enrichment are generally attributed to its capability of modulating brain plasticity18 and of increasing neuronal viability and available “reserves”15. In partial contrast with our expectations, EE did not seem to exert consistent effects at the level of brain metabolism. Yet, as discussed below, EE effects on brain metabolism were modulated and partially masked by JWH-018 administration during adolescence.
While the aforementioned main effects of EE and JWH-018 administration beget relevant information, the core aim of the present study was to investigate whether EE may modulate the effects of JWH-018 obtained in classical housing conditions. Ultimately, our main goal was to demonstrate that the conclusions concerning the cannabinoid agonist properties of a novel compound may be remarkably different if obtained in AFR (classical standard) or EE (the incoming standard requested by the Directive). Within this framework, we observed that two of the core domains traditionally affected by cannabinoid agonist administration – body temperature and pain perception – were sensitive to JWH-018, irrespective of environmental conditions. Yet, many of the other observations were markedly influenced by the environment in which experimental subjects were reared. Thus, a reduction in general locomotion in response to JWH-018 administration was observed only in EE subjects and not in AFR. The absence of effects of JWH-018 on locomotion in AFR subjects is in partial conflict with our previous findings22. This inconsistency is most likely due to the fact that while in the present study we tested weaning mice, in our previous report we tested adult subjects. Although hypothetical, it may be suggested that AFR-vehicle subjects showed absolute levels of locomotion sufficiently low not to be further reduced by JWH-018 administration (floor effect). Such floor effect was not observed in EE individuals as exposure to enriched cages resulted in increased locomotion. Additionally, the long-term consequences of a subchronic administration of JWH-018 during adolescence on anxiety-related behaviour were opposite in AFR and EE subjects. Specifically, while JWH-018 administration reduced anxiety-related responses in EE, it had opposite effects in AFR (see43 for a detailed description of the biological significance of the stretched-attend posture). This aspect is particularly relevant whereby it may partly explain why anxiety-related data in response to cannabinoids vary across different studies44,45,46. Whilst some authors observed a reduction in anxiety-related parameters47, some others obtained opposite results44. Thus, leveraging its effects on brain plasticity, EE may have also regulated individual reactivity towards the stimulation of the endocannabinoid system. Ultimately, we observed that environmental enrichment radically modified the effects of JWH-018 administration on brain metabolism. Specifically, within the prefrontal cortex, we observed that JWH-018 administration resulted in increased factor 1 values (NAA, GPC + PCh, NAA + NAAG, and Cr + PCr) in AFR and decreased in EE subjects. Factor 1 in the PFC may constitute an indicator of energetic metabolism both at the level of Cr + PCr (which favour the transition of a phosphoric group to ADP to form ATP48,) and at the level of the other metabolites as described above. Regardless of the specific direction of the effects of JWH-018, we note that they were opposite if evaluated in AFR or EE conditions. Likewise, while JWH-018 administration altered Glu and Glu + Gln values in the PFC of AFR subjects, it had no long-term consequences in EE individuals. Thus, whereas data obtained in AFR conditions may indicate variations at the level of excitatory neurotransmission49, data obtained in EE conditions would not support this conclusion. Similar considerations may also pertain to neuronal energetic metabolism and excitatory activity in the hippocampus (factors 1 and 3, see above). Beside the considerations regarding the possibility to consider JWH-018 a cannabinoid agonist, these data further support the view that rearing conditions may strongly influence individual reactivity, and in turn its vulnerability, towards the effects of psychoactive compounds50.
While understanding the basic mechanisms explaining the environment × treatment interaction extended beyond the scopes of this manuscript, herewith we wish to reiterate the need to carefully consider environmental conditions during the compulsory transition from traditional to EE housing systems. The provision of enriched and more complex environmental stimuli to laboratory animals certainly constitutes a needed objective; yet, such increased complexity may raise several issues related to the validity of experimental data. To exemplify our concern, in the present study, we investigated whether a novel compound possessing in vitro cannabimimetic properties exerted in vivo behavioural effects. Although two core findings support the view that JWH-018 acts as a traditional cannabinoid agonist irrespective of the rearing environment, many other observations contradict this view. Thus, the conclusion as to whether JWH-018 shall be considered a cannabinoid agonist may strongly depend on the specific conditions in which mice are reared. With respect to this aspect, it is important to consider the nature of the environmental enrichment strategy adopted in the present study and the potential solutions to be proposed. In the present study we selected an EE strategy in which most of the European Directive recommendations on animal housing and experimentation have been applied: space of sufficient complexity, control and choice over the environment, extension of the range of activities (including promotion of physical exercise, foraging, social interaction, manipulative and cognitive activities). However, although our EE strategy does constitute a foreseeable scenario, we acknowledge that milder forms of enrichment can be proposed and applied. For example, Wuerbel and Garner (2007) systematically analysed different forms of EE and concluded that some consensus may be achieved regarding the provision of a standard minimal enrichment (shelter material) capable of promoting individual activities while minimising inter-individual competition51. We note, however, that the proposition of a univocal enrichment standard may conflict with the Directive itself as it explicitly states that environmental enrichment “shall be adapted to the species and individual needs”. Thus an a priori definition of a standard enrichment may not always meet individual needs. This aspect is particularly relevant whereby it implicitly suggests that rearing and breeding conditions shall not be standardised, but rather adjusted to individual needs. An absence of standardization across different facilities has been proposed to affect the reproducibility of experimental findings52. This would ultimately increase the number of animals to be used to achieve a given experimental objective. By the same token, neglecting that different individuals may have different needs would contradict the Directive and potentially hamper the external validity of experimental data. In line with other scholars, we argue that standardization should not neglect variation, but rather, that it should “prohibit variation at random”52. Within this framework, several recent studies are proposing the adoption of systematic variations in housing conditions (environmental heterogenization) as a way to design externally valid and reproducible results53,54,55. Specifically, these studies propose the adoption of test strategies entailing experimental subjects housed in systematically variable conditions. These studies show that the effects of given independent variables are more robust if tested in subjects belonging to heterogeneous rather than to homogeneous environmental conditions (be the latter EE or AFR53). Our study is in partial agreement with this view whereby some of the classical effects of cannabinoid compounds have been observed in the entire experimental population (i.e. main effect of JWH-018 administration regardless of housing conditions). We believe that future efforts should focus on the following goals: systematic comparison of different forms of environmental enrichment with respect to variations in basal conditions and potential environment × treatment interactions; indication, within the methods' section of published manuscripts, of exact details of the environmental enrichment strategies adopted. Beside the parameters traditionally reported, such description should include a precise description of pre- and post-weaning housing conditions, separations and re-grouping, cage composition and nature and displacement of enrichment materials. Additionally, commercial breeders should precisely disclose the exact details regarding rearing, housing, and shipping conditions of laboratory rodents used across different facilities.
Methods
Animals
Twenty-one outbred CD-1 pregnant female were purchased from a commercial breeder (Charles River®, Italy). Out of 21 dams, 16 delivered litters of appropriate size (8–14 pups). Animals were housed individually in standard polycarbonate cages (33.0 × 13.0 × 14.0 cm) with sawdust bedding. Water and food were available ad libitum (enriched standard diet, Mucedola, Settimo Milanese, Italy). Mice maintained on a reversed 12:12 h light-dark cycle (lights on at 1900 h) with temperature at 21 ± 1°C and relative humidity of 60 ± 10%. Dams were inspected daily at 0930 h for delivery and day of birth was designated as postnatal day 1 (P1). Between delivery and weaning (p23) all subjects were kept under standard facility rearing (AFR) conditions (cage cleaning once a week). Litters were not culled. At weaning, each dam contributed 4 male offspring, which were further allocated to standard facility rearing conditions (AFR) or environmental enrichment (EE) (see below for details). Only male offspring were used for this experiment. Animal handling and experimental procedures were performed according to European Communities guidelines (EC Council Directive 86/609), Italian legislation on animal experimentation (Decree by law 116/92). The study has been approved by the Service for Biotechnology and Animal Welfare of the Istituto Superiore di Sanità and authorized by the Italian Ministry of Health (Decree Nr. 217/2010-B). All efforts were made to minimise animal suffering, and reduce the number of animals used.
Housing and environmental enrichment
At weaning (P23) mice were transferred to standard or enriched cages and kept in these conditions until the end of the experiments. The standard laboratory conditions (animal facility rearing, AFR) were defined as a pair of unrelated mice housed in standard polycarbonate cages (33.0 × 13.0 × 14.0 cm) with sawdust bedding and ad libitum water and rodent pellets. Environmental enrichment consisted of four unrelated subjects housed in larger cages (40.0 × 25.0 × 30.0 cm) provided with a running wheel (15 cm), red plastic see-through shelters, plastic tubes and balls, and chewable tubes and cardboard houses. The enrichment objects were changed twice a week. The bottle of water was suspended above the ceiling and food pellets were provided on the floor. Cage cleaning was performed once a week in association with measurement of individual body weight. In order to avoid litter effects, littermates were attributed to different experimental groups.
Drug treatment during adolescence
JWH-018 (naphthalen-1-yl-(1-pentylindol-3-yl) methanone) was dissolved in a vehicle (VEH) solution of 0.9% saline (99%), and ethanol (1%) and administered i.p. at a volume of 10 ml/kg body weight. All animals were injected once daily for 11 consecutive days during adolescence (P23-33) with vehicle or JWH-018 (0.3 mg/kg). Thus the general experimental design consisted of four groups: AFR-VEH (N = 16); AFR-JWH (N = 16); EE-VEH (N = 16); EE-JWH (N = 16). The dose of JWH-018 was chosen on the basis of our previous study22.
Body temperature
Body temperature was measured through a rectal thermometer CMA/150 (CMA Microdialysis, Solna, Sweden). The probe was inserted into the rectum 1.5 cm for 60 s. Rectal temperature was determined at 0, 30 and 60 minutes after the injection during the first and last day of drug administration.
Locomotor activity: apparatus and schedule
Spontaneous locomotion was monitored continuously for 2 hours on the first and last day of JWH-018 administration, in cages identical to the home-cages in which mice were kept between birth and weaning (33.0 × 13.0 × 14.0 cm), starting 30 minutes after the injection. Spontaneous locomotion was monitored through an automatic device using small passive infrared sensors positioned on the top of each cage (ACTIVISCOPE system, NewBehaviour Inc., Zurich, Switzerland)56. With respect to the first day of JWH-018 administration, locomotion was measured 2.5 hours after mice were assigned to their post-weaning conditions (AFR or EE). Exact details on the procedure are reported in25 and in the Supplementary Information. The sensors (20 Hz) detected any movement of mice with a frequency of 20 events per second. Data were recorded by an IBM computer with dedicated software. No movements were detected by the sensors when mice were sleeping, inactive, or performed moderate self-grooming. Scores were obtained during 1-hr intervals and expressed as counts per minute (cpm). The position of cages in the rack was such that mice of each group were equally distributed in rows and columns. The access of the authorized personnel to the animal room was not restricted and followed the routine schedule.
Hot-plate test
The apparatus consisted of a metal plate 25 × 25 cm (Socrel Mod. DS-37, Ugo Basile, Italy) heated to a constant temperature of 55 ± 1°C, above which a transparent Plexiglas cylinder, 20 cm in diameter and 18 cm high, was placed. Hind-paw liking has been adopted as the main end-point and cut-off time was set at 60 seconds57. Additionally, we also scored fore-paw licking latency. Ultimately, since mice may exhibit jumping as an alternative pain-relieving strategy, we included this behaviour in our ethogram and planned to interrupt the session if a subject jumped earlier than hind-paw licking. This decision was based on the fact that we aimed at limiting the time spent on the heated surface. However, since all experimental mice exhibited hind-paw licking earlier than jumping, data on jumping are not reported in the manuscript. The test was conducted on the first and the last day of treatment 30 minutes after drug administration. Animals were tested at P23 and P33.
Rotarod test
To evaluate motor coordination in adult mice (P60), we performed the rotarod test. The apparatus consists of five compartments divided by circular separators in order to test five mice at the same time, a rotating rod of 4.5 cm in diameter (suspended at a height that does not harm the animal during the fall, but enough to create a feeling of emptiness below) that has horizontal grooves to ensure a good grip to the animal during the rotation. Five levers located below each compartment were connected to a timer detecting the fall latency (seconds) of each subject. The timer was started, lifting the lever, when mice were placed on the rotating rod. The fall of the animal caused a lever pressure and subsequent stopping of the timer. The rotation accelerated steadily by 4 rotations per minute (rpm) every 24 seconds and started from 4 rpm until achieving a maximum of 40 rpm in four minutes (cut off). The test parameter used was the mouse latency to fall.
Elevated 0-maze (EOM) test
To evaluate the exploration of an environment imposing on the animal an approach-avoidance conflict, mice were tested on the elevated 0-maze, a paradigm originally described by Shepherd and colleagues58. We recorded the following parameters59: spatiotemporal measures comprised the frequencies of sector entries (sector entries were defined as the animal entering the respective sector with all four paws) and the time spent in the open or closed parts of the maze in 5 min. Furthermore, we scored the latency to the first open sector entry. We used the percentage of entries into the open sectors as an inverse index of anxiety58. The sessions started placing the animal in one corner of the sector. Animals were tested at P65. Please see Supplementary Information for further details.
Progressive ratio operant procedure
At age 3–4 months, mice were trained and tested in the progressive ratio (PR) schedule for reinforcement. We used the same procedure used in25; further details are reported in the Supplementary Information.
Magnetic resonance imaging and spectroscopy
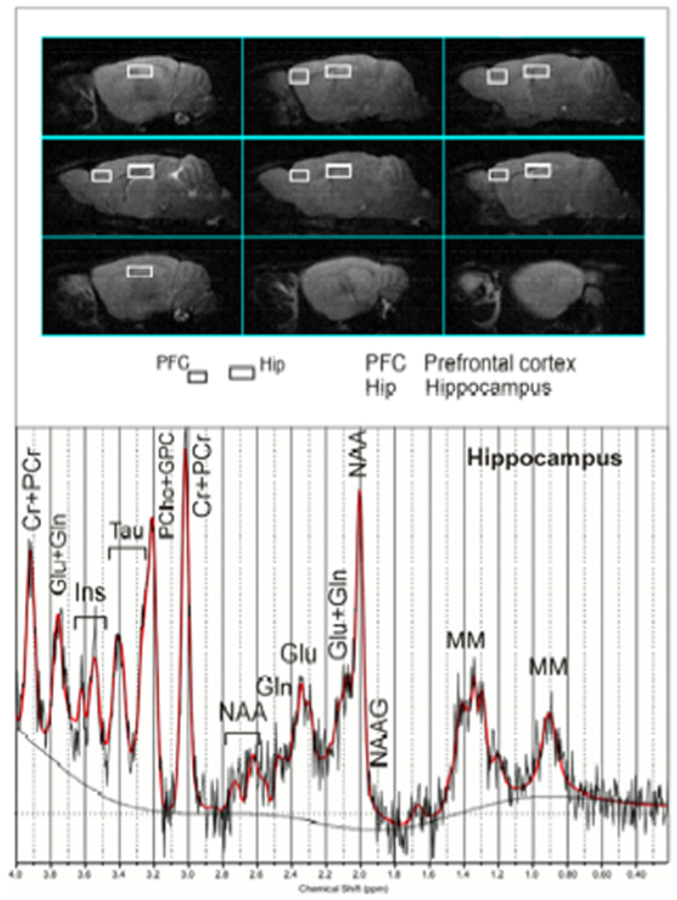
Adult mice (P > 130) underwent MRI/MRS analyses in order to characterise differences in the metabolism of relevant brain areas among the different groups. 1H MRI and MRS analyses were performed on a 4.7 T Varian/Agilent Inova animal system (Agilent Inc. Palo Alto, CA, USA), equipped with actively shielded gradient system (max 200 mT/m, 12 cm bore size). A 6-cm diameter volume coil was used for transmission in combination with an electronically decoupled receive-only surface coil (Rapid Biomedical, Rimpar, Germany). The procedure adopted in the present study was analogous to that used in25. Single voxel localised 1H MR spectra (PRESS, TR/TE = 4000/23 ms, ns = 256 or 512) were collected from relevant brain areas: hippocampus (Hip, 11.7 μl) and prefrontal cortex (PFC, 5.9 μl) as shown in fig. 8. The accuracy of voxel positioning was guaranteed by choosing voxels which are inscribed within the region of interest, as it is defined in the mouse brain atlas60 (see Supplementary information for further details).
Figure 8. MRI panel: Example of in vivo sagittal T2-weighted spin-echo images (TR/TE = 3000/70 ms, slice thickness 0.8 mm, NS = 2, FOV = 20 × 20 mm2, matrix 128 × 128).
Voxels localised on PFC and Hip are indicated by the white rectangles. MRS panel – Examples of in vivo 1H spectra (as a black trace), acquired from the Hip (PRESS, TR/TE = 4000/23 ms, NS = 256). The result of LCModel fit is shown as a red trace overimposed on the spectrum. Metabolite assignments: Ins, inositol; Cr, creatine; PCr, phospho-creatine; Glu, glutamine; Gln, glutamate; Tau, taurine; PCho, phospho-choline; GPC, glicero-phospho-choline; NAA, N-acetyl-aspartate; NAAG, N-acetyl-aspartyl-glutamate; MM, macromolecules.
Statistical analysis
Data on body weight gain, temperature and behavioural responses were analysed through general ANOVA for split-plot designs. The general model was a 2 housing (AFR vs. EE) × 2 drug (VEH vs. JWH-018) × k (repeated measurement, variable depending on the specific parameter). Housing and drug were between subject factors while repeated measurements were within subject factors. MRS data were analysed through Principal Component Analysis (PCA). Specifically, PCA was performed on mean-centred metabolic data in order to reduce the number of correlated variables to a reduced number of orthogonal factors. Score-weights were then used to calculate the corresponding factor value for each individual. Computed data were then analysed through general ANOVA with housing (AFR vs. EE) and drug (VEH vs. JWH-018) as between subject factors.
Author Contributions
G.L., S.M. and C.C. conceived the study; C.C. performed the experiments; R.C. and L.A. performed the experiments, analysed the data, and wrote the section concerning MRI/MRS investigation; C.C. and S.M. analysed the data; S.M. and C.C. wrote the manuscript; all the authors have seen and approved the manuscript.
Supplementary Material
Supplementary Methods
Acknowledgments
The authors are grateful to Augusto Vitale for critical reading and to Giovanni Dominici for technical support. This work was supported by the grant “ECS-EMOTION” from the Department of Antidrug Policies c/o Presidency of the Council of Ministers, Italy to G.L. and S.M., and by the EU-FP7 framework project EMTICS under grant agreement n° 278367. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Ricceri L. & Vitale A. The law through the eye of a needle. How and when to apply the new European Directive on animals used in research. EMBO rep. 12, 637–640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer D. P. et al. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432, 821–822 (2004). [DOI] [PubMed] [Google Scholar]
- Fox C., Merali Z. & Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav. Brain Res. 175, 1–8 (2006). [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A. et al. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms? Pharmacol. Biochem. Behav. 73, 233–245 (2002). [DOI] [PubMed] [Google Scholar]
- Jurgens H. A. & Johnson R. W. Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain Behav. Immun. 26, 1006–1016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi V., Barnekow A. & Sachser N. Effects of environmental enrichment on males of a docile inbred strain of mice. Physiol. Behav. 82, 765–776 (2004). [DOI] [PubMed] [Google Scholar]
- Workman J. L., Fonken L. K., Gusfa J., Kassouf K. M. & Nelson R. J. Post-weaning environmental enrichment alters affective responses and interacts with behavioral testing to alter nNOS immunoreactivity. Pharmacol. Biochem. Behav. 100, 25–32 (2011). [DOI] [PubMed] [Google Scholar]
- Crabbe J. C., Wahlsten D. & Dudek B. C. Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672 (1999). [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Monkeyluv: And Other Essays on Our Lives as Animals. (Scribner, 2006). [Google Scholar]
- West-Eberard M. J. Developmental Plasticity and Evolution. (Oxford University Press, 2003). [Google Scholar]
- Fischer A., Sananbenesi F., Wang X., Dobbin M. & Tsai L. H. Recovery of learning and memory is associated with chromatin remodelling. Nature 447, 178–182 (2007). [DOI] [PubMed] [Google Scholar]
- Solinas M., Thiriet N., El Rawas R., Lardeux V. & Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology 34, 1102–1111 (2009). [DOI] [PubMed] [Google Scholar]
- Bezard E. et al. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J. Neurosci. 23, 10999–11007 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick K. M., Stearns N. A., Pan J. Y. & Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn. Mem. 10, 187–198 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosini L. et al. On whether the environmental enrichment may provide cognitive and brain reserves. Brain res. Rev. 61, 221–239 (2009). [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J., Levis H. & Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol. learn. Mem. 81, 200–210 (2004). [DOI] [PubMed] [Google Scholar]
- Rampon C. et al. Effects of environmental enrichment on gene expression in the brain. Proc. Natl. Acad. Sci. U S A 97, 12880–12884 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H., Kempermann G. & Gage F. H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
- Laviola G., Hannan A. J., Macri S., Solinas M. & Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol. Dis. 31, 159–168 (2008). [DOI] [PubMed] [Google Scholar]
- Huffman J. W. et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 13, 89–112 (2005). [DOI] [PubMed] [Google Scholar]
- Fattore L. & Fratta W. Beyond THC: The New Generation of Cannabinoid Designer Drugs. Front. Behav. Neurosci. 5, 60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrì S. et al. Behavioral Responses to Acute and Sub-chronic Administration of the Synthetic Cannabinoid JWH-018 in Adult Mice Prenatally Exposed to Corticosterone. Neurotox. Res. 24, 15–28 (2013). [DOI] [PubMed] [Google Scholar]
- Wiley J. L., Marusich J. A., Martin B. R. & Huffman J. W. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 123, 148–153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E., Perchuk A., Hall F. S., Uhl G. R. & Onaivi E. S. Behavioral methods in cannabinoid research. Methods Mol. Med. 123, 269–290 (2006). [DOI] [PubMed] [Google Scholar]
- Macrì S., Ceci C., Canese R. & Laviola G. Prenatal stress and peripubertal stimulation of the endocannabinoid system differentially regulate emotional responses and brain metabolism in mice. PLoS One 7, e41821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E. M. et al. Enhancement of endocannabinoid signalling during adolescence: Modulation of impulsivity and long-term consequences on metabolic brain parameters in early maternally deprived rats. Pharmacol. Biochem. Behav. 86, 334–345 (2007). [DOI] [PubMed] [Google Scholar]
- Maddock R. J. & Buonocore M. H. MR Spectroscopic Studies of the Brain in Psychiatric Disorders. Curr. Top. Behav. Neurosci. (2012). [DOI] [PubMed] [Google Scholar]
- Licata S. C. & Renshaw P. F. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann. N. Y. Acad. Sci. 1187, 148–171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anavi-Goffer S. & Mulder J. The polarised life of the endocannabinoid system in CNS development. Chembiochem. 10, 1591–1598 (2009). [DOI] [PubMed] [Google Scholar]
- Fride E. et al. The endocannabinoid system during development: emphasis on perinatal events and delayed effects. Vitam. Horm. 81, 139–158 (2009). [DOI] [PubMed] [Google Scholar]
- Canese R. et al. Characterisation of in vivo ovarian cancer models by quantitative (1) H magnetic resonance spectroscopy and diffusion-weighted imaging. NMR Biomed. 25, 632–645 (2011). [DOI] [PubMed] [Google Scholar]
- De Vry J., Jentzsch K. R., Kuhl E. & Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav. Pharma. 15, 1–12 (2004). [DOI] [PubMed] [Google Scholar]
- Wiley J. L., Smith F. L., Razdan R. K. & Dewey W. L. Task specificity of cross-tolerance between Delta9-tetrahydrocannabinol and anandamide analogs in mice. Eur. J. Pharmacol. 510, 59–68 (2005). [DOI] [PubMed] [Google Scholar]
- Eisenstein S. A., Holmes P. V. & Hohmann A. G. Endocannabinoid modulation of amphetamine sensitization is disrupted in a rodent model of lesion-induced dopamine dysregulation. Synapse 63, 941–950 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton G. C. et al. Cannabis use and mental health in young people: cohort study. BMJ 325, 1195–1198 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow M. H. The Vertebrate Brain, Evidence of Its Modular Organization and Operating System: Insights into the Brain's Basic Units of Structure, Function, and Operation and How They Influence Neuronal Signaling and Behavior. Front. Behav. Neurosci. 5, 5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Procopio J., Lima M. M., Pithon-Curi T. C. & Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem. Funct. 21, 1–9 (2003). [DOI] [PubMed] [Google Scholar]
- Whitaker J. et al. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab. animal 38, 24–34 (2009). [DOI] [PubMed] [Google Scholar]
- Bayne K. Potential for unintended consequences of environmental enrichment for laboratory animals and research results. ILAR J. 46, 129–139 (2005). [DOI] [PubMed] [Google Scholar]
- Yan L., DeMars L. C. & Johnson L. K. Long-term voluntary running improves diet-induced adiposity in young adult mice. Nutr. Res. 32, 458–465 (2012). [DOI] [PubMed] [Google Scholar]
- Bell R. R., Spencer M. J. & Sherriff J. L. Voluntary exercise and monounsaturated canola oil reduce fat gain in mice fed diets high in fat. J. Nutr. 127, 2006–2010 (1997). [DOI] [PubMed] [Google Scholar]
- Munn E. et al. Reversed light-dark cycle and cage enrichment effects on ethanol-induced deficits in motor coordination assessed in inbred mouse strains with a compact battery of refined tests. Behav. Brain Res. 224, 259–271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. C., Griebel G. & Blanchard R. J. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 25, 205–218 (2001). [DOI] [PubMed] [Google Scholar]
- Bambico F. R., Nguyen N. T., Katz N. & Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol. Dis. 37, 641–655 (2010). [DOI] [PubMed] [Google Scholar]
- Biscaia M. et al. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 170, 301–308 (2003). [DOI] [PubMed] [Google Scholar]
- Schneider M. & Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28, 1760–1769 (2003). [DOI] [PubMed] [Google Scholar]
- Rutkowska M., Jamontt J. & Gliniak H. Effects of cannabinoids on the anxiety-like response in mice. Pharmacol. Rep. 58, 200–206 (2006). [PubMed] [Google Scholar]
- Greenhaff P. L. The creatine-phosphocreatine system: there's more than one song in its repertoire. J. Physiol. 537, 657 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S., Giove F. & Dinuzzo M. Metabolic pathways and activity-dependent modulation of glutamate concentration in the human brain. Neurochem. Res. 37, 2554–2561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley-Fletcher S. et al. Prenatal stress affects 3,4-methylenedioxymethamphetamine pharmacokinetics and drug-induced motor alterations in adolescent female rats. Eur. J. Pharmacol. 489, 89–92 (2004). [DOI] [PubMed] [Google Scholar]
- Wuerbel H. & Garner J. P. Refinement of rodent research through environmental enrichment and systematic randomization. NC3Rs J. 9, 1–9 (2007). [Google Scholar]
- Van der Staay F. J. & Steckler T. The fallacy of behavioral phenotyping without standardisation. Genes Brain Behav. 1, 9–13 (2002). [DOI] [PubMed] [Google Scholar]
- Richter S. H., Garner J. P., Auer C., Kunert J. & Wurbel H. Systematic variation improves reproducibility of animal experiments. NaT. Methods 7, 167–168 (2010). [DOI] [PubMed] [Google Scholar]
- Richter S. H., Garner J. P. & Wurbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat. Methods 6, 257–261 (2009). [DOI] [PubMed] [Google Scholar]
- Macrì S. On the incongruity between developmental plasticity and methodological rigidity. Front. Behav. Neurosci. 6, 93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Omo G. et al. Early behavioural changes in mice infected with BSE and scrapie: automated home cage monitoring reveals prion strain differences. Eur. J. Neurosci. 16, 735–742 (2002). [DOI] [PubMed] [Google Scholar]
- Macrì S. et al. Moderate neonatal stress decreases within-group variation in behavioral, immune and HPA responses in adult mice. PLoS One 2, e1015 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. K., Grewal S. S., Fletcher A., Bill D. J. & Dourish C. T. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 116, 56–64 (1994). [DOI] [PubMed] [Google Scholar]
- Holmes A. & Rodgers R. J. Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci. Biobehav. Rev. 23, 971–980 (1999). [DOI] [PubMed] [Google Scholar]
- Paxinos G. & Franklin K. B. J. The Mouse Brain in Stereotaxic Coordinates. (Academic Press, 2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods