ABSTRACT
BACKGROUND:
Adenosquamous carcinoma of the pancreas (ASCAP) is a rare histologic type of pancreatic carcinoma that constitutes 1% to 4% of all pancreatic exocrine malignancies. It has a clinical presentation similar to that of adenocarcinoma of the pancreas (ACP), but may have a worse overall prognosis, with most patients surviving for less than 2 years.
METHODS:
This was an institutional, retrospective, cohort analysis of 237 patients who underwent resection of pancreatic cancer with curative intent.
RESULTS:
Of the 237 cases examined, we identified 7 (2.9%) with histologically confirmed ASCAP. Demographics, comorbidities, risk factors, presenting symptoms, survival data, tumor characteristics, and types of treatment for each patient were included in the analysis. Risk factors for development of ASCAP were not conclusive. Although human papilloma virus (HPV) has been implicated in other squamous cell cancers, in our cohort, its involvement in ASCAP was 0%. Presurgical fine-needle aspiration failed to identify the invasive squamous cell component in all cases. In this cohort analysis, overall survival ranged from 3 to 25 months, with 2 patients surviving more than 20 months after surgical resection. With a median follow-up of 2.9 years, our data demonstrate a trend to worse median overall survival for ASCAP than for ACP (8.2 vs. 20.4 months; P = .23), with a limited number of long-term survivors.
CONCLUSIONS:
Although recommended, adjuvant treatment was inconsistently provided for patients in this ASCAP cohort. Published data show variability in overall survival, but our findings support that surgical resection is one of the few options for control of this rare, poorly understood pancreatic malignancy. Further research is necessary to define risk factors and adjuvant and neoadjuvant treatments, to help improve patient outcomes.
Adenosquamous carcinoma of the pancreas (ASCAP) is a rare histologic type of pancreatic cancer that is estimated to constitute 1% to 4% of all pancreatic exocrine malignancies.1,2 Adenosquamous carcinoma is more common in other organs, such as the anus, esophagus, intestines, uterus, cervix, and vagina.3 Much of the published literature in ASCAP is based on small series or single case reports. The first report describing ASCAP was published in 1907 by Gotthold Herxheimer, who referred to it as cancroide.1,4 As in adenocarcinoma of the pancreas (ACP), ASCAP typically presents with similar symptoms, making it difficult to initially differentiate these 2 malignancies based on history alone.5–7 Some reports suggest that patients with ASCAP present with larger primary tumor size and more severe abdominal symptoms than do their counterparts with adenocarcinoma.8
Histologically, ASCAP has been defined to consist of at least 30% malignant squamous cell carcinoma with coexisting ductal adenocarcinoma.6,7 Multiple theories have been proposed regarding the pathophysiology of ASCAP, since normal pancreatic tissue does not have a squamous epithelium. These have been referred to as the squamous metaplasia, collision, and differentiation theories. The first theory suggests that squamous metaplasia may occur as a result of chronic inflammation caused by obstruction by a tumor or chronic pancreatitis with subsequent transformation into a malignant tumor.1,4,5,8,9 The collision theory proposes that 2 histologically distinct neoplastic cell populations arise independently and subsequently combine to form one cohesive unit.1 Clear evidence confirming the collision theory has not been found, but squamous components have been shown to present initially mixed with the adenocarcinoma component.8 The differentiation theory suggests that the squamous and adenocarcinoma elements arise from the same pluripotent stem cells.4,8,10 Given the rarity of this condition, little is formally known about its pathophysiology.
ASCAP is historically regarded to be more clinically aggressive and connotes a worse prognosis than ACP. Despite aggressive surgical management, the median survival of patients with ASCAP has been reported to be consistently less than 1 year. No standard adjuvant therapy has been established for this entity of pancreatic cancer.1,4,5,8 This study was undertaken to characterize this malignant subtype in the context of a contemporary ACP cohort. The findings will allow for a better understanding of ASCAP and provide a better treatment strategy for this rare subtype of pancreatic cancer.
METHODS
A retrospective analysis was conducted of all patients with surgical pathology available who underwent definitive pancreatic resection with curative intent for ACP at the University of Florida between 2001 and 2011. The project was approved by the Institutional Review Board. The pathology reports were individually reviewed for any evidence of a squamous component or keratinizing feature. Seven cases were identified and reviewed by 2 pathologists to confirm the diagnosis of ASCAP consistent with previously reported criteria.6,7 Patient characteristics such as demographics, comorbidities, risk factors, presenting symptoms, tumor characteristics, treatment provided, and clinical outcomes were obtained. Laboratory data, when available, such as CA 19-9, carcinoembryonic antigen (CEA), and direct bilirubin before or immediately after the tissue diagnosis, were recorded. In situ hybridization (ISH) for high-risk human papilloma virus (HPV) genotypes using the Ventana automated methodology (INFORM HPV III Family 16 Probe) was performed on associated, formalin-fixed, paraffin-embedded tissue. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death. Descriptive statistics were calculated, with t-tests used to determine differences.
RESULTS
Seven (2.9%) of 237 resected pancreatic adenocarcinomas were confirmed to be ASCAP. The characteristics of patients with ASCAP compared to ACP are described in Table 1. Patients with ASCAP were equally balanced for sex, with a median age of 68.7 years (range, 55–82). Five patients were Caucasian, 1 was Japanese, and 1 was Hispanic. In 1 patient, diagnosis was made incidentally during abdominal imaging as part of routine follow-up for gastric dysplasia. All other patients presented with symptoms such as abdominal pain, weight loss, and jaundice, similar to patients with ACP. In the former case, this tumor mass was not seen on the CT scan completed 3 months earlier, implying a rapid rate of growth. There were no differences in serum tumor marker positivity or levels between the 2 groups, with no ASCAP patient having hypercalcemia.
Table 1.
Characteristics of patients with ASCAP compared with ACP
| ASCAP (n = 7) | ACP (n = 230) | P | |
|---|---|---|---|
| Mean age, range | 68.7 (55–82) | 65.4 (37–87) | NS |
| Male, % | 57 | 51 | NS |
| Caucasian, % | 75 | 91 | NS |
| Never-smoker, % | 57 | 31.8 | NS |
| Head of pancreas location, % | 14 | 78 | <0.001 |
| Pathologic node involvement, % | 57 | 59 | NS |
| Median overall survival, months | 8.2 | 20.4 | NS (P = 0.23) |
| 3-year overall survival, % | 0 | 19 | NS |
More than half (57%) of the ASCAP patients were life-long nonsmokers, compared with 31.8% of the patients with ACP (P = .4). Alcohol use was denied in approximately 50% of the ASCAP patients, with no ASCAP patient having a documented history of chronic pancreatitis. One patient with ASCAP had a history of squamous cell carcinoma of the tongue definitively treated years before the diagnosis of ASCAP. None of the ASCAP cases demonstrated the presence of HPV genomic integration. In all cases of ASCAP, the diagnosis was made after surgical resection, whereas preoperative fine-needle aspiration biopsies revealed only the adenocarcinoma component. Table 2 describes the tumor characteristics of the ASCAP cohort.
Table 2.
Tumor characteristics on diagnosis and outcomes of patients with ASCAP
| Case | Location | Greatest dimension (cm) | Histologic grade | Margin | Adeno (%) | Squamous (%) | PNI/LVI | Pathologic stage | HPV status | Adjuvant therapy | Survival (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tail | 7 | Moderate to poor | R1 | 10 | 90 | Y/N | T3N1 | Neg | Unknown | 20 |
| 2 | Tail | 3.5 | Moderate | R0 | 20 | 80 | Y/Y | T2N0 | Neg | Yes (Chemo + EBRT) | Lost to f/u |
| 3 | Body-Tail | 9.5 | Moderate to poor | R1 | 60 | 40 | Y/Y | T3N1 | Neg | Unknown | 4.5 |
| 4 | Body | 3 | Moderate | R0 | 70 | 30 | Y/N | T3N1 | Neg | No | 3 |
| 5 | Tail | 3.5 | Moderate to poor | R2 | 70 | 30 | Y/Y | T3N1 | Neg | Unknown | 3 |
| 6 | Tail | 3 | Poor | R0 | 5 | 95 | Y/N | T3N1 | Neg | Unknown | Lost to f/u |
| 7 | Head | 3.4 | Moderate | R0 | 10 | 90 | Y/N | T2N0 | Neg | No | 25 |
PNI: perineural invasion; LVI: lymphovascular invasion; Y: yes; N: no; HPV: human papilloma virus; Neg: negative; Pos: positive; EBRT: external beam radiotherapy.
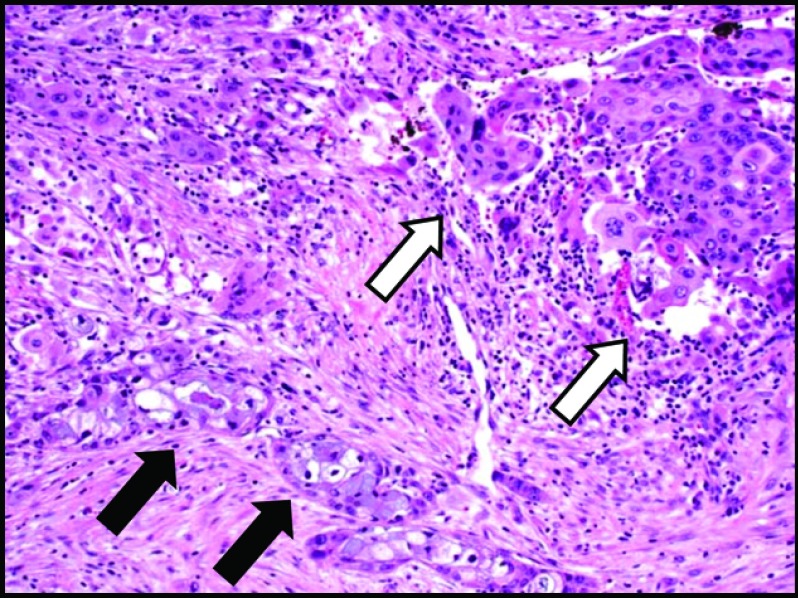
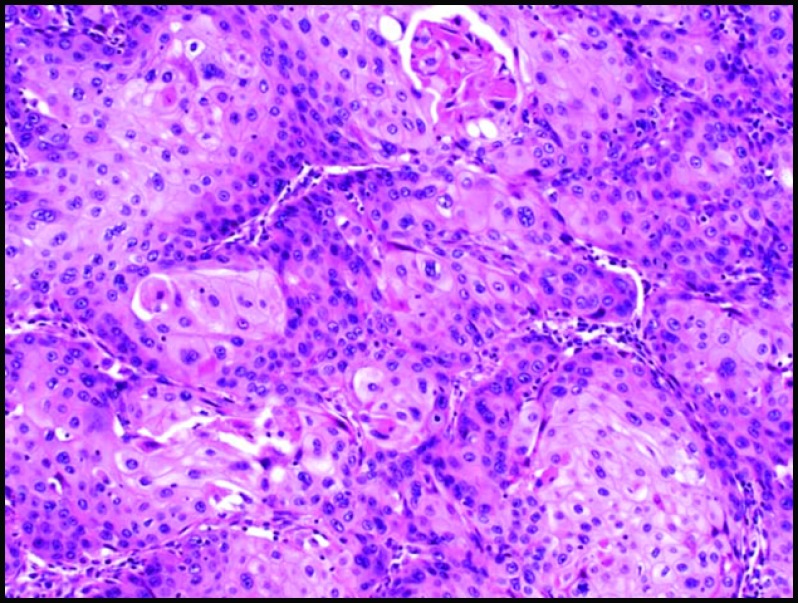
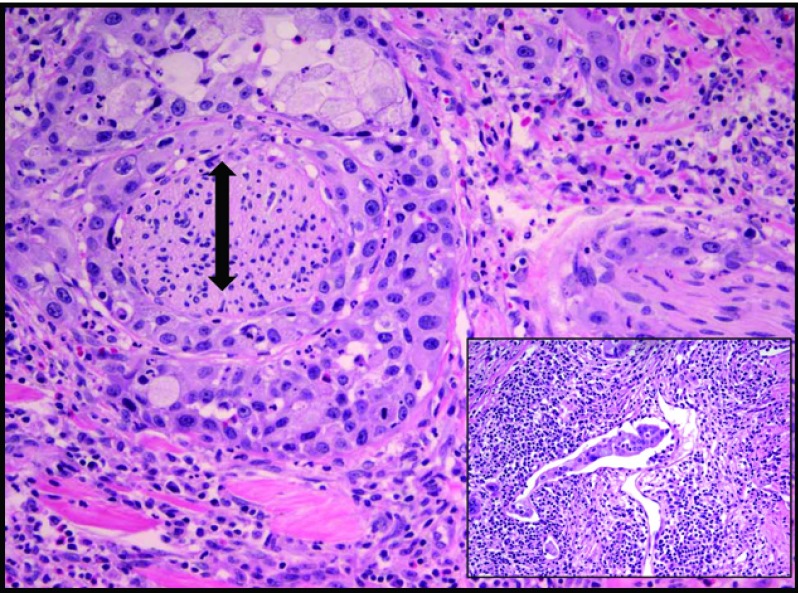
Pathologic review of the ASCAP cases confirmed that the proportion of squamous component ranged from 30% to 95%, with 4 of 7 cases showing a predominance of this element over the adenocarcinoma constituent (Figures 1 and 2). Most tumors were histologically moderate or poorly differentiated, with perineural invasion identified in all cases. Lymphovascular invasion, sometimes involving large-caliber vessels, was documented in 3 of the 7 cases (Figure 3). Lymph node metastases were found in 5 cases, with 3 showing only involvement by adenocarcinoma and the remaining 2 demonstrating a mixture of adenocarcinoma and squamous cell carcinoma. Of note, the lymph nodes with both adenocarcinoma and squamous cell carcinoma components were involved by direct extension in contrast to those solely involved by adenocarcinoma, which were separate from the main body of the tumor. Examination of the surrounding pancreatic tissue revealed pancreatic intraductal neoplasia (PanIN) in 2 cases and extensive intraductal papillary mucinous neoplasm (IPMN) in 1 case.
Figure 1.
Glandular component (black arrows) and squamous cell component (white arrows) in ASCAP (H&E stain, 10× magnification).
Figure 2.
Squamous cell carcinoma component with dyskeratotic cells (H&E stain, 10× magnification).
Figure 3.
Predominately squamous cell carcinoma component with extensive perineural invasion (double-headed arrow). Inset: focus of lymphovascular invasion (H&E stain, 20× magnification).
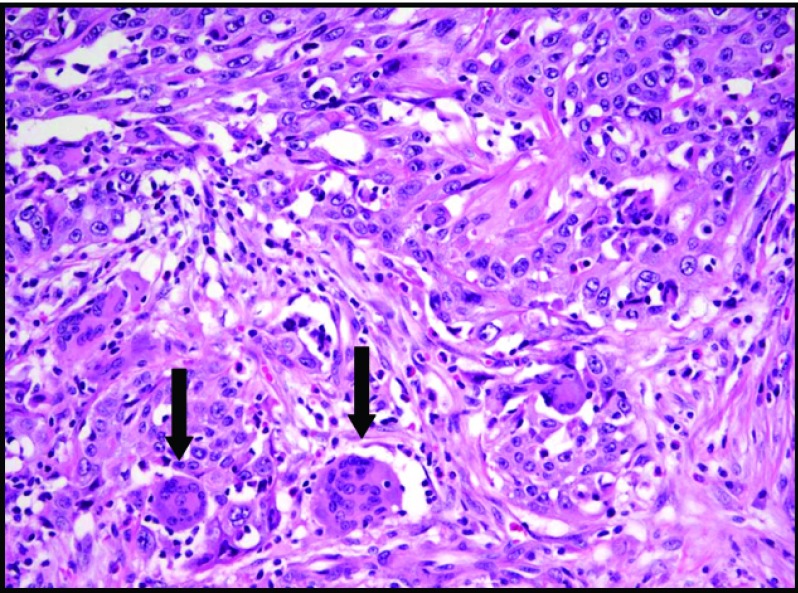
Although all 7 cases met the aforementioned qualifications for the diagnosis of adenosquamous carcinoma of the pancreas, 2 were particularly distinct. One contained a striking inflammatory component as well as numerous multinucleated giant cells (Figure 4), and another demonstrated a prominent Crohn-like lymphoid infiltrate with extensive areas of dense sclerosis. Neither of these patients had underlying diagnosed autoimmune conditions.
Figure 4.
Prominent inflammatory infiltrate within ASCAP and abundant multinucleated giant cells (arrows) (H&E stain, 20× magnification).
Most ASCAP tumors (86%) were located in the body and/or tail of the pancreas. Surgical margins were R0 (n = 4), R1 (n = 2), and R2 (n = 1). Per the AJCC (American Joint Committee on Cancer) 7th edition staging system, ASCAP was either stage IB (T2N0M0; n = 2) or IIB (T3N1M0; n = 5).
Median follow-up for all patients was 2.9 years after surgery. Median overall survival (OS) was worse for ASCAP than for ACP (8.2 vs. 20.4 months; P = .23). The range of survival for the ASCAP cohort was 3 to 25 months. None of the ASCAP patients received preoperative therapy. Postoperative adjuvant therapy was recommended to most of the patients, but was confirmed to have been received by only 1 with ASCAP.
DISCUSSION
ASCAP remains an uncommon and poorly understood malignancy, with a reportedly worse prognosis than ACP. Ethnic predisposition to ASCAP initially favored Asian populations,3 but more recent reports, including this series, may make this variable alone less reliable. The lack of identification of any ASCAP features on diagnostic fine-needle aspiration coupled with the relative paucity of patients with pancreatic cancer who are surgical candidates may result in underreporting of the disease.
The presentation of symptoms, including abdominal pain, weight loss, and jaundice, was consistent with previous reports of ASCAP and is very similar to that of ACP.5,6,10 Okabayashi and Hanazaki7 reviewed 39 patients who underwent resection for ASCAP and did not note any characteristic symptoms that would help facilitate the differentiation between ASCAP and ACP, making the diagnosis reliant on the histopathologic findings in the resection specimens.
Our ASCAP cohort demonstrated a predilection to present in the pancreatic tail and body more than in the head. In contrast, Kardon et al5 and Trikudanathan et al8 noted that a majority of patients had the primary tumor located in the head of the pancreas. A population-based analysis by Boyd et al6 described 45% of ASCAP tumors as located in the head of the pancreas. Taken together, ASCAP may not have a specific preferential site within the pancreas; rather, our cohort was biased toward tail locations, given that all our patients underwent attempts at definitive surgical resection. In their histologic analysis of 25 patients with ASCAP, Kardon et al5 found 20 tumors to have evidence of perineural and angiolymphatic invasion; the authors suggested that these features may contribute to the aggressive nature of this malignancy.5 In our cohort, all (7/7) patients had evidence of perineural invasion, and 3 had evidence of angiolymphatic invasion (Figure 3).
It has been proposed that squamous metaplasia of the pancreatic ductal epithelium, occurring in the setting of chronic pancreatitis, contributes to the development of ASCAP.4,8 Half of the patients in our cohort were noted to have pathologic changes of chronic pancreatitis, although, interestingly, no patients had this as a clinically recognized medical condition. Three patients reported occasional alcohol use on retrospective review, of whom all were noted to have histologic evidence of chronic pancreatitis. These findings support the hypothesis that subclinical pancreatitis is associated with or possibly a causative risk factor for ASCAP, with or without prior alcohol use. Although not statistically different, tobacco use appeared common in patients with ASCAP and ACP, perhaps mediated by aggressive biologic behavior affecting the Src pathway through nicotine.11
The role of HPV in squamous cell carcinomas of the cervix, head and neck, and anal canal is well established. An association has never been reported or evaluated in ASCAP. HPV status in our cohort was determined through standard ISH analysis and was uniformly negative. Thus, the development of ASCAP is more likely to be related to underlying inflammation or other carcinogenic etiologies and not to HPV infection.
It has been shown that CA 19-9 and CEA have immunoreactivity in both the squamous and adenocarcinoma components in pancreatic malignancies. Studies have shown that more than 70% of patients with ASCAP have elevated serum CA 19-9 and CEA levels.8 In this cohort series, 4 (57%) of 7 patients had elevated CA 19-9 levels at diagnosis. CEA was available in only 1 patient and was elevated. These trends are consistent with those in prior research and also illustrate that adenosquamous carcinoma is similar to adenocarcinoma in reactivity of common serum tumor markers.
Kobayashi et al12 and Inoue et al13 reported humoral hypercalcemia of malignancy (HHM) in ASCAP, which was associated with a particularly poor prognosis. Both studies commented on the rarity of HHM in ASCAP and proposed that a possible origin for the elevated PTH-rp and subsequent hypercalcemia was from production in the coexisting glandular component, which is associated with rapid tumor growth and short survival. No patient in our ASCAP cohort showed evidence of HHM, yet 3 had a survival of less than 6 months. Thus, we cannot determine whether HHM is truly prognostic of poor survival beyond other biologic variables.
Madura et al1 discussed a series of 6 cases over an 8-year period that demonstrated the inherent aggressiveness of the tumor despite intensive surgical management, with a median OS of 5 months. Similarly, Hsu and colleagues3 reported that 11 of 12 patients with ASCAP died within 12 months despite aggressive surgical management along with adjuvant chemotherapy and radiation, with a median OS of 4.41 months and a 1-year OS rate of 8.3%. However, several of the patients in these studies had metastatic disease at diagnosis. In 39 patients with ASCAP who had undergone curative surgical resection, Okabayashi and colleagues7 noted a worse 3-year OS rate of 14% (median OS, 6.8 months) than that of ACP. Voong et al14 reviewed outcomes of 38 patients with ASCAP at Johns Hopkins and reported a median OS of 10.9 months (range, 2.1–140.6). Adjuvant chemoradiation therapy was associated with superior overall survival in patients with ASCAP (P = .005). Seventy-five percent of patients who survived longer than 2 years received adjuvant chemoradiation. Smoot et al15 reported that patients with ASCAP who underwent curative resection had a median survival of 13.1 months, but 0% survival at 2 years. Katz et al16 performed a historical analysis of cases in the California Cancer Registry database, of which 95 were ASCAP and 14,746 were ACP. In contrast to other reports, as a group, the median overall survival of all patients with ASCAP was 4 months (95% CI, 3–6), which was similar to that of all patients with ACP (P = .41). In addition, the median overall survival duration of all patients with ASCAP was similar to that of all patients with ACP after adjustment for age, sex, ethnicity, socioeconomic status, stage of disease, and first treatment strategy (HR = 1.091; 95% CI, 0.870–1.367; P = .45). In patients with locoregional cancers who underwent resection, the median overall survival of patients with each histopathologic diagnosis was similar after adjustment for those variables and the receipt of adjuvant therapy (HR, 0.886; 95% CI, 0.530–1.482; P = .65). Patients who underwent resection had an improved median OS of 12 months (95% CI, 8–52), compared with 5 months (95% CI, 1–12) for those who did not (P = .018). This analysis showed that early stage, resection, and radiation or chemotherapy were favorable independent prognostic factors among patients with adenosquamous carcinoma. Following resection, Boyd and colleagues6 reported 1- and 2-year OS rates of 50.7% and 29%, respectively—significantly inferior to rates in ACP patients (60.1% and 35.8%, respectively; P < .0001). Table 3 summarizes the outcomes of ASCAP patients in the published literature. Given the lack of prospective studies, the true benefit of adjuvant therapy beyond surgical resection for this population of patients remains unclear.
Table 3.
Outcomes of resected ASCAP from published case reports and series
| Author | Total ASCAP patients (n) | Treatment |
Survival (ASCAP) |
Median ACP OS (resected, mo) | ||
|---|---|---|---|---|---|---|
| Curative surgery |
Adjuvant chemo and/or radiation (n) | Median OS |
2 year OS |
|||
| (n) | (mo) | (%) | ||||
| Madura et al1 | 6 | 6 | 3 | 5 | 0 | NR |
| Hsu et al3 | 12 | 7 | 5 | 6.5 | 0 | 9.7 (P = 0.018) |
| Kardon et al5 | 25 | 8 | 2 | 11.3 | NR | NR |
| Okabayashi and Hanazaki7 | 39 | 39 | 15 | 6.8 | 14 | NR |
| Voong et al14 | 38 | 38 | 19 | 10.9 | 11 | NR |
| Smoot et al15 | 23 | 12 | 5 | 13.1 | 0 | NR |
| Katz et al16 | 95 | 26 | 25 | 12 | 1.2 | 12 |
| Boyd et al6 | 415 | 86 | NR | 12 | 29 | 16 (P < 0.0001) |
NR, not reported.
In our cohort of patients with resected tumors, the median OS of 8.2 months was notably shorter than the 20.4 months of contemporary ACP cohort patients (3-year OS 0 vs. 19%), but the difference was not statistically significant (P = .23). Three patients survived less than 4 months and 2 survived 20 months or more. One patient left the country after surgery and the status is unknown. One patient died of immediate postoperative complications including prolonged respiratory failure, gastrointestinal bleeding, and sepsis. However, in this particular case, the tumor likely had high potential for recurrence, given rapid tumor growth, as this mass was not evident on CT scan 3 months before the diagnosis. Although definitive surgical resection offers the only chance for cure and is likely to prolong survival, the impact of adjuvant chemoradiotherapy in ASCAP is less well defined.
A clear statistical limitation of our study is reflected in the small sample size (n = 7) of patients with ASCAP who underwent resection, for whom limited adjuvant treatment data are available. We deliberately focused on outcomes only in the cohort of operable patients with pancreatic cancer at our institution. With direct comparisons to outcomes in ACP patients during an identical period, survival of patients with ASCAP relative to ACP may be more clinically relevant than that in the metastatic or unresectable settings. Having secondary pathologic confirmation of ASCAP diagnostic criteria in our cohort strengthens our confidence in the true diagnosis, although again, the limited sample size and inconsistent postoperative treatment and follow-up limit conclusive results.
CONCLUSIONS
ASCAP remains a very uncommon and poorly understood exocrine pancreatic neoplasm. It may be underreported, given the diagnostic limitations of fine-needle aspiration. Although there is variability in the published literature regarding the true prognosis, it appears to be worse than in ACP, but it is still potentially curable. Surgical resection remains the best therapeutic option for patients with ASCAP, with consideration of adjuvant or potentially neoadjuvant therapy. Although further research to determine specific tumor genetic markers, the role of neoadjuvant or adjuvant treatment, and potentially targeted therapy is recommended, identification of risk factors and primary prevention may be a more optimal goal.
Acknowledgments
Presented in part at the 2013 Gastrointestinal Cancers Symposia, San Francisco, CA, January 24–26, 2013.
Footnotes
Funding for the study was provided through the University of Florida Department of Medicine Young Investigator Award.
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Madura JA, Jarma BT, Doherty MG, et al. : Adenosquamous carcinoma of the pancreas. Arch Surg 134:599–603, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Rahemtullah A, Misdraji J, Pitman MB: Adenosquamous carcinoma of the pancreas cytologic features in 14 cases. Cancer Cytopathol 99;372–378, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Hsu J, Han-Ming C, Ren-Chin W, et al. : Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol 6:95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skafida E, Grammatoglou X, Glava C, et al. : Adenosquamous carcinoma of the pancreas: a case report. Cases J 3:41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kardon DE, Thompson LD, Przygodzki RM, et al. : Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol 14;443–451, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. : 415 Patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res 174:12–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okabayashi T, Hanazaki T: Surgical outcomes of adenosquamous carcinoma of the pancreas. World J Gastroenterol 14;6765–6770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trikudanathan G, Dasanu CA: Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J 103;903–908, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Terada T: Adenosquamous carcinoma and pure squamous cell carcinoma of the pancreas: report of two cases. Case Reports Gastroenterol 4;369–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Na YJ, Shim KN, Cho MS, et al. : Primary adenosquamous cell carcinoma of the pancreas: a case report with a review of the Korean literature. Korean J Int Med 26:348–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Treviño JG, Pillai S, Kunigal S, et al. : Nicotine induces inhibitor of differentiation-1 in a Src-dependent pathway promoting metastasis and chemoresistance in pancreatic adenocarcinoma. Neoplasia 14;1102–1114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi N, Higurashi T, Iida H, et al. : Adenosquamous carcinoma of the pancreas associated with humoral hypercalcemia of malignancy (HHM). J Hepatobiliary Pancreatic Surg 15:531–535, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Inoue T, Nagao S, Tajima H, et al. : Adenosquamous pancreatic cancer producing parathyroid related protein. J Gastroenterol 39:176–180, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Voong K, Davison J, Pawlik TM, et al. : Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol 41:113–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smoot RL, Zhang L, Sebo TJ, et al. : Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg 207;368–370, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Katz MH, Taylor TH, Al-Refaie WB, et al. : Adenosquamous versus adenocarcinoma of the pancreas: a population-based outcome analysis. J Gastroenterol Surg 15:165–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]