CASE REPORT
A 57-year-old Caucasian male with no significant medical history was admitted to the intensive care unit and intubated for airway protection after he was found unresponsive at home, followed by a grand mal seizure en route to the hospital. The patient, who had recently won a body-building contest, had no other complaints except for recent unintentional weight loss of 20 pounds and right shoulder pain. He had no history of alcohol or tobacco use and was not on any medications. A screening colonoscopy in 2010 was normal. Findings in the admission laboratory work-up were normal except for mild hyponatremia (sodium, 127 mmol/L) and elevated alkaline phosphatase (ALP, 184 U/L). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain (Figure 1) revealed multiple ring enhancing lesions of various sizes within both the cerebral and cerebellar hemispheres, with minimal edema. Contrast CT of the chest and abdomen/pelvis (Figure 2) showed an infiltrating mass in the neck, body, and tail of the pancreas, with arterial and venous encasement, along with multiple lesions in the lung and liver suspicious for metastases. A CT-guided biopsy of the liver lesions revealed a poorly differentiated carcinoma with immunohistochemistry positive for CK7 and polyclonal CEA (carcinoembryonic antigen) and negative for CK20, CDX-2, TTF-1, and Hep Par 1.
Figure 1.
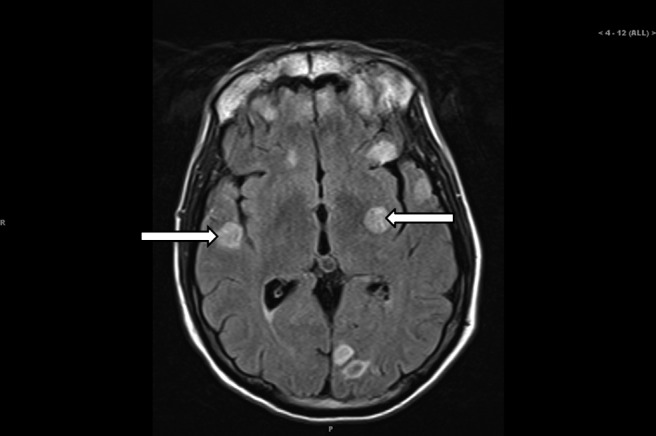
Brain MRI showing multiple ring enhancing masses (arrows).
Figure 2.
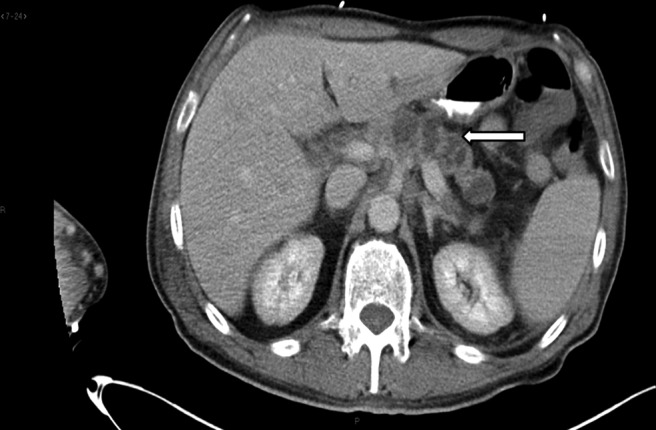
CT scan of the abdomen showing an infiltrating pancreatic neoplasm involving the neck, body, and tail encasing the celiac axis and portal venous system (arrow).
Given the markedly elevated CA 19-9 (21,080 U/mL) and the clinical and radiologic findings, the patient was given a diagnosis of metastatic pancreatic cancer. A bone scan performed for elevated ALP and persistent right shoulder pain demonstrated increased uptake in multiple areas, suggesting diffuse skeletal metastases. Following extubation and during recovery on the oncology floor, the patient had a sudden onset of weakness in his right leg, neck stiffness, and photophobia and an episode of urinary incontinence. An MRI of the spine (Figure 3) revealed enhancement of multiple nerve roots of the cauda equina suggestive of carcinomatous meningitis. A repeat MRI of the brain showed findings suspicious for leptomeningeal involvement. An analysis showed clear cerebrospinal fluid (CSF), 32 RBCs, 6 WBCs, 3% neutrophils, and 55% lymphocytes, with an elevated protein at 84 mg/dL (range, 12–45). CSF cytology showed rare atypical cells.
Figure 3.
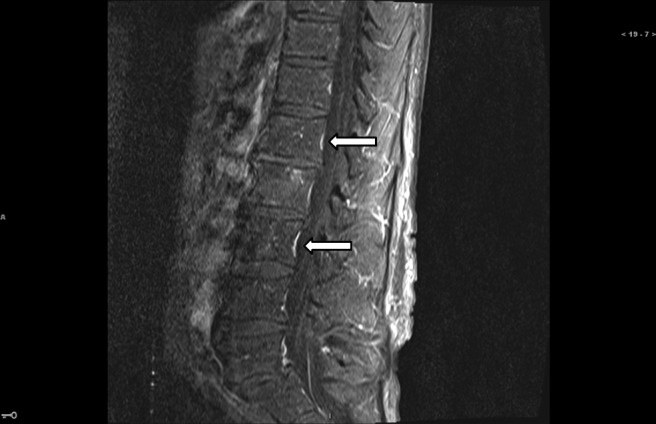
Spine MRI showing multiple roots of the cauda equina.
A diagnosis of metastatic leptomeningeal carcinomatosis (LC) from primary pancreatic cancer was based on the clinical, radiologic (MRI), and CSF findings. After a lengthy discussion regarding the extent of his disease and the prognosis, the patient expressed his desire to pursue treatment as long as he could tolerate it and then a preference for transition to hospice. He underwent whole-brain radiation and palliative radiation to the spine and right shoulder. Intrathecal chemotherapy was deferred because of potential toxicity with concurrent radiation therapy and because of the urgent need to start systemic chemotherapy for systemic control of the disease. After completion of radiation therapy, he received palliative chemotherapy with the FOLFIRINOX regimen (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin), with minimal side effects. After two cycles of chemotherapy, he showed significant deterioration in performance status and was appropriately transitioned to hospice care.
DISCUSSION
Pancreatic cancer is commonly seen in the elderly population and is the fourth most common cause of cancer-related deaths in the United States.1 Unfortunately, less than 20% of patients present with localized, potentially curable tumors. With a high annual incidence of mortality, the 5-year overall survival rate is <5%, with most patients dying within 2 years of diagnosis.1,2 Despite the recent advances that have been made with regard to the molecular biology, diagnosis, staging, and treatment of early-stage pancreatic cancer, progress has been limited in the areas of prevention, screening, and treatment of advanced-stage disease. About 40% of the patients are diagnosed with locally advanced disease and another 40% with metastatic disease, with the most common sites of metastasis being the liver and peritoneum, followed by the lung.3 Incidence of skeletal metastasis is between 5% and 20%.3 Metastases to the brain and central nervous system (CNS) is very rare (∼0.3%).4 On our literature review through Medline, we found only 6 other cases of pancreatic cancer with leptomeningeal metastasis.5–10 In all cases, LC was found later as the disease progressed, with onset ranging from months to years. Based on the literature review, we believe that ours is the first described case of pancreatic cancer with LC at the initial presentation and also the first reported case of pancreatic cancer with synchronous carcinomatosis meningitis and intraparenchymal brain metastasis.
First described by Eberth10 as early as 1870, LC is the infiltration of the leptomeninges by malignant cells. Previously considered to be an uncommon manifestation of malignancy, the incidence of LC appears to be increasing due to improvement in imaging modalities, such as MRI, and the longer survival of cancer patients. LC occurs in 5% to 8% of cancer patients, and studies have found that 20% of patients with neurologic manifestations are found to have LC at autopsy. LC represents advanced disease and carries a poor prognosis. Most commonly observed with solid tumors, such as breast, lung, melanoma, and gastrointestinal malignancies and cancers of unknown primary site,11,12 LC is also reported in some leukemias and lymphomas. Sporadic cases have been reported in head and neck, cervical, ovarian, renal, and bladder cancer.11,12 Leptomeningeal infiltration can occur from either hematogenous or lymphatic spread or by direct extension from pre-existing malignant lesions.10
Patients can present with various neurologic signs and symptoms, depending on the site of leptomeningeal invasion. Symptoms are due to CSF flow obstruction or to direct tumor infiltration and can present as neck rigidity, headache, and other signs of meningismus. Cerebral involvement can present with encephalopathy and seizures. Cranial nerve involvement usually manifests as diplopia but multiple cranial nerves can be involved, with occulomotor, trochlear, abducens, and facial being the most common.13 In addition, patients can present with leg weakness and bladder and bowel incontinence if the lumbosacral roots are involved.14 A high index of suspicion and thorough clinical examination can identify early signs of central nervous system (CNS) involvement.10,14 The diagnosis of LC, however, can sometimes be difficult and challenging. It typically requires the demonstration of malignant cells in the CSF. Gadolinium-enhanced, T1-weighted images can support the diagnosis in patients with negative cytology.14 CSF analysis and MRI are complementary, and the use of both increases diagnostic accuracy.15 Research has shown that MRI is more sensitive (76%–87%) than a single CSF cytologic examination.15
Typical MRI findings in the brain include thin, diffuse leptomeningeal contrast enhancement, multiple nodular deposits in the subarachnoid space, cerebellar folia, or cortical surface and tumor masses, especially at the base of the brain, with or without hydrocephalus.15,16 It is important to remember that CSF cytology will detect some cases that are not visible on MRI and vice versa. Malignant cells are found in the initial CSF sample in approximately 50% of patients with LC, and repeated cytologic examination has been found to improve the yield to approximately 90% after the third sample.13,14 Flow cytometry of CSF can be performed when LC is suspected in cases of leukemia and lymphoma. At the time of lumbar puncture, the elevated CSF opening pressure, elevated protein level, and/or a decrease in glucose level in the CSF and, in the appropriate clinical context, levels of specific tumor markers can help in the diagnosis.17
The treatment goal in LC is to improve or stabilize neurologic function and thus improve the quality of life. Unfortunately, very often patients with LC have limited life expectancy. Treatment decisions are based on risk stratification, where poor risk is defined as having a Karnofsky Performance Status (KPS) <60, multiple fixed neurologic defects, extensive systemic cancer without good treatment options, encephalopathy, or bulky CNS disease (NCCN Guidelines, version 1.2013). For these patients, palliative care is suggested unless the patient has a chemosensitive malignancy such as small cell lung cancer or lymphoma.14 For patients with good risk features, treatment options include intrathecal chemotherapy. Methotrexate and liposomal cytarabine are the most commonly used chemotherapy agents. Thiotepa, is an alternate choice, but used less frequently due to its short CSF half life.10,17 Superiority over systemic treatment has not been established in randomized, controlled trials. IT chemotherapy can be given either via lumbar puncture (LP) or, preferably, through an intraventricular (Ommaya reservoir) route.10,17 External beam radiation can be used to treat symptoms due to a radiographically bulky tumor. Skull base radiation has shown to be useful in patients with cranial neuropathies.11,15 Prognosis in LC depends on tumor histology, performance status, and systemic disease burden. Studies have found that patients with hematologic malignancies and breast cancer have a relatively better prognosis than do those with other tumors.12,14,16 Multiple studies have shown that, despite treatment with currently available chemotherapy and radiation therapy, the median survival for these patients is dismal at 3 to 6 months (These studies mainly comprised of cases of LC from breast cancer, lung cancer and melanoma).14,18,19,20
We would like to highlight this unique presentation of pancreatic cancer and also to emphasize that ours is the first case presenting with synchronous LC and intraparenchymal brain metastatic disease. It is still reasonable to consider aggressive therapy in patients who have a good performance status, in an attempt to improve their quality of life. Transition to hospice is appropriate when the overall condition deteriorates as the median overall survival in this clinical condition is poor.
CONCLUSIONS
LC associated with pancreatic cancer is extremely rare and can have a devastating impact on the prognosis and quality of life. Hence, neurologic manifestations in patients with pancreatic cancer should prompt an early work-up since therapy in carefully selected patients is critical in preserving neurologic function and quality of life.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Siegel R, Naishadham A, Jemal A: Cancer statistics. CA Cancer J Clinic 2013 [DOI] [PubMed] [Google Scholar]
- 2. Hidalgo M: Pancreatic cancer. N Engl J Med 362:1605–1617, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Borad MJ, Saadati H, Lakshmipathy A, et al. : Skeletal metastases in pancreatic cancer: a retrospective study and review of the literature. Yale J Biol Med 82:1–6, 2009 [PMC free article] [PubMed] [Google Scholar]
- 4. Park KS, Kim M, Park SH, et al. : Nervous system involvement by pancreatic cancer. J Neurooncol 63;313–316, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Yochikazu Y, Yasuaki N, Shigekazu N, et al. : A case of meningeal carcinomatosis from pancreatic cancer during chemotherapy using gemcitabine. Jpn J Gastroenterol Surg 39;1683–1688, 2006 [Google Scholar]
- 6. Hirota M, Yagi Y, Yamashita K, et al. : A long survival case of unresectable pancreatic cancer by chemo-radiotherapy with gemcitabine as the key drug (in Japanese). Gan To Kagaku Ryoho 35;2413–2416, 2008 [PubMed] [Google Scholar]
- 7. Kurzaj E, Kopczynski S, Barowska-Lehman J, et al. : Subdural haematoma associated with dural carcinomatosis in a patient with primary carcinoma of the pancreas. Neurochirurgia (Stuttg) 23:13–17, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Galatioti S, Savettieri G: Meningeal carcinomatosis secondary to a primary pancreatic tumour (in Italian). Acta Neurol (Napoli) 30:359–367, 1975 [PubMed] [Google Scholar]
- 9. Minchom A, Chan S, Melia W, et al. : An unusual case of pancreatic cancer with leptomeningeal infiltration. J Gastrointest Cancer 41:107–109, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira Filho AF, Cardoso F, Di Leo A, et al. : Carcinomatosis meningitis as a clinical manifestation of pancreatic carcinoma. Ann Oncol 12;1757–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Pavlidis N: The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol 15(Suppl 4):285–291, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Kaplan JG, DeSouza TG, Farkash A, et al. : Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 9:225–229, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Kesari S, Batchelor TT: Leptomeningeal metastases. Neurol Clin 21:25–66, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Wasserstrom WR, Glass JP, Posner JB: Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 49:759–772, 1982 [DOI] [PubMed] [Google Scholar]
- 15. Drappatz J, Batchelor T: Leptomeningeal metastasis. American Society of Clinical Oncology Educational Book. Alexandria, VA: American Society of Clinical Ongolocy, 2009, pp 100–105 [Google Scholar]
- 16. Straathof CS, de Bruin HG, Dippel DW, et al. : The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol 246:810–814, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Leal T, Chang JE, Mehta M, et al. : Leptomeningeal metastasis: challenges in diagnosis and treatment. Curr Cancer Ther Rev 7;319–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfeffer MR, Wygoda M, Siegal T: Leptomeningeal metastases-treatment results in 98 consecutive patients. Isr J Med Sci 24:611–618, 1988 [PubMed] [Google Scholar]
- 19. Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. : Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 116:561–569, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Glantz MJ, LaFollette S, Jaeckle KA, et al. : Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol 176:3110–3116, 1999 [DOI] [PubMed] [Google Scholar]


