Summary
All archaeal genomes encode RNA polymerase (RNAP) subunits E and F that share a common ancestry with the eukaryotic RNAP subunits A43 and A14 (Pol I), Rpb7 and Rpb4 (Pol II), and C25 and C17 (Pol III). By gene replacement, we have isolated archaeal mutants of Thermococcus kodakarensis with the subunit F-encoding gene (rpoF) deleted, but we were unable to isolate mutants lacking the subunit E-encoding gene (rpoE). Wild-type T. kodakarensis grows at temperatures ranging from 60 to 100 °C, optimally at 85°C, and the ΔrpoF cells grew at the same rate as wild-type at 70 °C, but much slower and to lower cell densities at 85 °C. The abundance of a chaperonin subunit, CpkB, was much reduced in the ΔrpoF strain growing at 85 °C and increased expression of cpkB, rpoF or rpoE integrated at a remote site in the genome, using a nutritionally-regulated promoter, improved the growth of ΔrpoF cells. RNAP preparations purified from ΔrpoF cells lacked subunit F and also subunit E and a transcription factor TFE that co-purifies with RNAP from wild-type cells, but in vitro, this mutant RNAP exhibited no discernible differences from wild-type RNAP in promoter-dependent transcription, abortive transcript synthesis, transcript elongation or termination.
Keywords: Archaea, RNA polymerase subunits E and F, ΔrpoF, TFE, Thermococcus kodakarensis
Introduction
Archaea have only single-type of RNA polymerase (RNAP) which is most similar to eukaryotic RNAP II [Pol II; (Kwapisz et al., 2008; Thomm, 2007; Werner, 2007)]. Archaeal promoter recognition and basal transcription initiation in vitro requires only two transcription factors, TBP and TFB, archaeal homologs of eukaryotic TATA-box binding protein and transcription factor IIB. All archaeal genomes also encode TFE and TFS, proteins with sequence homologies to the α-subunit of TFIIE and to TFIIS, respectively (Bell and Jackson, 1998; Ouhammouch, 2004). TFE stimulates transcription initiation in vitro from some promoters, and under sub-optimal conditions (Bell et al., 2001; Hanzelka et al., 2001), and has been shown to participate in promoter DNA melting and early transcript elongation (Grunberg et al., 2007; Micorescu et al., 2008; Naji et al., 2007). TFS stimulates cleavage of transcripts present in stalled elongation complexes in vitro (Lange and Hausner, 2004).
The structure of an archaeal RNAP purified from Sulfolobus solfataricus has been determined by X-ray crystallography at 3.4 Å resolution. This enzyme has a nine subunit core plus an extended stalk, formed by subunits E and F, that protrudes from the base of the core polymerase-clamp domain (Hirata et al., 2008). Studies of archaeal RNAP sub-complexes reconstituted from purified subunits have established that a nine-subunit core is capable of promoter-dependent transcription initiation and transcript synthesis (Ouhammouch et al., 2004; Werner and Weinzierl, 2002), but the reconstituted core initiated transcription much less efficiently than a complete enzyme at low temperatures (Naji et al., 2007). TFE co-purifies through multiple steps with certain archaeal RNAPs (Micorescu et al., 2008; Santangelo et al., 2007) and in vitro reconstitution of RNAP subcomplexes has identified the E subunit as being required for TFE interactions with archaeal RNAP (Naji et al., 2007). Consistent with this, transcription initiation by an archaeal RNAP core, lacking subunits E and F, was not stimulated by TFE (Ouhammouch et al., 2004).
The three eukaryotic RNAPs (Pol I, II and III) also have a catalytic core plus two-subunit stalk structure with the extension formed by polypeptides that share common ancestries with the archaeal E and F subunits, namely A43 and A14 in Pol I, Rpb7 and Rpb4 in Pol II, and C25 and C17 in Pol III (Armache et al., 2003; Bushnell and Kornberg, 2003; Fernandez-Tornero et al., 2007; Jasiak et al., 2006; Kuhn et al., 2007). The Rpb7 plus Rpb4 structure in Pol II is most similar to the archaeal E plus F stalk but, unlike the archaeal RNAP core, Pol II requires Rbp7 plus Rpb4 for promoter-dependent transcription initiation in vitro (Edwards et al., 1991; Orlicky et al., 2001). Mutagenesis has established that Rpb7 is essential in yeast but that Rpb4 is only essential under stress conditions, for growth above 34°C or below 12°C, and for retaining viability during starvation (Choder and Young, 1993; McKune et al., 1993; Woychik and Young, 1989). In the absence of Rpb4, a global reduction was observed in yeast gene expression at high temperature (Miyao et al., 2001), although loss of Rbp4 has also been reported to change the expression of a much smaller number of genes under both normal growth and stress conditions (Pillai et al., 2003). Over-expression of Rpb7 has been shown to suppress some yeast phenotypes resulting from the loss of Rpb4 (Sheffer et al., 1999).
Given the in vitro assembly results and the close relationships of the archaeal and eukaryotic RNAP subunits, it was of interest and importance to determine if the archaeal RNAP subunits E and F were essential for viability but, without genetics, this was impossible. With the development of genetics for Thermococcus kodakarensis, this technical barrier was removed (Matsumi et al., 2007; Sato et al., 2003, 2005), and here we report the isolation of a T. kodakarensis mutant with the subunit F encoding gene deleted [ΔrpoF; (ΔTK0901)]. Growth defect associated from ΔrpoF mutation was investigated, and transcription activity of the mutant RNAP purified from ΔrpoF cells was characterized by in vitro transcription assay.
Results
Deletion of the T. kodakarensis subunit F-encoding gene (TK0901)
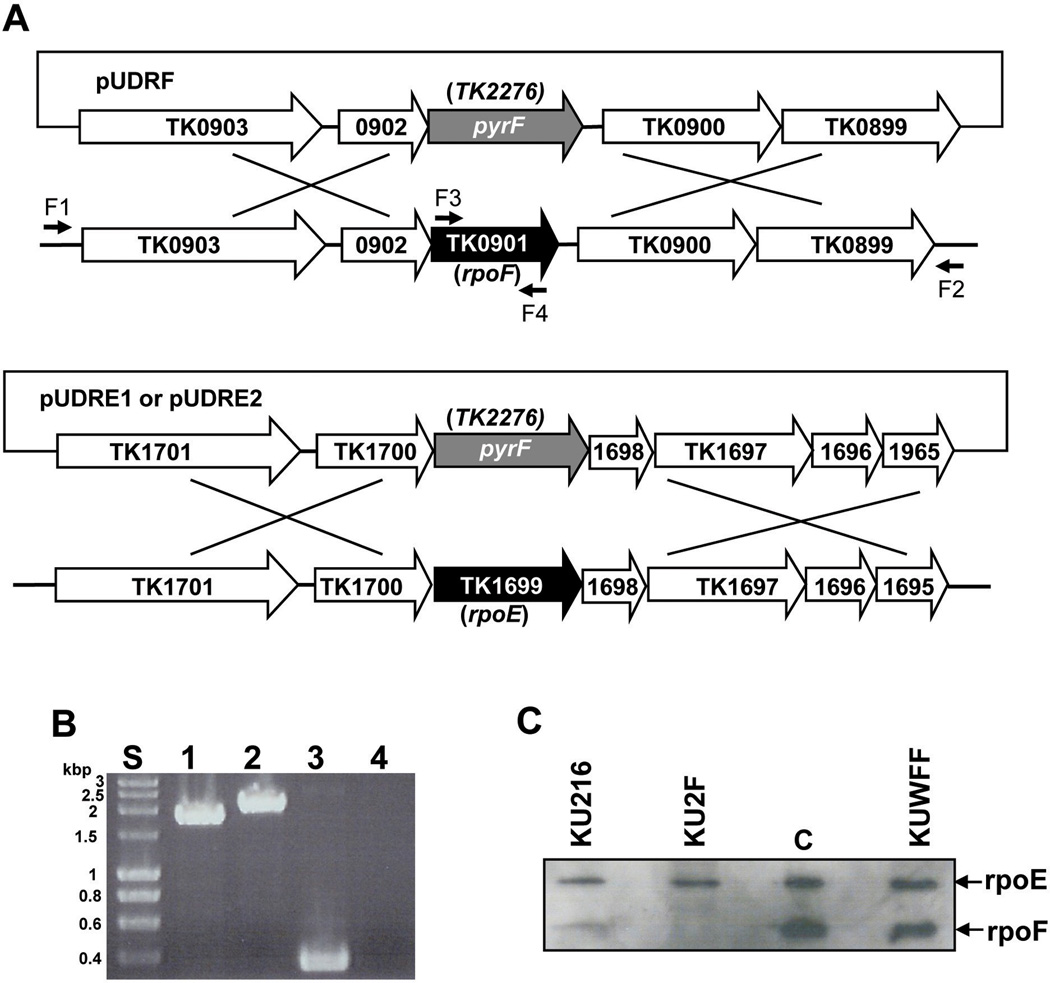
In the T. kodakarensis genome, TK0901 encodes the RNAP subunit F and appears to be the second gene in a two-gene operon in which TK0902 encodes a ribosomal protein, L21 (Fig. 1A top). To construct plasmid pUDRF, TK0901 was replaced by pyrF (TK2276) and this was flanked by ~700 bp of upstream sequence (TK0903-TK0902) and ~700 bp of downstream intergenic plus TK0900-TK0899 sequence (Fig. 1A; Table 1). Plasmid pUDRF DNA was used to transform T. kodakarensis strain KU216 (ΔpyrF) and transformants were selected by their ability to grow in the absence of uracil (Sato et al., 2003, 2005). All of the ~20 transformants screened that grew without uracil at 85 °C had an intact TK0901 that confirmed by PCR based genotyping. Most likely, this resulted from a single crossover event of the entire pUDRF sequence integrated into the T. kodakarensis genome, and the integrated DNA was unstable and lost when cultures were grown with uracil. In contrast, 9 of 12 uracil-independent transformants that were isolated at 70 °C had lost the chromosomal copy of TK0901. PCR amplification and DNA sequencing of DNA amplified from the genomes of these transformants confirmed that double crossover events had resulted in pyrF replacement of TK0901 (Fig. 1B). A representative of these T. kodakarensis (ΔpyrF; ΔrpoF∷pyrF) transformants was designated T. kodakarensis strain KU2F (Table 1). Western blot analyses of KU2F cell lysates confirmed the absence of the F subunit and revealed that this did not change the abundance of the E subunit in cells (Fig. 1C). By isolating KU2F (ΔpyrF; ΔrpoF∷pyrF), we established that the F subunit was not essential for viability, but the inability to delete TK0901 at 85 °C suggested that the rpoF is required for cell growth at higher temperatures.
Fig. 1. Construction of plasmids designed to delete TK0901 (rpoF) and TK1699 (rpoE), and confirmation of deletion (ΔTK0901; ΔrpoF) inT. kodakarensis KU2F.
(A) Plasmids pUDRF, pUDRE1 and pUDRE2 were constructed with pyrF (TK2297) flanked by ~0.7 to 1 kbp of the T. kodakarensis genome as illustrated. These plasmids were used to transform KU216 (ΔpyrF). The pairs of oligonucleotides (F1 and F2; F3 and F4; sequences in Supplementary Table 1) used to PCR amplify genomic DNA from pUDRF-generated uracil-independent transformants are indicated. (B) Electrophoresis of DNA molecules PCR amplified from T. kodakarensis KU216 (lanes 1 and 3) and KU2F (lanes 2 and 4) using primer pairs F1 and F2 (lanes 1 and 2), or F3 and F4 (lanes 3 and 4). The DNA molecules amplified using primers F1 and F2 from T. kodakarensis KU216 and KU2F were, as predicted, 1.8 and 2.1 kbp, respectively. Lane S contained size standards. (B) Western blot analysis of the polypeptides present in aliquots of lysates of T. kodakarensis KU216, KU2F and KUWFF separated by SDS-PAGE. The control lane (C) contained a sample of the purified E plus F subunits used to generate the rabbit anti-subunit E and anti-subunit F antibodies. Total 10 ng proteins were applied to each lane of the gel.
Table 1.
T. kodakarensis strains and plasmids used and constructed for this study
| Strains | Genome deletions (Δ); insertions (∷) | Reference/Construction |
|---|---|---|
| KU216 | ΔpyrF | Sato et al., 2005 |
| KUW1 | ΔpyrF; ΔtrpE | Sato et al., 2005 |
| KW128 | ΔpyrF; ΔtrpE∷pyrF | Sato et al., 2003 |
| KU2F | ΔpyrF; ΔrpoF∷pyrF | KU216 with rpoF (TK0901) replaced by pyrF (TK2276) |
| KUWF | ΔpyrF; ΔtrpE; ΔrpoF∷pyrF | KUW1 with rpoF replaced by pyrF |
| KUWFB | ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-cpkB | KUWF with the cpkB (TK2303) expression cassette in chiA |
| KUWFE | ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-rpoE | KUWF with the rpoE (TK1699) expression cassette in chiA |
| KUWFF | ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-rpoF | KUWF with the rpoF expression cassette in chiA (TK1769) |
| TS413 | ΔpyrF; ΔtrpE∷pyrF; ΔrpoL∷rpoL∷HA-his6∷trpE | KW128 with rpoL (TK1167) replaced by rpoL-HA-his6trpE |
| KUWL | ΔtrpE; ΔpyrF; ΔrpoL∷rpoL-his6 | KUW1 with rpoL replaced by rpoL-his6-his6 |
| KUWLF | ΔtrpE; ΔpyrF; ΔrpoF∷pyrF; ΔrpoL∷rpoL-his6 | KUWL with rpoF replaced by pyrF |
| KUWLFB | ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-cpkB; ΔrpoL∷rpoL-his6 | KUWFL with the cpkB (TK2303) expression cassette in chiA |
| Plasmids | ||
| pUD2 | pyrF cloned in pUC118 | Sato et al., 2005 |
| pUDRF | TK0903-TK0902-pyrF-TK0900-TK0899 cloned in pUC18 | DNA fragment was inserted between KpnI and XbaI sites |
| pUDRE1 | TK1701-TK1700-pyrF-TK1689-TK1965 cloned in pUC18 | DNA fragment was inserted between KpnI and PstI sites |
| pUDRE2 | TK1701-TK1700-pyrF-TK1689-TK1965 cloned in pUC18 with pyrF promoter | DNA fragment was inserted between KpnI and PstI sites |
| pCTF | TK1765∷trpE-PfbprpoF-TK1766 in pUC18 | DNA fragment was inserted at HincII site |
| pCTB | TK1765∷trpE-PfbpcpkB-TK1766 in pUC18 | DNA fragment was inserted at HincII site |
| pCTE | TK1765∷trpE-PfbprpoE-TK1766 in pUC18 | DNA fragment was inserted at HincII site |
| pUDL-His | rpoL-his6 cloned in pUD2 | DNA fragment was inserted between EcoRI and BamHI sites |
The E subunit is encoded by TK1699, and this is apparently the second gene in a six gene operon (Fig. 1A bottom). Three proteins encoded in this operon have unidentified functions, but the two promoter-distal genes (TK1696 and TK1695) encode ribosomal proteins. Two plasmids were constructed, pUDRE1 with pyrF, and pUDRE2 with pyrF plus the pyrF promoter replacing TK1699, flanked by ~1 kbp of operon DNA from immediately upstream and downstream of TK1699 (Table 1). Uracil-independent transformants of T. kodakarensis KU216 (ΔpyrF) were obtained after transformation with these plasmid DNAs at both 70 °C and 85 °C. However, all of the transformants screened, regardless of the isolation temperature, retained the intact chromosomal copy of TK1699. Although care was taken to avoid adding sequences that might cause intrinsic transcription termination (Santangelo and Reeve, 2006), it remains possible that operon polarity, rather than subunit E essentiality, prevented the isolation a T. kodakarensis ΔTK1699 mutant.
Temperature sensitive growth of T. kodakarensis KU2F
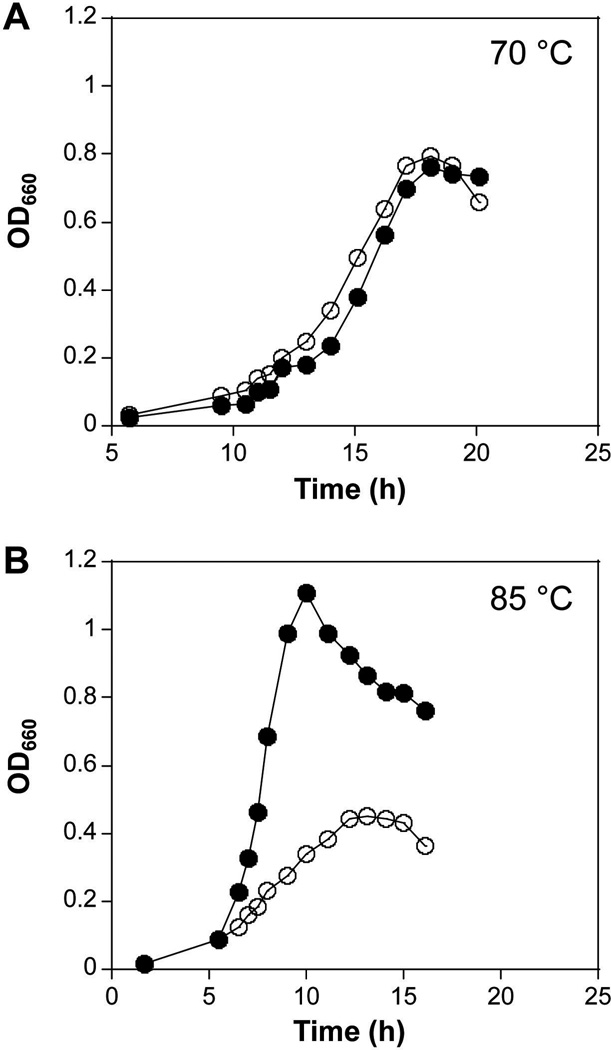
At 70 °C, cultures of KU216 and KU2F grew in nutrient-rich medium (ASW-YT-S0-Pyr) at the same rate (doubling times of ~2.1 h, which were determined with growth curves between OD660 0.2 and 0.4) and reached the same final cell densities (OD660 of ~0.8; Fig. 2A). In contrast, when incubated at 85 °C, KU216 grew faster, with generation times of ~0.9 h, whereas cultures of KU2F doubled in cell density only every ~3.9 h and reached final cell densities only ~50 % of that of KU216 cultures. When incubated at 90 °C, KU216 grew but there was no detectable growth of KU2F. These data indicated that the rpoF is required for cell growth at higher temperatures.
Fig. 2. Growth ofT. kodakarensis KU216 (●) and KU2F(○) cultures at 70 and 85 °C.
Aliquots of overnight cultures were diluted into ASW-YT-S0 medium containing 1% (w/v) sodium pyruvate and the cultures were then incubated (A) at 70 °C and (B) at 85 °C.
Chaperonin synthesis is reduced in T. kodakarensis KU2F
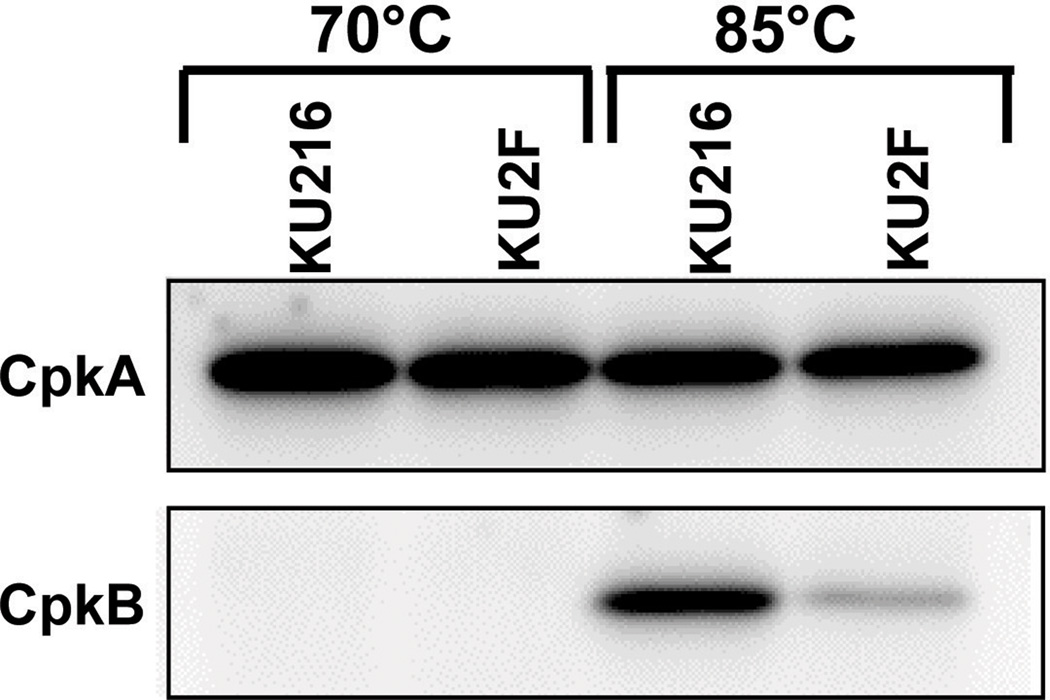
CpkA (TK0678) and CpkB (TK2303) are subunits of a T. kodakarensis thermosome that vary in abundance in vivo depending on growth temperature (Izumi et al., 2001). At 70 °C, CpkA predominates but CpkB increases at higher temperatures and almost entirely replaces CpkA in cells grown at temperatures >85 °C. As shown in Fig. 3, western blot analyses revealed that there were no detectable differences in the abundance of CpkA in KU216 and KU2F grown at 70 °C or at 85 °C, and confirmed that neither strain synthesized detectable amounts of CpkB at 70 °C. Both strains did express CpkB at 85 °C, but the abundance of CpkB at 85 °C was much lower in KU2F than in KU216.
Fig. 3. Western blot analysis of CpkA and CpkB synthesis at 70 and 85 °C.
The polypeptides in aliquots (5 µg) of lysates of T. kodakarensis KU216 and KU2F cells grown at 70 or 85 °C were separated by SDS-PAGE. CpkA and CpkB were detected by western blot analysis using antibodies specific for T. kodakarensis CpkA (top) or CpkB (bottom).
Synthesis of RpoF, RpoE or CpkB suppresses the temperature sensitive phenotype of T. kodakarensis KU2F
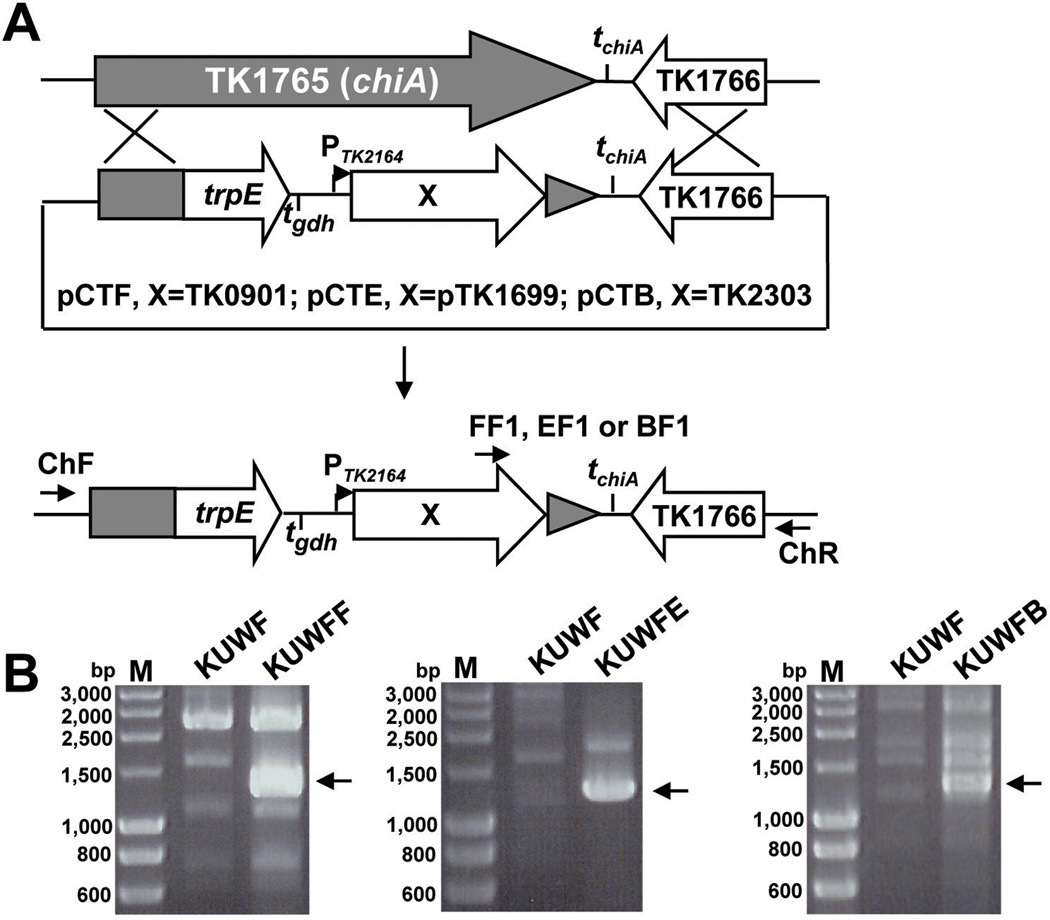
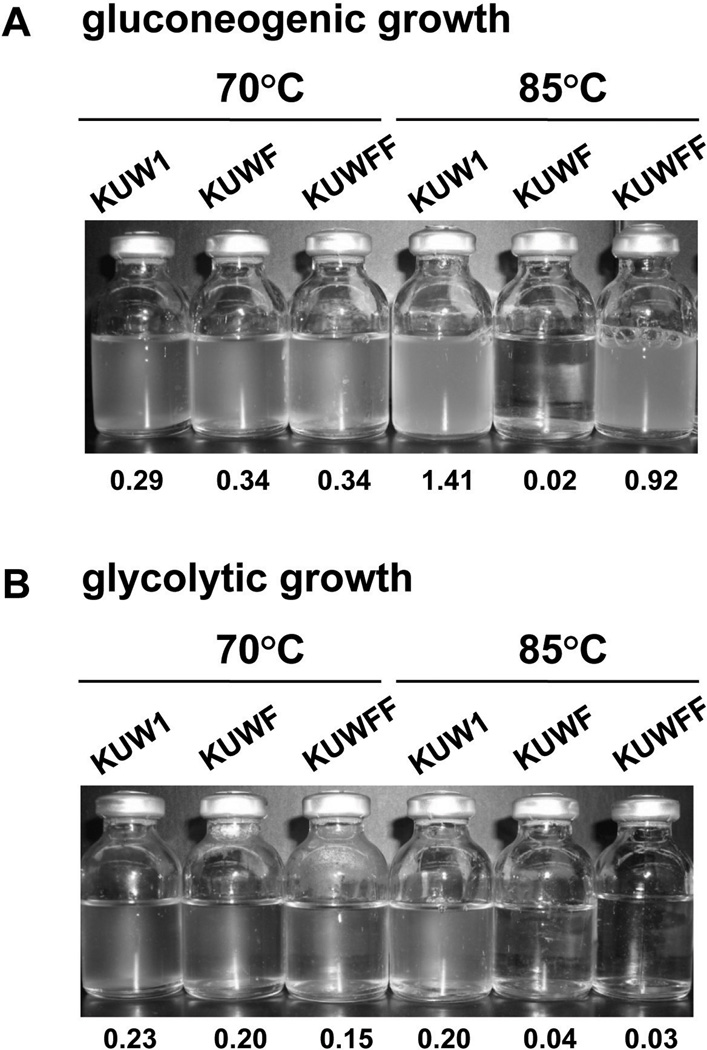
A conditional expression system was developed to determine if expression of TK0901 (rpoF) was sufficient to remove the temperature sensitivity of KU2F. Transcription of TK2164 that encodes fructose-1,6-bisphosphatase (FBPase) was known to increase ~15-fold under gluconeogenic versus glycolytic growth conditions (Kanai et al., 2007) and, in plasmid pCTF (Fig. 4, Table 1), TK0901 (rpoF) was positioned immediately downstream from the TK2164 promoter (PTK2164). To provide a selectable marker, trpE (TK0254) was cloned adjacent to the rpoF construct, and this selection-expression cassette was ligated between by 5'- and 3'-sequences from TK1765 (chiA), a gene that is not required for T. kodakarensis growth in nutrient rich media (K. Sato, T. Fukui, H. Atomi, and T. I, unpublished result). Plasmid pCTF was used to transform T. kodakarensis KUWF (ΔpyrF; ΔtrpE; ΔrpoF∷pyrF) with transformants selected by colony growth at 70 °C on plates containing ASW-AA medium without tryptophan. Insertion of the expression-selection cassette into TK1765 (chiA) was confirmed by PCR amplification (Fig. 4B left) from a representative transformant designated T. kodakarensis KUWFF (ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-rpoF). Synthesis of the RNAP subunit F in KUWFF was confirmed by western blot analysis (Fig. 1C). Cultures of KUWFF and KUW1 grew almost identically at 85 °C under gluconeogenic conditions (Fig. 5A), but under glycolytic growth conditions, KUWFF retained the temperature-sensitivity of KUWF (Fig. 5B). Nutrient regulation of the PTK2164 promoter did therefore provide a mechanism to regulate gene expression in vivo in T. kodakarensis, and permissive expression of TK0901 (rpoF) was sufficient to eliminate the temperature-sensitivity of T. kodakarensis KUWF.
Fig. 4. Structure of the selection-expression cassettes and integration into the TK1765-TK1766 region of theT. kodakarensis genome.
(A) The three plasmids constructed, pCTB, pCTE and pCTF (Table 1) contained the 5' and 3' regions of TK1765 (chiA), the intergenic region downstream of TK1765 with the tchiA transcription terminator and TK1766. The selection-expression cassettes cloned between the 5' and 3' regions of TK1765 contained the selectable marker gene (trpE; TK0254), a transcription terminator (tgdh), the regulated PTK2164 promoter (PFBPase), and either TK2303 (cpkB; pCTB), TK1699 (rpoE; pCTE) or TK0901 (rpoF; pCTF). These plasmids were used to transform T. kodakarensis KUWF (ΔrpoF∷pyrF; ΔtrpE). Integration of the expression cassettes into the TK1765-TK1766 region of the KUWF genome was confirmed by PCR amplification of genomic DNAs using primer sets including: 1) ChF and ChR; and 2) ChR and FF1, EF1 or BF1 (sequences in Supplementary Table 1) that hybridized at the locations indicated black arrows. (B) Electrophoresis of DNA molecules PCR amplified from T. kodakarensis KUWF, KUWFF, KUWFE and KUWFB using primer pairs FF1 and ChR (left panel), EF1 and ChR (middle panel), or BF1 and ChR (right panel). The DNA molecules amplified using these primer pairs were indicated by arrows. Lane M contained size standards.
Fig. 5. Media-dependent growth ofT. kodakarensis strains at 70 and 85°C.
Aliquots of overnight cultures of T. kodakarensis KUW1 (ΔpyrF; ΔtrpE), KUWF (ΔrpoF∷pyrF; ΔtrpE) and KUWFF (ΔrpoF∷pyrF; ΔtrpE; ΔchiA∷trpE-PTK2164-rpoF) were diluted into (A) ASW-YT-S0-Pyr medium (gluconeogenic growth condition; PTK2164 active) or (B) ASW-YT-Mdx medium (glycolytic growth condition; PTK2164 inactive) and incubated for 16 h at 70°C or 85°C. The OD600 reached after the 16 h incubation period is listed below the culture.
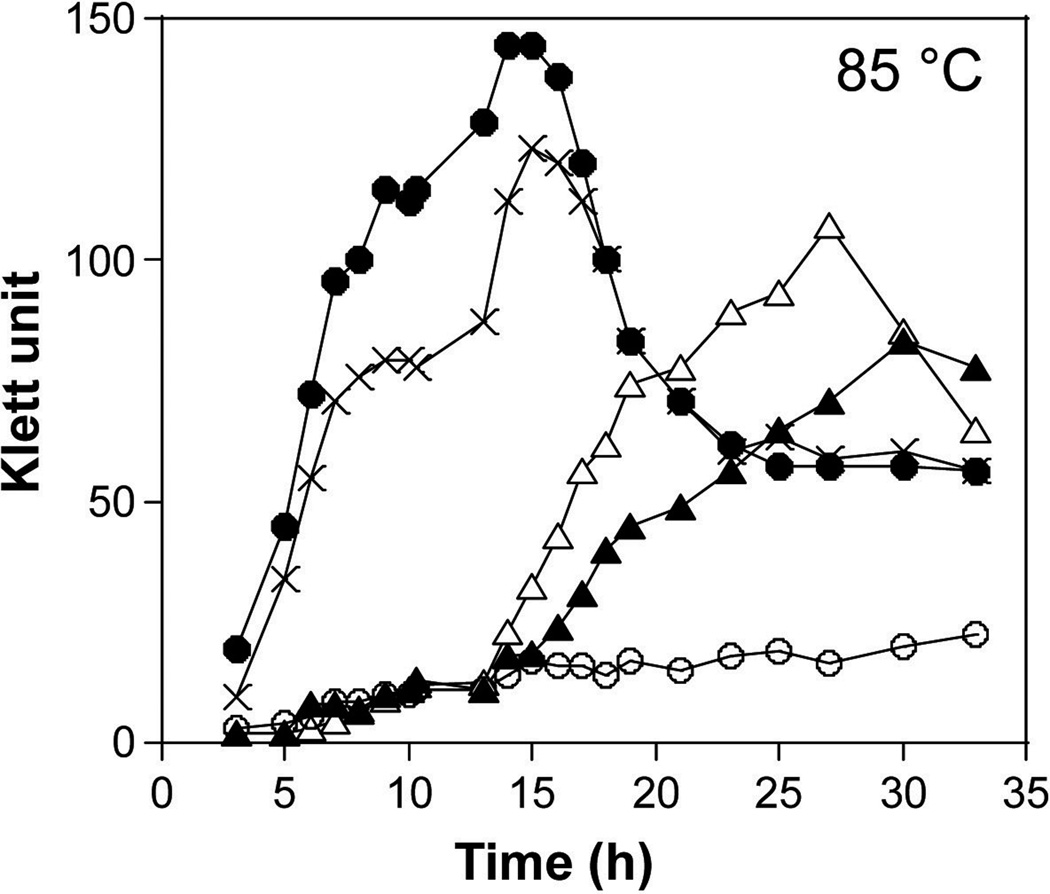
As growth defects of yeast mutants lacking RPB4 were suppressed by over-expression of RPB7 (Sheffer et al., 1999), it was of interest to determine if increased synthesis of subunit E, or of CpkB, might suppress the temperature sensitivity of KUWF. Plasmids pCTE and pCTB were constructed from pCTF by replacing TK0901 (rpoF) with TK1699 (rpoE) or TK2303 (cpkB), respectively (Fig. 4, Table 1). These plasmids were used to transform KUWF with transformants selected by colony growth at 70 °C on plates lacking tryptophan. Insertion of the trpE-rpoE or trpE-cpkB cassette into TK1765 (chiA) was confirmed by PCR amplification (Fig. 4B middle and right) and representative transformants, designated T. kodakarensis KUWFE (ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-rpoE) and KUWFB (ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-cpkB) (Table 1). Cultures of KUW1, KUWFF, KUWFE and KUWFB grew identically at 70 °C in ASW-YT-S0-Pyr medium (gluconeogenic conditions; PTK2164 active, data not shown). At 85 °C, KUWFE and KUWFB cultures grew slower than KUW1, but did reach 58~74 % of final cell density in this medium compared with KUW1. In contrast, in the absence of suppression of the ΔrpoF mutation, at 85°C, cultures of KUWF grew much lower (~16 %) cell densities (Fig. 6). The increased synthesis of subunit E or CpkB, directed by PTK2164 transcription of TK1699 (in KUWFE) or TK2303 (in KUWFB) did therefore reduce the temperature sensitivity that would otherwise have resulted from the ΔrpoF mutation present in these strains. Given the growth of KUWFB at 85 °C, the reduction in CpkB at 85 °C in KUWF (Fig. 3) does apparently play a causative role in the temperature sensitivity of KUWF.
Fig. 6. Partial suppression of the temperature-sensitive growth phenotype of KUWF (ΔrpoF) by rpoE and cpkB expression.
Cultures of T. kodakarensis KUW1 (ΔpyrF; ΔtrpE;●), KUWF (ΔrpoF∷pyrF; ΔtrpE;○), KUWFF (ΔrpoF∷pyrF; ΔtrpE; ΔchiA∷trpE-PTK2164-rpoF; X), KUWFE (ΔrpoF∷pyrF; ΔtrpE; ΔchiA∷trpE-PTK2164-rpoE; ∆) and KUWFB (ΔrpoF∷pyrF; ΔtrpE; ΔchiA∷trpE-PTK2164-cpkB, ▲) were grown at 85°C in ASW-YT-S0-Pyr (gluconeogenic growth condition; Pfbp active). Turbidity measurements were made using a Klett spectrophotomer (100 Klett units was equivalent to an OD660 of 0.65).
Using a T. kodakarensis strain having an extra copy of the rpoE integrated at the chiA locus, we have attempted to delete the TK1699 (rpoE) from the T. kodakarensis genome by homologous recombination. However, we could not isolate any transformed cell lacking the TK1699 suggesting that the rpoE cannot be deleted from the T. kodakarensis genome most likely due to an operon polarity. A role of the rpoE to T. kodakarensis cell viability remains uncertain.
Isolation and in vitro transcription by T. kodakarensis RNAP lacking subunits E and F
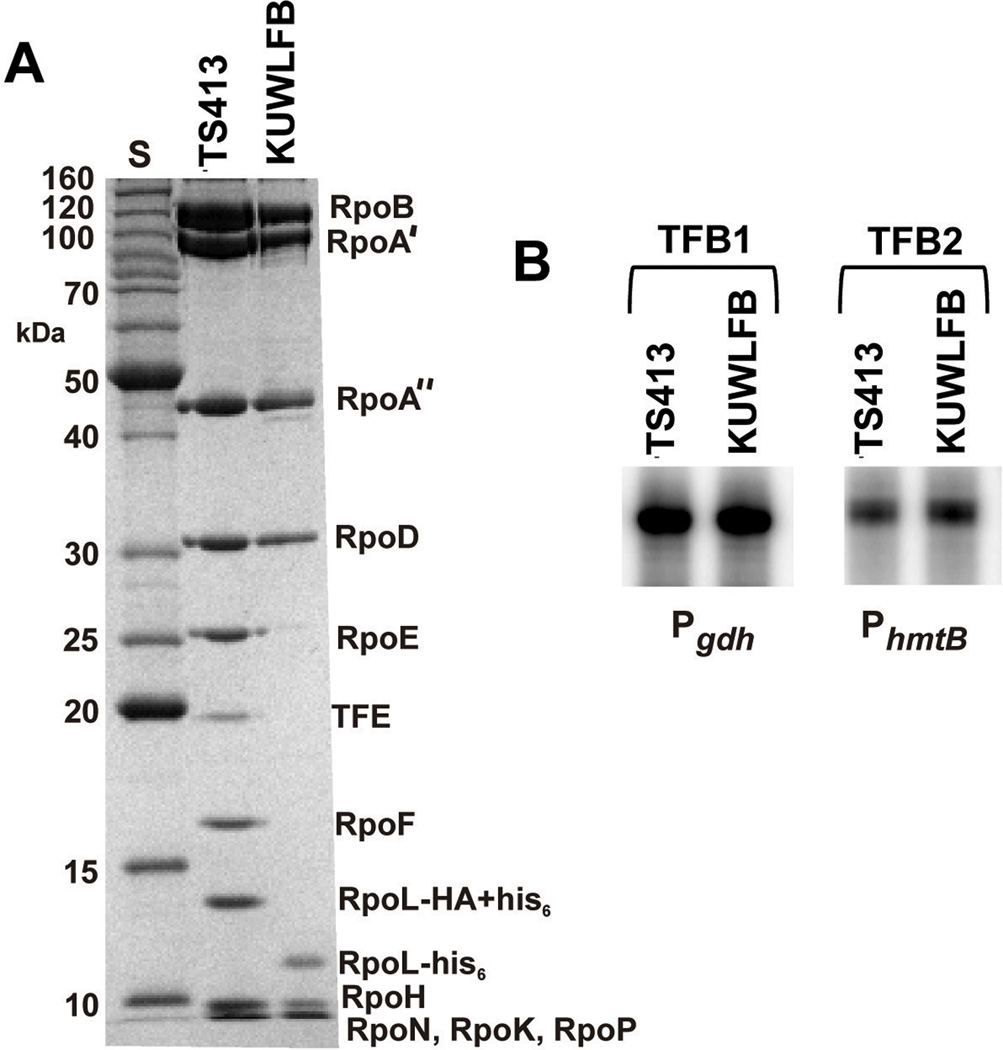
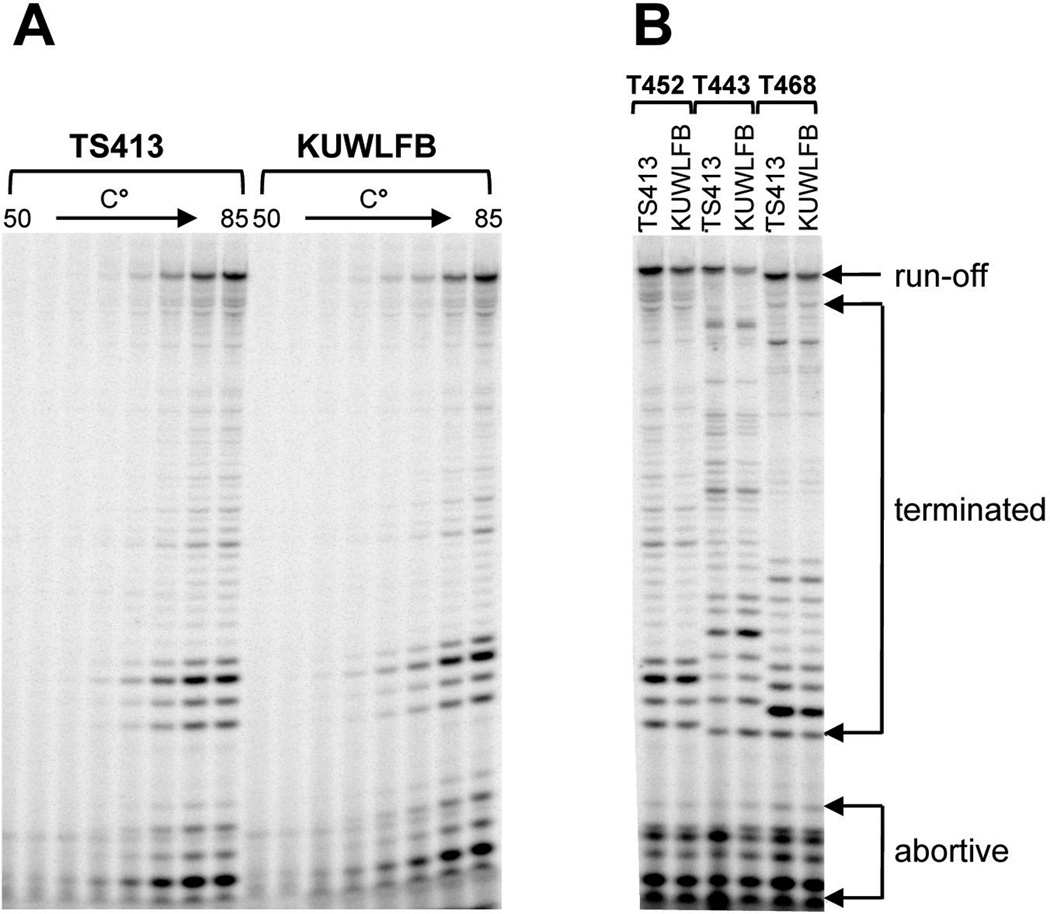
RNAP preparations were purified directly from T. kodakarensis TS413 (rpoL-HA-his6) and KUWLFB (ΔpyrF; ΔtrpE; ΔrpoF∷pyrF; ΔchiA∷trpE-PTK2164-cpkB; ΔrpoL∷rpoL-his6) cell lysates by binding to a Ni2+-charged matrix, washing and imidazole elution (Santangelo et al., 2008). As reported previously for archaeal RNAPs purified by multiple column chromatographic steps (Micorescu et al., 2008; Santangelo et al., 2007), the RNAP preparations purified by Ni2+-affinity from T. kodakarensis TS413 contained the eleven RNAP subunits plus a polypeptide identified as TFE (TK2024) (Fig. 7A). In contrast, the RNAP preparations purified by Ni2+-affinity from T. kodakarensis KUWLFB contained only the nine core subunits. They did not contain subunits E or F, or TFE (Fig. 7A), but nevertheless, exhibited virtually identical activity in all in vitro transcription assays when compared with the Ni2+-affinity-purified complete RNAP (Figs. 7B and 8). Transcription was initiated with the same efficiency from different archaeal promoters in reaction mixtures that contained T. kodakarensis TBP and TFB1 or TFB2, either of the two TFB homologues present in T. kodakarensis (Fig. 7B). A Pyrococcus furiosus RNAP core reconstituted in vitro from individual subunits without subunits E and F was very inefficient in transcription initiation at 60 °C when compared with a reconstituted complete RNAP (Naji et al., 2007). In contrast, the T. kodakarensis RNAP core assembled in T. kodakarensis KUWLFB, initiated transcription with essentially the same efficiency in vitro as the complete enzyme at all temperatures assayed from 50 to 85 °C (Fig. 8A). Archaeal transcription is sensitive to termination by oligoT-rich template sequences (Santangelo and Reeve, 2006) and when templates were used that contained such archaeal intrinsic terminators, identical patterns of abortive, intrinsically terminated and run-off transcripts were generated by the RNAPs from T. kodakarensis TS413 and KUWLFB (Fig. 8B).
Fig. 7. Ni2+-affinity purification andin vitro transcription byT. kodakarensis RNAPs.
(A) Commassie blue staining after SDS-PAGE of aliquots of RNAP (7.5 µg) purified from T. kodakarensis TS413 and KUWLFB. The control lane (S) contained size standards (kDa). RNAP subunits E (RpoE) and F (RpoF) and TFE are present only in RNAP preparations from T. kodakarensis TS413. The L subunits in the RNAPs from T. kodakarensis TS413 and KUWLFB have HA+his6 and his6 C-terminal extensions, respectively, that result in different electrophoretic mobilities. (B) Comparison of transcripts synthesized in vitro directed by the constitutive archaeal promoters Pgdh or PhmtB in reaction mixtures that contained either TFB1 (TK1280) or TFB2 (TK2287) and RNAP from TS413 or KUWLFB (Santangelo et al. 2006; 2007). Aliquots of RNAP (40 nM) were incubated with 80 nM TBP, 80 nM TFB1 (1) or TFB2 (2) and 10 nM of a template for 5 min at 85°C, and transcription then initiated by adding 500 µM ATP, CTP and GTP,50 µM UTP plus 0.5 µCi of [32P]-α-UTP. The [32P]-labeled transcripts synthesized during 5 min incubation at 85°C were precipitated, separated by electrophoresis and visualized using a Storm 840 phosphorimager. The image shows the relative amounts of the full-length run-off transcripts synthesized from the two promoters from initiation complexes containing either TFB1 or TFB2.
Fig. 8. Temperature and template-dependent transcript synthesis in vitro by T. kodakarensis RNAPs.
(A) Electrophoretic separation of the transcripts synthesized by RNAP from T. kodakarensis TS413 or KUWLFB from template T452 (sequence in Supplementary Table 2) during incubation for 5 min at temperatures increasing from 50 to 55, 60, 65, 70, 75, 80 and 85 °C. Transcription was initiated from PhmtB and the transcripts synthesized were labeled at their 5'-terminus by [32P]-ApC incorporation. (B) Electrophoretic separation of the transcripts synthesized by RNAP from T. kodakarensis TS413 or KUWLFB from templates T452, T443 or T468 (sequence in Supplementary Table 2) during incubation for 5 min at 85 °C. These templates all contain the same PhmtB promoter and transcription was initiated at the same location. The downstream transcribed DNA in T452 contained an archaeal protein coding sequence (T452), whereas T443 and T468 contained archaeal intergenic sequences. All transcripts synthesized were labeled at their 5'-terminus by [32P]-ApC incorporation. As indicated, additional investigations confirmed the identity of transcripts that resulted from abortive initiation, template-directed intrinsic termination and template run-off (TJS, unpublished results).
Discussion
Based on their sequences (Kwapisz et al., 2008) and on high-resolution structures (Hirata et al., 2008; Todone et al., 2001), archaeal and eukaryotic RNAPs are closely related, and here we provide the first evidence of conservation of archaeal and eukaryotic RNAP subunit functions in vivo. It was known that yeast mutants lacking Rpb4 were viable, but temperature and stress sensitive, and we were able to delete the archaeal Rpb4 homolog (Fig. 1) and the T. kodakarensis (ΔrpoF) mutant generated does have a temperature-sensitive phenotype (Fig. 2). In yeast, Rpb7 is essential (McKune et al., 1993) and Rpb7 over-expression can partially compensate for the loss of Rpb4 (Sheffer et al., 1999). Similarly, we were unable to delete the Rpb7 homolog, and increased synthesis of subunit E gene reduced the temperature sensitivity imposed on T. kodakarensis by a ΔrpoF (Fig. 6). The loss of subunit F also led to a reduction in the abundance of CpkB (Fig. 3), a component of a thermosome at 85 °C, and increased synthesis of CpkB also partially suppressed the temperature sensitivity the T. kodakarensis ΔrpoF mutant (Fig. 6). Using DNA microarray, we are now determining if the ΔrpoF mutation directly reduces CpkB synthesis, or if the reduction in CpkB is a downstream event in a larger cascade of gene expression and protein stability changes.
In the S. solfataricus RNAP structure, the subunit E plus F complex is attached to the core primarily through subunit E interactions (Hirata et al., 2008). The F subunit binds to the E subunit between two sub-domains of the E subunit (Todone et al., 2001), but as loss of the F subunit results in an enzyme lacking both the E and F subunits (Fig. 7A), subunit F binding to subunit E may be required to stabilize subunit E-core polymerase interactions. This conclusion was drawn from the observation that loss of Rpb4 results in a yeast Pol II enzyme that also lacks Rpb7 (Edwards et al., 1991; Sheffer et al., 1999). Alternatively, E subunits synthesized in the absence of F subunits may not fold correctly which would be consistent the observation that increased synthesis of subunit E or the chaperonin CpkB can partially compensate for the loss of the F subunit and facilitate the growth of T. kodakarensis (ΔrpoF) strains at 85 °C (Fig. 6).
TFE co-purified with archaeal RNAPs purified by sequential column chromatography (Micorescu et al., 2008; Santangelo et al., 2007) or by Ni2+-affinity (Fig. 7A), but did not co-purify with the RNAP core enzyme isolated from T. kodakarensis KUWLFB. This is consistent with TFE stimulation of archaeal RNAP activity requiring subunit E (Naji et al., 2007; Ouhammouch et al., 2004) and with reports of stimulatory transcription factor interactions with the homologous complexes in Pol I, II and III. The extensions formed in Pol I by A43 plus A14, and in Pol III by C25 plus C17 interact with polymerase-specific transcription initiation factors that recruit Pol I and Pol III to the appropriate promoters (Kassavetis et al., 2001; Peyroche et al., 2000). The Rpb7 plus Rpb4 extension of Pol II has been shown by cryo-electron microscopy to interact with TFIIF as an essential step in the assembly of a Pol II transcription pre-initiation complex (Chung et al., 2003). Given these observations, it seems most likely that the extensions formed by the archaeal subunits E plus F, and eukaryotic subunits A43 plus A14 (Pol I), Rpb4 plus Rpb7 (Pol II) and C25 plus C17 (Pol III) provide targets for transcription factor binding, and so facilitate RNAP recruitment and transcription factor activation of the transcription machinery embodied in the core structures of these enzymes.
All in vitro transcription studies to date argue that only RNAP and two general transcription factors, TBP and TFB, are required for archaeal basal transcription although some Archaea have more than one TBP and/or TFB homologue (Thomm, 2007). The results reported demonstrate that the F subunit of RNAP is not essential for T. kodakarensis viability and as RNAP isolated from the T. kodakarensis ΔrpoF mutant also lacks subunit E, it seems possible that subunit E is also not essential for archaeal transcription in vivo. Consistent with this, the core enzyme purified from T. kodakarensis KUWLFB, that lacks both subunits E and F, was fully active in transcription initiation, elongation and termination in vitro (Figs. 7 and 8). If correct, this would further predict that any TFE-RNAP interaction that requires the participation of subunit E is also not essential in vivo, at least at the lower growth temperatures where the T. kodakarensis ΔrpoF mutant grows well.
With the development of gene deletion and insertion technologies, we could inactivate and manipulate non-essential genes in T. kodakarensis (Santangelo et al., 2007; Santangelo et al., 2008; Sato et al., 2003, 2005). With the addition of PTK2164- based conditional expression, we can now also mutate and investigate the activities of essential genes. For example, our inability to delete the genes encoding TFE (TK2024) and TFS (TK0533) (T.J.S. and J.N.R., unpublished observation) predicts that these genes are required for viability. These negative results can now be tested directly by expression of these genes from PTK2164 in T. kodakarensis cells cultured under permissive versus non-permissive conditions. If the loss of TFE and/or TFS is lethal, then we can also determine if viability can be recovered by mutations introduced into the T. kodakarensis genes that encode RNAP subunits.
Experimental Procedures
Strains and growth media
The T. kodakarensis strains and plasmids used and constructed in this study are listed in Table 1. Cultures were grown under anaerobic conditions at temperatures ranging from 70 to 95 °C in either nutrient rich medium (ASW-YT) that contained yeast extract (Y) and tryptone (T) dissolved in artificial seawater (ASW) (Sato et al., 2003), or in a minimal medium (ASW-AA) that contained a mixture of vitamins, trace minerals and the 20 canonical amino acids dissolved in ASW (Atomi et al., 2004). For the conditional growth studies, cultures grown overnight (~10 hrs) were diluted 100-fold into ASW-YT supplemented with S0 and 1% (w/v) sodium pyruvate (ASW-YT-S0-Pyr; gluconeogenic growth), or ASW-YT medium containing 1% (w/v) Amycol #3-L (ASW-YT-Mdx; glycolytic growth). Amycol #3-L (Nippon Starch Chemical, Osaka, Japan) contains a mixture of malto-oligosaccharides with lengths from 1 to 12 glucose units. Culture growth was followed by measuring the increase in adsorption using an Ultraspec 3300 spectrophotometer or by measuring turbidity (Klett) increase using a Klett-Summerson photoelectric colorimeter with a green filter (Klett Manufacturing Co., NY). In growth studies in Fig. 6, triplicate measurements were made from at least three independent cultures.
Construction of ΔrpoF and ΔrpoE plasmids and transformation of T. kodakarensis KU216
DNA molecules that carried TK0901 (rpoF) or TK1699 (rpoE), plus their flanking genes, were PCR amplified from T. kodakarensis genomic DNA using the primers listed in Supplementary Table 1. The primer sequences were taken from the genome sequence (Fukui et al., 2005). Using standard molecular biology techniques, the desired DNA fragments were cloned from the amplified DNA into plasmid pUC18 to generate plasmids pUDRF, pUDRE1 and pUDRE2 (Fig. 1A; Table 1). These plasmids were amplified and purified from Escherichia coli Mach1-T1 and used to transform competent T. kodakarensis KU216 cells, prepared as previously described (Sato et al., 2003). Transformed cells were cultured in ASW-AA-S0 liquid medium at 70 °C in the absence of uracil to select the uracil prototroph transformants. The cell cultures were then spread on ASW-AA-S0 plate lacking uracil and incubated for 5 days at 70 or 85°C. PCR amplification and DNA sequencing was used to determine the structure of the region of interest in representative transformant genomes.
Construction of trpE-PTK2164-based selection-expression cassettes
The required regions of the T. kodakarensis genome were PCR amplified from genomic DNA and cloned into pUC18 derivative by standard molecular biology techniques resulting plasmids pCTB, pCTE and pCTF (Fig. 4; Table 1). These plasmids contain a 5'-region of TK1765 (chiA), TK0254 (trpE), the intrinsic transcription terminator from downstream of TK1431 (gdh), the nutritionally-regulated PTK2164 (FBPase) promoter, the 3'-region of TK1765 plus the downstream gene TK1766. In plasmids pCTB, pCTE or pCTF, TK2303 (cpkB), TK1699 (rpoE) or TK0901 (rpoF) was positioned, respectively, immediately downstream from PTK2164. These plasmids were amplified and purified from E. coli Mach1-T1 and used to transform competent cells of T. kodakarensis KUWF. Transformants were selected by colony formation on ASW-AA-S0 plates that lacked tryptophan incubated for 5 days at 70 °C. The structures of the relevant region of the genomes of representative transformants, designated T. kodakarensis KUWFB, KUWFE and KUWFF (Table 1), were determined by PCR amplification. To differentiate between single- and double-crossover events, we designed PCR primers having complementary sequences at outside of 5’ and 3’ flanking regions (ChF and ChR in Fig. 4A, Supplementary Table 1) and confirmed their genotypes by PCR (data not shown).
Construction of T. kodakarensis KUWLFB
The chromosomal copy of the rpoL gene was replaced by the rpoL-his6 allele in which the ORF is extended by six histidine codons using the pop-in/pop-out strategy. The strategy is generally used for deletion, mutation or replacement of chromosomal genes in many organisms, such as yeast (Kerscher et al., 2002) or halophilic archaea (Peck et al., 2000). The pop-in/pop-out strategy consists of two steps; a single crossover integration of the plasmid into the chromosome followed by a second recombination that removes the marker gene while simultaneously exchanging the target gene. The vector used for transformation was constructed as follows. The first PCR reaction produced a fragment (~1,000 bp) corresponding to the region upstream from the rpoL stop codon using the primer set rpoL-1/rpoL-his-2 (Supplementary Table 1). The second PCR reaction produced a fragment (~900 bp) corresponding to the region downstream from the rpoL stop codon using the primer set rpoL-his-1/rpoL-2 (Supplementary Table 1). Both PCR products, with an extra 18 bp overhang region corresponding to the hexahistidine tag, were mixed and used as the template for a fusion PCR reaction using the primer set rpoL-1/rpoL-2 (Supplementary Table 1). The amplified fragment was inserted into the EcoRI and the BamHI sites of plasmid pUD2, a plasmid in which the pyrF marker gene is inserted into the HincII site of pUC118 (Sato et al., 2005), and the constructed vector (pUDL-His, Table 1) was used to transform T. kodakaraensis KUW1 (ΔtrpE, ΔpyrF). The transformed cells that have undergone single crossover integration can be selectively grown in a liquid medium in the absence of uracil. The uracil prototrophs are then spread on a solid, nutrient-rich medium supplemented with 5-fluoroorotic acid. In this medium, only strains that have undergone a second (pop-out) recombination step can grow. In principle, among the 5-fluoroorotic acid resistant strains, half should harbor a genotype equivalent to the original host strain, whereas the other half should result in a genotype where the rpoL-his6 gene is inserted. PCR analyses indicated that the intended gene replacement had occurred in 3 colonies out of 12. One strain that was confirmed to have the correct rpoL-his6 sequence was selected (designated as KUWL) and used for further recombination. Using pUDRF, ΔrpoF strain was constructed from KUWL (designated as KUWLF, Table 1). Finally, KUWLFB was constructed from KUWLF using pCTB.
Western blot analyses
Purified recombinant T. kodakarensis RNAP subunits E and F were used to vaccinate rabbits (Convance, PA). Dilutions of the resulting rabbit antisera, and dilutions of anti-CpkA and anti-CpkB antisera, donated by S. Fujiwara, were used in a western blot analyses.
RNAP purification and in vitro transcription
RNAP preparations were purified from T. kodakarensis TS413 and KUWLFB by binding to a Ni2+-charged affinity matrix and imidazole elution, as previously described (Santangelo et al., 2008). The polypeptides in these RNAP preparations were separated by electrophoresis through denaturing 15% (w/v) polyacrylamide gels, visualized by Coomassie blue staining, and identified by mass spectrometry. The preparations of recombinant T. kodakarensis TBP, TFB1 and TFB2, the template DNAs carrying the archaeal Pgdh and PhmtB promoters, optimized solution conditions and 32P-UTP incorporation protocol used to document and quantify promoter-dependent transcription by T. kodakarensis RNAP have been described previously in detail (Santangelo and Reeve, 2006; Santangelo et al., 2007).
To compare the patterns of transcripts synthesized and terminated in reaction mixtures that contained 1 mM concentrations of all four NTPs, reaction mixtures were assembled that contained 40 nM RNAP, 80 nM TBP, 80 nM TFB2, 10 nM T452, T443 or T468 template DNA (sequences in Supplementary Table 2) and 75 µM 32P-labeled ApC, the 5'-dinucleotide common to all the promoter-directed transcripts synthesized from these templates. The reaction mixtures were incubated at the experimental temperature for 10 min, to facilitate open complex formation, and the four rNTPs were then added at 1 mM final concentrations. Transcription was allowed for 5 min, and the 32P-labeled transcripts synthesized were separated by electrophoresis through denaturing 22.5% (w/v) polyacrylamide gels, visualized and quantified using a Storm 840 phosphorimager (GE Healthcare). By using this procedure, transcripts were [32P]-labeled only at their 5'-terminus, and all transcripts were labeled to the same extent regardless of length. The relative amounts of the transcripts synthesized from a template were then visually apparent, and readily quantified by phosphorimaging.
Supplementary Material
Acknowledgment
We thank D. Ikegami for the construction of the strain KUWL, S. Fujiwara for the gifts of anti-CpkA and anti-CpkB antisera, J. E. Brenchley for use of the Klett colorimeter, and E. P. Geiduschek and J. G. Ferry for critical help with the manuscript. This research was supported at Pennsylvania State University by the Pew Scholars Program in the Biomedical Science and by a grant from the National Institutes of Health [NIH; (GM071897)] to KSM; at Kyoto University, by the Japan Society for the Promotion of Science under grant-in-aid for Creative Scientific Research (project no. 18GS0421) to TI; and at Ohio State University by grants from the Department of Energy (DE-FG02–87ER13731) and NIH (GM53185) to JNR, and a NIH fellowship (GM073336) to TJS.
References
- Armache KJ, Kettenberger H, Cramer P. Architecture of initiation-competent 12-subunit RNA polymerase II. Proc Natl Acad Sci U S A. 2003;100:6964–6968. doi: 10.1073/pnas.1030608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea. 2004;1:263–267. doi: 10.1155/2004/204953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SD, Jackson SP. Transcription and translation in Archaea: a mosaic of eukaryal and bacterial features. Trends Microbiol. 1998;6:222–228. doi: 10.1016/s0966-842x(98)01281-5. [DOI] [PubMed] [Google Scholar]
- Bell SD, Brinkman AB, van der Oost J, Jackson SP. The archaeal TFIIEalpha homologue facilitates transcription initiation by enhancing TATA-box recognition. EMBO Rep. 2001;2:133–138. doi: 10.1093/embo-reports/kve021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell DA, Kornberg RD. Complete, 12-subunit RNA polymerase II at 4.1-A resolution: implications for the initiation of transcription. Proc Natl Acad Sci U S A. 2003;100:6969–6973. doi: 10.1073/pnas.1130601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M, Young RA. A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol Cell Biol. 1993;13:6984–6991. doi: 10.1128/mcb.13.11.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WH, Craighead JL, Chang WH, Ezeokonkwo C, Bareket-Samish A, Kornberg RD, Asturias FJ. RNA polymerase II/TFIIF structure and conserved organization of the initiation complex. Mol Cell. 2003;12:1003–1013. doi: 10.1016/s1097-2765(03)00387-3. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Kane CM, Young RA, Kornberg RD. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J Biol Chem. 1991;266:71–75. [PubMed] [Google Scholar]
- Fernandez-Tornero C, Bottcher B, Riva M, Carles C, Steuerwald U, Ruigrok RW, Sentenac A, Muller CW, Schoehn G. Insights into transcription initiation and termination from the electron microscopy structure of yeast RNA polymerase III. Mol Cell. 2007;25:813–823. doi: 10.1016/j.molcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Fukui T, Atomi H, Kanai T, Matsumi R, Fujiwara S, Imanaka T. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 2005;15:352–363. doi: 10.1101/gr.3003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg S, Bartlett MS, Naji S, Thomm M. Transcription factor E is a part of transcription elongation complexes. J Biol Chem. 2007;282:35482–35490. doi: 10.1074/jbc.M707371200. [DOI] [PubMed] [Google Scholar]
- Hanzelka BL, Darcy TJ, Reeve JN. TFE, an archaeal transcription factor in Methanobacterium thermoautotrophicum related to eucaryal transcription factor TFIIEalpha. J Bacteriol. 2001;183:1813–1818. doi: 10.1128/JB.183.5.1813-1818.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Klein BJ, Murakami KS. The X-ray crystal structure of RNA polymerase from Archaea. Nature. 2008;451:851–854. doi: 10.1038/nature06530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Fujiwara S, Takagi M, Fukui K, Imanaka T. Two kinds of archaeal chaperonin with different temperature dependency from a hyperthermophile. Biochem Biophys Res Commun. 2001;280:581–587. doi: 10.1006/bbrc.2000.4154. [DOI] [PubMed] [Google Scholar]
- Jasiak AJ, Armache KJ, Martens B, Jansen RP, Cramer P. Structural biology of RNA polymerase III: subcomplex C17/25 X-ray structure and 11 subunit enzyme model. Mol Cell. 2006;23:71–81. doi: 10.1016/j.molcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kanai T, Akerboom J, Takedomi S, van de Werken HJ, Blombach F, van der Oost J, Murakami T, Atomi H, Imanaka T. A global transcriptional regulator in Thermococcus kodakaraensis controls the expression levels of both glycolytic and gluconeogenic enzyme-encoding genes. J Biol Chem. 2007;282:33659–33670. doi: 10.1074/jbc.M703424200. [DOI] [PubMed] [Google Scholar]
- Kassavetis GA, Letts GA, Geiduschek EP. The RNA polymerase III transcription initiation factor TFIIIB participates in two steps of promoter opening. Embo J. 2001;20:2823–2834. doi: 10.1093/emboj/20.11.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher S, Drose S, Zwicker K, Zickermann V, Brandt U. Yarrowia lipolytica, a yeast genetic system to study mitochondrial complex I. Biochim Biophys Acta. 2002;1555:83–91. doi: 10.1016/s0005-2728(02)00259-1. [DOI] [PubMed] [Google Scholar]
- Kuhn CD, Geiger SR, Baumli S, Gartmann M, Gerber J, Jennebach S, Mielke T, Tschochner H, Beckmann R, Cramer P. Functional architecture of RNA polymerase I. Cell. 2007;131:1260–1272. doi: 10.1016/j.cell.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Kwapisz M, Beckouet F, Thuriaux P. Early evolution of eukaryotic DNA-dependent RNA polymerases. Trends Genet. 2008;24:211–215. doi: 10.1016/j.tig.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lange U, Hausner W. Transcriptional fidelity and proofreading in Archaea and implications for the mechanism of TFS-induced RNA cleavage. Mol Microbiol. 2004;52:1133–1143. doi: 10.1111/j.1365-2958.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- Matsumi R, Manabe K, Fukui T, Atomi H, Imanaka T. Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J Bacteriol. 2007;189:2683–2691. doi: 10.1128/JB.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKune K, Richards KL, Edwards AM, Young RA, Woychik NA. RPB7, one of two dissociable subunits of yeast RNA polymerase II, is essential for cell viability. Yeast. 1993;9:295–299. doi: 10.1002/yea.320090309. [DOI] [PubMed] [Google Scholar]
- Micorescu M, Grunberg S, Franke A, Cramer P, Thomm M, Bartlett M. Archaeal transcription: function of an alternative transcription factor B from Pyrococcus furiosus. J Bacteriol. 2008;190:157–167. doi: 10.1128/JB.01498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyao T, Barnett JD, Woychik NA. Deletion of the RNA polymerase subunit RPB4 acts as a global, not stress-specific, shut-off switch for RNA polymerase II transcription at high temperatures. J Biol Chem. 2001;276:46408–46413. doi: 10.1074/jbc.M107012200. [DOI] [PubMed] [Google Scholar]
- Naji S, Grunberg S, Thomm M. The RPB7 orthologue E' is required for transcriptional activity of a reconstituted archaeal core enzyme at low temperatures and stimulates open complex formation. J Biol Chem. 2007;282:11047–11057. doi: 10.1074/jbc.M611674200. [DOI] [PubMed] [Google Scholar]
- Orlicky SM, Tran PT, Sayre MH, Edwards AM. Dissociable Rpb4-Rpb7 subassembly of rna polymerase II binds to single-strand nucleic acid and mediates a post-recruitment step in transcription initiation. J Biol Chem. 2001;276:10097–10102. doi: 10.1074/jbc.M003165200. [DOI] [PubMed] [Google Scholar]
- Ouhammouch M. Transcriptional regulation in Archaea. Curr Opin Genet Dev. 2004;14:133–138. doi: 10.1016/j.gde.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ouhammouch M, Werner F, Weinzierl RO, Geiduschek EP. A fully recombinant system for activator-dependent archaeal transcription. J Biol Chem. 2004;279:51719–51721. doi: 10.1074/jbc.C400446200. [DOI] [PubMed] [Google Scholar]
- Peck RF, Dassarma S, Krebs MP. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol Microbiol. 2000;35:667–676. doi: 10.1046/j.1365-2958.2000.01739.x. [DOI] [PubMed] [Google Scholar]
- Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M. The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. Embo J. 2000;19:5473–5482. doi: 10.1093/emboj/19.20.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai B, Verma J, Abraham A, Francis P, Kumar Y, Tatu U, Brahmachari SK, Sadhale PP. Whole genome expression profiles of yeast RNA polymerase II core subunit, Rpb4, in stress and nonstress conditions. J Biol Chem. 2003;278:3339–3346. doi: 10.1074/jbc.M112180200. [DOI] [PubMed] [Google Scholar]
- Santangelo TJ, Reeve JN. Archaeal RNA polymerase is sensitive to intrinsic termination directed by transcribed and remote sequences. J Mol Biol. 2006;355:196–210. doi: 10.1016/j.jmb.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Santangelo TJ, Cubonova L, James CL, Reeve JN. TFB1 or TFB2 is sufficient for Thermococcus kodakaraensis viability and for basal transcription in vitro. J Mol Biol. 2007;367:344–357. doi: 10.1016/j.jmb.2006.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo TJ, Cubonova L, Reeve JN. Shuttle vector expression in Thermococcus kodakaraensis: contributions of cis elements to protein synthesis in a hyperthermophilic archaeon. Appl Environ Microbiol. 2008;74:3099–3104. doi: 10.1128/AEM.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Fukui T, Atomi H, Imanaka T. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol. 2003;185:210–220. doi: 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Fukui T, Atomi H, Imanaka T. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl Environ Microbiol. 2005;71:3889–3899. doi: 10.1128/AEM.71.7.3889-3899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer A, Varon M, Choder M. Rpb7 can interact with RNA polymerase II and support transcription during some stresses independently of Rpb4. Mol Cell Biol. 1999;19:2672–2680. doi: 10.1128/mcb.19.4.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M. Transcription: mechanism and regulation. In: Cavicchioli R, editor. Archaea: Molecular and Cellular Biology. Washington, DC: American Society for Microbiology Press; 2007. pp. 139–157. [Google Scholar]
- Todone F, Brick P, Werner F, Weinzierl RO, Onesti S. Structure of an archaeal homolog of the eukaryotic RNA polymerase II RPB4/RPB7 complex. Mol Cell. 2001;8:1137–1143. doi: 10.1016/s1097-2765(01)00379-3. [DOI] [PubMed] [Google Scholar]
- Werner F, Weinzierl RO. A recombinant RNA polymerase II-like enzyme capable of promoter-specific transcription. Mol Cell. 2002;10:635–646. doi: 10.1016/s1097-2765(02)00629-9. [DOI] [PubMed] [Google Scholar]
- Werner F. Structure and function of archaeal RNA polymerases. Mol Microbiol. 2007;65:1395–1404. doi: 10.1111/j.1365-2958.2007.05876.x. [DOI] [PubMed] [Google Scholar]
- Woychik NA, Young RA. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol Cell Biol. 1989;9:2854–2859. doi: 10.1128/mcb.9.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.