Abstract
Malignant brain tumors are aggressive in both children and adults. Despite recent improvements in diagnostic techniques, therapeutic approaches remain disappointing and unsuccessful. There is an urgent need for promising anticancer agents to improve overall survival of patients with brain cancer. β-Elemene has been shown to have antiproliferative effects on many types of carcinomas. In this study, we compared the cytotoxic efficacy of β-elemene and its synthetic analogs in the brain tumor cell lines A172, CCF-STTG1, and U-87MG. β-Elemene exhibited cytotoxicity towards the tumor lines, effectively suppressing tumor cell survival. The inhibitory effect of β-elemene was mediated by the induction of apoptosis, as demonstrated by three assays. The annexin V assay showed that β-elemene increased the percentage of early- and late-apoptotic cells. Apoptotic nuclei were detected in cancer cells in situ by the terminal deoxynucleotidyltransferase-mediated deoxy-UTP-fluorescein nick end labeling (TUNEL) staining, and the number of TUNEL-positive cells was significantly increased at 24–72 h following drug treatment of the cell lines. Cell death enzyme-linked immunosorbent assay (ELISA) gave similar results. Furthermore, β-elemene increased caspase-3/7/10 activity, up-regulated protein expression of BAX, and down-regulated the one of BCL-2, BCL-XL, and of X-linked inhibitor of apoptosis (XIAP) in the cells, suggesting that apoptotic signaling pathways are involved in the responses triggered by β-elemene. Compared with β-elemene, only three of the 10 synthetic β-elemene analogs studied here, exerted comparable cytotoxic efficacy towards the three brain tumor lines: the analogs Lr-1 and Lr-2 had the same antitumor efficacy, while Lr-3 was less potent than β-elemene. Thus, some synthetic analogs of β-elemene may inhibit brain cancer cell growth and proliferation, and the synthetic analogs Lr-1 and Lr-2 may have great potential as alternatives to β-elemene for anticancer therapy. Overall, this study provides, to our knowledge, the first evidence showing that synthetic analogs of β-elemene hold promise for patients with brain tumors.
Keywords: Apoptosis, brain cancer, Chinese medicine, β-elemene, A172, U-87MG, CCF-STTG1 cells, synthetic analogs
Malignant brain cancer is one of the most challenging health issues for both children and adults. It is the second leading cause of cancer-related death in children and has significant morbidity and mortality in adults (1–6). According to the World Health Organization (WHO) classification (smw-1), glioblastomas (WHO grade IV) and anaplastic gliomas (astrocytomas, oligoastrocytomas, and oligodendrogliomas) (WHO grade III) are collectively referred to as malignant gliomas. Glioblastoma, the most common and most devastating glial tumor, is associated with extremely poor prognosis and high likelihood of relapse. It accounts for more than 50% of primary brain tumors. Despite significant advances in diagnostic techniques, the classical therapeutic agents are largely palliative and remain unsuccessful in providing long-term survival for patients with brain cancer. The conventional therapies used today remain similar to those used half a century ago (7–12). Thus, there is an urgent need for novel anticancer compounds that are non-toxic, efficacious, and able to significantly improve overall treatment in patients with malignant brain tumors.
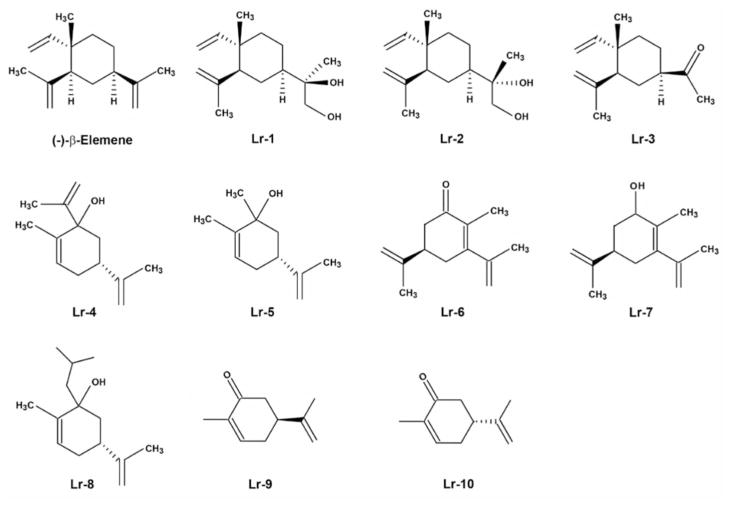
β-Elemene (β-1-methyl-1-vinyl-2,4-di-isopropenyl-cyclohexane), one of the active components in the essential oil of the traditional Chinese herbal medicine Curcuma wenyujin (13, 14), has been reported to exhibit a variety of pharmacological effects on tumor proliferation, oxidative stress, and fibrosis (15–22). As a multifunctional herbal medicine with few side-effects and strong bone marrow protection, β-elemene has attracted the attention of clinicians and scientists around the world (15–19, 23). In recent years, β-elemene has been intensively investigated due to its potent inhibition of various types of cancer cells both in vitro and in vivo (15–19, 23). Chemists and scientists are also ambitiously searching for synthetic β-elemene analogs that will reduce costs and achieve better results. A series of β-elemene synthetic analogs currently being tested are shown in Figure 1.
Figure 1.
Chemical structures of β-elemene and its synthetic analogs.
Apoptosis, or programmed cell death, plays an essential role in regulating a broad range of biological processes, such as cell differentiation and proliferation, as well as development, immunity, and tissue homeostasis. Dysregulation of apoptosis is associated with a variety of human diseases, including cancer, autoimmune diseases, infections, and neurodegenerative diseases (24–27). Apoptosis is the most important therapeutic target and the most common mechanism by which chemotherapeutic agents kill cancer cells and eradicate tumor tissues. The induction of apoptosis is regarded as the most efficient strategy for eliminating cancer cells (28, 29).
Given that there have been no substantial improvements in malignant brain tumor chemotherapy, we were motivated to evaluate β-elemene and its synthetic analogs using in vitro tests to determine: (i) whether β-elemene can effectively inhibit brain cancer cell growth and proliferation; (ii) whether its efficacy is mediated through the induction of apoptosis; (iii) how the apoptotic signaling pathways are regulated in the process; and (iv) whether synthetic β-elemene analogs preserve the same antitumor efficacy as β-elemene against brain cancer cells. The present study employed the three most commonly used malignant brain tumor cell lines, namely A-172, CCF-STTG1, and U-87MG, and focused primarily on β-elemene and three analogs: Lr-1 [(R or S)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinylcyclohexyl)-propane-1,2-diol]; Lr-2 [(S)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-propane-1,2-diol and (R)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-propane-1,2-diol]; and Lr-3 [1-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-ethanone].
Materials and Methods
Chemicals and reagents
(−)-β-Elemene (98% purity) and its 10 synthetic analogs were obtained from Yuanda Pharmaceuticals, Ltd. Inc. (Dalian, PRC). Dimethylsufoxide (DMSO) and propidium iodide (PI) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Antibodies against BAX, BCL-2, BCL-XL, XIAP, β-actin, peroxidase-labeled anti-rabbit immunoglobulin G (IgG), Blotto B, and ECL western blotting system were all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell lines and cell culture conditions
The brain glioblastoma cell lines A-172, U-87MG and brain astrocytoma cell line CCF-STTG1 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in monolayer using RPMI-1640 medium (Invitrogen, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). All cell lines were grown in logarithmic phase at 37°C, in a humidified atmosphere consisting of 5% CO2 and 95% air. Cells were routinely tested for mycoplasma infection using a commercial assay system (MytoTect; Invitrogen), and new cultures were established each month from frozen stocks. All media and reagents contained <0.1 ng/ml endotoxin, as determined by Limulus polyphemus amebocyte lysate assay (Whittaker Bioproducts, Walkersville, MD, USA). Before starting the experiments, cells were grown to 70–80% confluence after sub-culturing. β-Elemene was serially-diluted in culture medium to obtain the desired concentrations.
Drug treatment and cell survival assay
The effects of β-elemene and its 10 synthetic analogs on cell survival were measured by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay, as described previously (15–19, 23). In brief, cells were harvested using 0.25% trypsin-EDTA and resuspended to a final concentration of 5×104 cells/ml in fresh medium containing 10% fetal calf serum. Aliquots of 0.1 ml from each cell suspension were distributed evenly into 96-well cell culture plates (Corning, Inc., Corning, NY, USA). One column from each plate contained medium-alone as a blank control, and another column contained cells without drug exposure as an untreated control. After 24 h of incubation with fresh medium, serial dilutions of β-elemene or its synthetic analogs (20 to 180 μg/ml) were added individually, and the cells were incubated for an additional 24, 48, 72, 96, and 120 h, respectively. The effects of β-elemene and its analogs on growth of human brain cancer cells were determined using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The absorbance readings (A) were taken using a 96-well Opsys MR™ Microplate Reader (ThermoLab Systems, Chantily, VA, USA) and Revelation™ QuickLink Software at 490 nm. The blank control wells were used for zeroing absorbance. The percentage of cell survival was calculated using the background-corrected absorbance as follows: % Cell viability=100× (1-A of experimental well)/A of untreated control well. All experiments were performed at least three times with representative data presented.
Annexin V assay
Annexin V assay was used to detect apoptotic cells by staining cells with both annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI). Cells were treated with 0, 30, 50, and 70 μg/ml β-elemene, respectively. After 48-h treatment, 1×106 cells/ml were washed twice with PBS and resuspended in 300 μl binding buffer [10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, pH 7.4], then 3 μl of annexin V-FITC (Caltag Laboratories, Burlingame, CA, USA) was added to the cells. Cells were incubated on ice in the dark for 1.5 h and then washed with 4 ml HEPES buffer once before adding 10 μl PI (50 μg/ml) to the cells. Cells were then incubated for another 20 min and analyzed within 30 min by FACSCalibur (Becton-Dickinson, San Diego, CA, USA). CellQuest Pro software (Becton-Dickinson) and ModFit LT software (Verity Software House, Inc., Topsham, MN, USA) were used to determine the distribution of apoptotic cells. The same experiments were repeated at least three times.
Terminal deoxynucleotidyltransferase-mediated deoxy-UTP-fluorescein nick end labeling (TUNEL) assay
For in situ detection of apoptotic nuclei, TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the procedures described by the manufacturer. In brief, after desired treatment, cells were washed with PBS and then were placed in Bouin’s fixative for at least 24 h, dehydrated in alcohol, and paraffin-embedded using standard protocols. Sections of 5-μm thickness were mounted on glass slides and stained with hematoxylin and eosin. After rehydration, sections were post-fixed in 4% paraformaldehyde-PBS (pH 7.2), rinsed in PBS, and incubated for 1 h at room temperature with a mixture containing fluorescein-deoxy-UTP and terminal deoxynucleotidyltransferase, following the manufacturer’s instructions. Cells with brown granules in nuclei were considered TUNEL-positive cells. Over 200 cells were counted under a light microscope to determine the percentage of positive cells.
DNA fragment detection by enzyme-linked immunosorbent assay (ELISA)
Cells were seeded at a density of 104 cells/well in 96-well plates overnight. After desired treatment of cells with 0, 30, 50, or 70 μg/ml β-elemene for 24, 48, and 72 h, cells were incubated in lysis buffer for 30 min to obtain cytoplasmic lysates. A cell death detection ELISA kit (Cell Death Detection ELISAplus; Boehringer Mannheim, Indianapolis, IN, USA) was used to quantitatively determine cytoplasmic histone-associated DNA oligonucleosome fragments associated with apoptotic cell death according to the manufacturer’s instructions. Briefly, samples were incubated in microtiter plates adsorbed with mouse anti-histone antibody (clone H11-4) to bind histone-associated DNA oligonucleosomes uncovered by endonuclease-mediated DNA nicking. Plates were washed, and non-specific binding sites were saturated with blocking buffer. Bound samples were then reacted with anti-mouse DNA monoclonal antibody (MCA-33) and then conjugated with peroxidase. To determine the amount of retained peroxidase, 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS) was added as a substrate. The absorbance was read using a 96-well Opsys MR™ Microplate Reader (ThermoLab Systems) at 405 nm. The enrichment of mono- and oligonucleosomes released into the cytoplasm was calculated as the absorbance of sample cells divided by the absorbance of control cells. The apoptotic index was calculated as an enrichment factor, that is, the ratio of the result compared with the control set arbitrarily at 1.0. Each experiment was performed in triplicate, and means and standard deviations were calculated.
Caspase-3/7/10 enzymatic activity assay
Caspase-3/7/10 enzymatic activity was measured using the CasPASE™ Apoptosis Assay kit (Bioworld, Atlanta, GA, USA) according to the manufacturer’s protocol. In brief, after treatment with β-elemene for 24, 48, and 72 h, a total of 1×107 cells were harvested and washed twice with ice-cold PBS. Cytosolic extracts were prepared by five repeated cycles of freezing and thawing in 200 μl of lysis buffer, then by centrifugation at 12,000 rpm for 30 min at 4°C. Cell lysates (50 μl) were diluted with 2×CasPASE™ buffer (50 μl) in 96-well plates, and incubated at 37°C for 2 h with 5 μl of 1 mM caspase substrates. The absorbance of the cleaved substrate was measured by a 96-well Opsys MR™ Microplate Reader (ThermoLab Systems) at a test wavelength of 405 nm. Non-apoptotic cell lysate was used as the negative control. Buffer without cell lysate was used as the blank. Each experiment was performed in triplicate, and means and standard deviations were calculated.
Protein extraction and western blot analysis
Cells treated with β-elemene were harvested by trypsinization following 48 h of incubation. After washing with ice-cold PBS, the cells were lysed on ice for 30 min in a mammalian cell lysis buffer (Quality Biological, Inc., Gaithersburg, MD, USA), containing 10 μl/ml of 200 mM phenylmethylsulfonyl fluoride (PMSF), 10 μl/ml of 100 mM sodium orthovanadate, and 10 μg/ml aprotinin. Cellular extracts were clarified by centrifugation at 12,000 rpm at 4°C for 30 min, and protein concentrations were determined using the Bradford assay (Bio-Rad, Richmond, CA, USA). Sixty micrograms of proteins from whole-cell lysates were mixed 1:1 with 2×sodium dodecyl sulfate (SDS) protein gel solution (Quality Biological, Inc., Gaithersburg, MD, USA), heated for 5 min at 95°C, then separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Schleicher & Schuell BioScience, Inc., Keene, NH, USA). After blocking in Blotto B (Santa Cruz) for 1 h at room temperature, membranes were incubated overnight at 4°C with the specific primary antibodies (dilutions were 1:100–1:300). Membranes were washed with TBS/0.1% Tween20 solution, incubated with anti-rabbit peroxidase-conjugated secondary antibody (dilution was 1:1000), washed again, and developed with enhanced chemiluminescence substrate (Santa Cruz) according to the manufacturer’s instructions. The protein bands were visualized using X-ray films (Eastman Kodak, Rochester, NY, USA). All blots are representative of three independent experiments.
Statistical data analysis
Data are presented as the mean±SD. The Student’s t-test was used to analyze the difference between the means of the treatment groups and the control group. Differences with a p-value of less than 0.05 were considered statistically significant.
Results
β-Elemene was cytotoxic towards human brain tumor cells
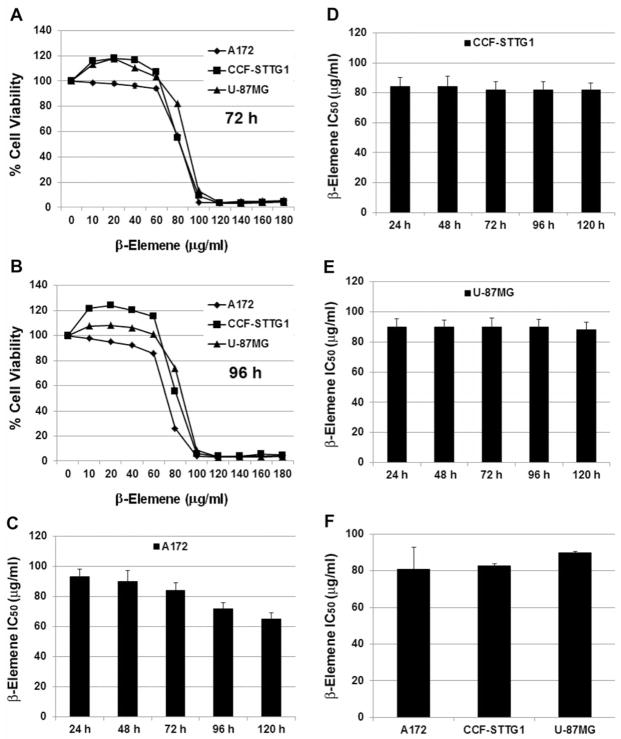
Three different human malignant brain tumor cell lines were used to investigate the effect of β-elemene on tumor cell growth and proliferation. The MTT assay showed that β-elemene at low concentrations (10–40 μg/ml) slightly increased cell growth, possibly because of a shock response of the cells to the drug. At higher concentrations (>40 μg/ml), β-elemene exerted dramatic cytotoxicity towards all three tumor cell lines. At 100 μg/ml or higher concentrations, β-elemene killed 90% or more of the cells (Figure 2A and B). The average half-maximal inhibitory concentration (IC50) of β-elemene was 80.8±11.9 μg/ml for A172 cells, 82.8±1.1 μg/ml for CCF-STTG1 cells, and 88.6±0.89 μg/ml for U-87MG cells (Figure 2C-F). The data indicate that β-elemene effectively inhibits human brain tumor cell growth and proliferation.
Figure 2.
Cytotoxicity of β-elemene towards three human brain tumor cell lines. Exponentially growing cells were treated with β-elemene at the indicated concentrations (10–180 μg/ml) for 24, 48, 72, 96, or 120 h. A and B: Cell survival was assessed by the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and cell growth values were expressed relative to those of untreated cells (100% control value). C-E: The average half-maximal inhibitory concentration (IC50) values at each time point were calculated for the three cell lines. F: The average IC50 values of β-elemene were 80.8±11.9 μg/ml in A172 cells, 82.8±1.1 μg/ml in CCF-STTG1 cells, and 88.6±0.89 μg/ml in U 87MG cells. The results represent the mean of at least three independent experiments.
Anticancer efficacy of β-elemene and its synthetic analogs towards human brain tumor cells
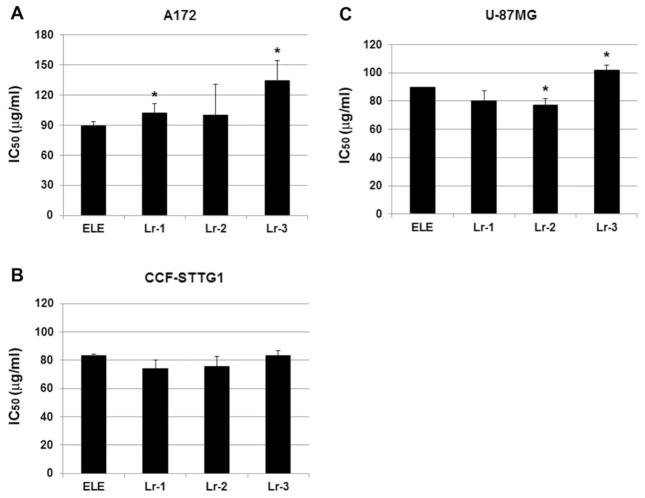
To compare the cytotoxic efficacy of β-elemene and its 10 synthetic analogs, we exposed brain cancer cells to serially diluted concentrations of each compound for 24, 48, and 72 h and then performed a routine MTT assay. Our preliminary results showed that Lr-1, Lr-2, Lr-3, and Lr-8 [(1R,5S)-1-isobutyl-3,5-di-isopropenyl-2-methyl-cyclohex-2-enol and (1S,5S)-1-isobutyl-3,5-diisopropenyl-2-methyl-cyclohex-2-enol] had anticancer efficacy comparable to that of β-elemene, whereas the other six analogs did not (data not shown). We focused on Lr-1, Lr-2, and Lr-3 in the subsequent experiments. Based on MTT assay results for A172 cells, the average IC50 values were 89±4.5 μg/ml (β-elemene), 102.4±8.8 μg/ml (Lr-1), 100±30.4 μg/ml (Lr-2), and 134.2±20.0 μg/ml (Lr-3), indicating that Lr-1 and Lr-3 were less potent than β-elemene (p<0.05 for each), whereas Lr-2 and β-elemene had equivalent cytotoxicity (Figure 3A). For CCF-STTG1 cells, all three synthetic compounds had the same cytotoxic efficacy as β-elemene, based on the average IC50 values: 83.3±1.15 μg/ml (β-elemene), 73.84±6.99 μg/ml (Lr-1), 75.55±7.62 μg/ml (Lr-2), and 83.14±6.07 μg/ml (Lr-3) (p>0.05 for each; Figure 3B). For U-87MG cells, the average IC50 values were 90±10 μg/ml (β-elemene), 80.22±7.2 μg/ml (Lr-1), 77.27±4.34 μg/ml (Lr-2), and 102.06±3.57 μg/ml (Lr-3), suggesting that Lr-1 had the same cytotoxicity as β-elemene (p>0.05), Lr-2 was more potent than β-elemene (p<0.05), and Lr-3 was less potent than β-elemene (p<0.05) (Figure 3C). Thus, the three synthetic β-elemene analogs have different cytotoxic efficacies against the three brain tumor cell lines, as compared with β-elemene. Overall, Lr-1, Lr-2, and β-elemene exhibited the same anticancer efficacy in vitro, while Lr-3 was less potent than β-elemene towards brain carcinoma cells.
Figure 3.
Comparison of cytotoxic efficacy of β-elemene and its synthetic analogs towards three human brain tumor cell lines. Exponentially growing cells were treated with β-elemene and its analogs Lr-1, Lr-2, Lr-3, respectively, at concentrations of 60–140 μg/ml for 24, 48, and 72 h. The average half-maximal inhibitory concentration (IC50) values of β-elemene and its analogs at 24, 48, and 72 h are shown for A172 cells (A), CCF-STTG1 cells (B), and U-87MG cells (C). The data are representative of three independent experiments. Significant differences compared with β-elemene-treated cells are indicated as *p<0.05. ELE, β-Elemene; Lr-1, (R or S)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-propane-1,2-diol; Lr-2, (S)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-propane-1,2-diol, and (R)-2-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-propane-1,2-diol; Lr-3, 1-((1R,3S,4S)-3-isopropenyl-4-methyl-4-vinyl-cyclohexyl)-ethanone.
β-Elemene induced apoptosis in human brain tumor cells as detected by annexin V staining
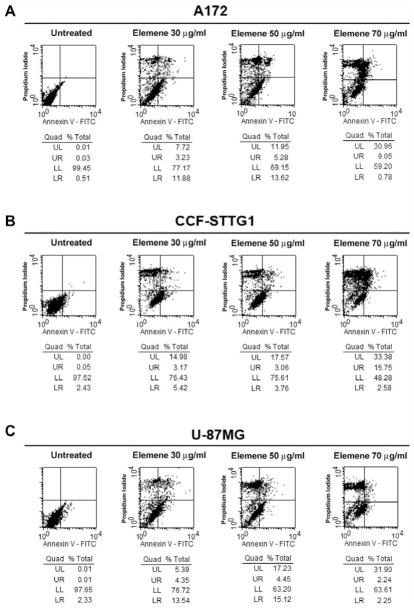
To examine apoptosis as a possible mechanism of β-elemene inhibition of tumor cell growth and proliferation, we performed flow cytometry to detect apoptotic cells based on annexin V staining on the outer cytoplasmic membrane. After 48 h, the annexin V-FITC level was significantly higher in tumor cells treated with β-elemene than in untreated cells. For A172 cells, the total percentage of early apoptotic, late apoptotic and necrotic cells was 0.55% for the untreated control cells, 22.83% for cells treated with β-elemene at 30 μg/ml, 30.85% for cells treated at 50 μg/ml, and 38.71% for cells treated at 70 μg/ml (Figure 4A). The respective values for CCF-STTG1 cells were 2.51%, 23.57%, 24.39%, and 51.72% (Figure 4B), and for U-87MG cells were 0.62%, 23.28%, 36.8%, and 36.39% (Figure 4C). These findings suggest that the apoptotic response contributes to the inhibitory effect of β-elemene on brain tumor cells.
Figure 4.
Detection of β-elemene-induced apoptosis in human brain tumor cell lines by flow cytometry. Cells were treated with β-elemene for 48 h, and then cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) to detect cell membrane changes caused by β-elemene. Untreated cells were used as a negative control. Annexin V-FITC staining is evident in A172 cells (A), CCF-STTG1 cells (B), and U-87MG cells (C). ELE 30, β-elemene 30 μg/ml. UL, Upper left, represents necrotic cells (%); UR, upper right, represents late apoptotic cells (%); LL, lower left, represents viable cells (%); LR, lower right, represents early apoptotic cells (%).
β-Elemene induced apoptosis in human brain tumor cells based on cell death detection ELISA and TUNEL staining
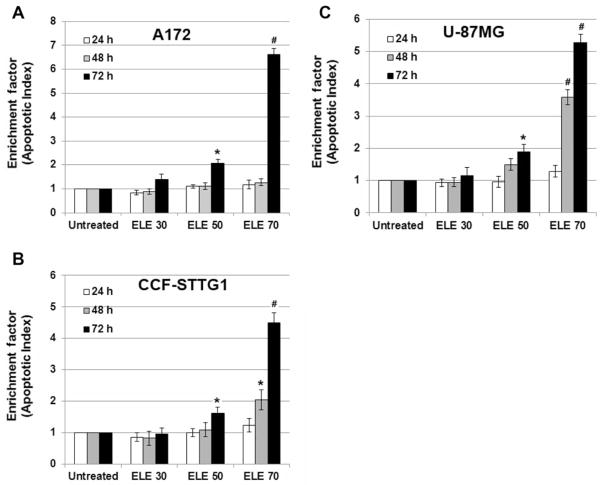
Small nucleotide fragments produced as a result of apoptosis were quantified using a cell death detection ELISA, and the enrichment factor, which is the fold increase in fragments for treated cells compared with untreated cells, was determined as a parameter of apoptosis. At 24 and 48 h, there was no appreciable change or only a moderate increase in the enrichment factor for all cell lines treated with either concentration of β-elemene (p>0.05), with the exception of CCF-STTG1 and U-87MG cells treated with β-elemene at 70 μg/ml for 48 h (p<0.01 and p<0.05, respectively, compared with untreated cells). However, after 72 h, the enrichment factor was significantly increased for all three brain tumor cell lines treated with β-elemene at 50 and 70 μg/ml (p<0.01 and p<0.05, compared with untreated cells, respectively). These data suggest that β-elemene induced apoptotic cell death of human brain cancer cells in a dose- and time-dependent manner (Figure 5A–C).
Figure 5.
Detection of β-elemene-induced apoptosis in human brain tumor cell lines by cell death detection enzyme-linked immunosorbent assay (ELISA). Cells were treated with β-elemene at the indicated concentrations for 24, 48, or 72 h, and then small nucleotide fragments produced as a result of cell apoptosis were quantified by ELISA using a commercially available apoptosis detection kit. The enrichment factor, which is correlated with the number of apoptotic cells, is shown for A172 cells (A), CCF-STTG1 cells (B), and U-87MG cells (C). ELE 30, β-elemene 30 μg/ml. Data are representative of three independent experiments. Significant differences compared with untreated control cells are indicated as *p<0.05 and #p<0.01.
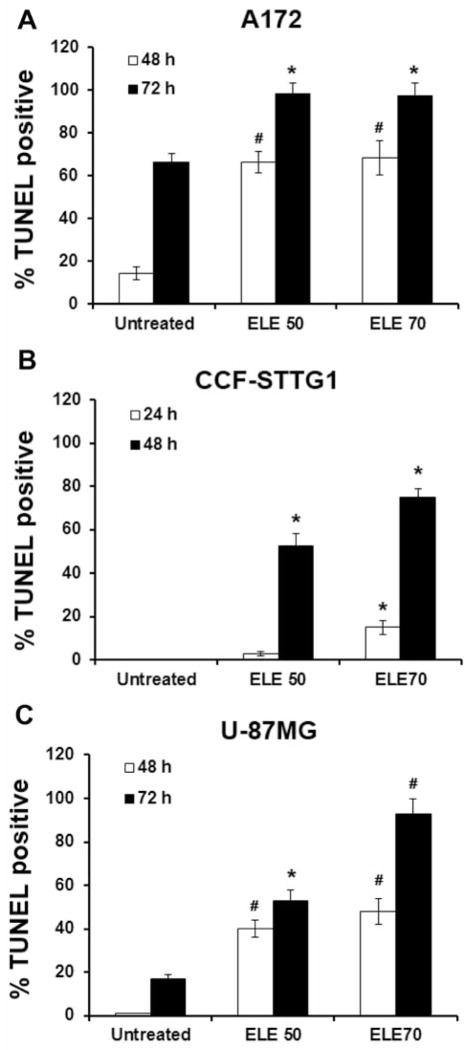
TUNEL staining was used to detect apoptotic nuclei in situ in brain cancer cells. Compared with TUNEL staining of untreated cells, the rate of positive TUNEL staining was increased in both A172 and U-87MG cells after treatment with β-elemene for 48 and 72 h (Figure 6A and C), and in CCF-STTG1 cells after treatment for 24 and 48 h (Figure 6B).
Figure 6.
Detection of β-elemene-induced apoptotic nuclei in situ in human brain tumor cells by terminal deoxynucleotidyltransferase-mediated deoxy-UTP-fluorescein nick end labeling (TUNEL) staining. Cells were incubated with β-elemene at the indicated concentrations. At the termination of incubation (48 and 72 h for A172 and U-87MG cells; 24 and 48 h for CCF-STTG1 cells), the cells were harvested, fixed with 4% formaldehyde, and labeled using TUNEL. The numbers of TUNEL-positive cells counted under a light microscope are shown for A172 cells (A), CCF-STTG1 cells (B), and U-87MG cells (C). Significant differences compared with untreated control cells are indicated as *p<0.05 and #p<0.01. ELE 50, β-elemene 50 μg/ml.
β-Elemene-induced apoptosis of human brain tumor cells was dependent on caspases
To further characterize the molecular mechanism by which β-elemene induced apoptosis in human brain cancer cells, the activity of caspase-3/7/10 was measured by ELISA. Caspase-3/7/10 activity was significantly increased in all three human brain tumor cell lines at 24, 48, and 72 h after treatment with β-elemene at 70 μg/ml (p<0.01 and p<0.05, compared with untreated cells, respectively). With β-elemene at 30 and 50 μg/ml, the changes in caspase activity varied among the three cell lines. Caspase activity was both time- and cell line-dependent (Figure 7A–C). The increase in caspase activity was blocked by the general caspase inhibitor Z-VAD- FMK (data not shown).
Figure 7.
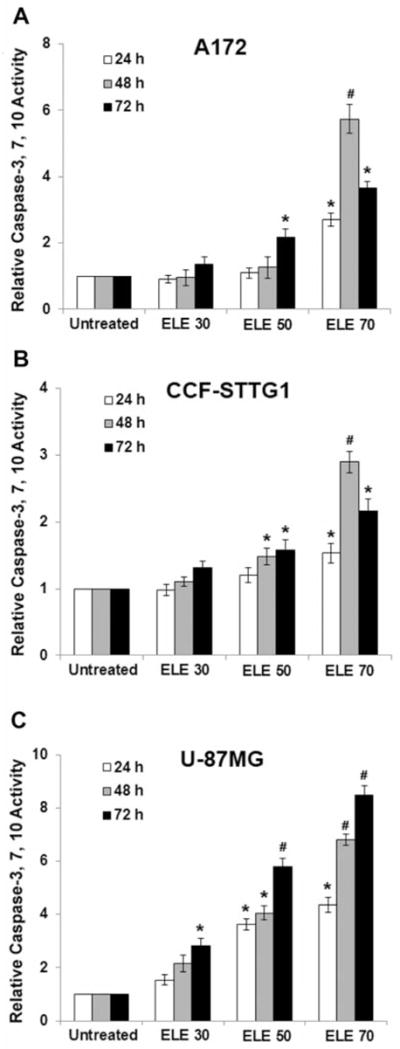
β-Elemene triggered caspase activation of human brain tumor cells. Cells were treated with β-elemene at 30, 50, or 70 μg/ml for 24, 48, and 72 h, and caspase-3/7/10 enzymatic activity was measured using a CasPASETM Apoptosis Assay kit. Non-apoptotic cell lysate was used as a negative control. Buffer without cell lysate was used as the blank. The relative caspase activity, determined by calculating the fold-increase of caspase activity in the treated cells compared with the untreated cells, is shown for A172 cells (A), CCF-STTG1 cells (B), and U-87MG cells (C). *p<0.05 and #p<0.01 indicate a significant difference compared with untreated control cells. ELE 30, β-elemene 30 μg/ml.
β-Elemene-induced apoptosis of human brain tumor cells was modulated by BCL-2 family proteins and XIAP
Western blot analysis revealed that β-elemene up-regulated BAX protein expression and down-regulated that of BCL-2, BCL-XL, and X-linked inhibitor of apoptosis (XIAP) in the three brain tumor cell lines. These results suggest that BCL-2 family proteins and XIAP are involved in the regulation of the apoptotic signaling pathway induced by β-elemene in human brain cancer cells (Figure 8).
Figure 8.
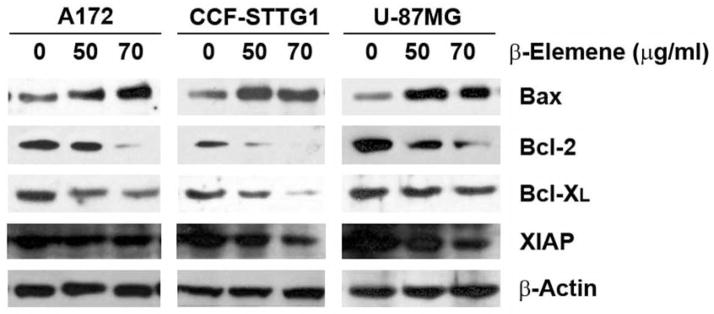
β-Elemene caused alterations in the levels of the BCL-2 family of proteins and X-linked inhibitor of apoptosis (XIAP) in human brain tumor cells. Cells were treated with β-elemene at the indicated concentrations for 48 h. The levels of BCL-2 family of proteins and XIAP were analyzed by western blotting as described in Materials and Methods. Equal protein loading was verified with an anti-β-actin antibody.
Discussion
β-Elemene, a natural product extracted from medicinal herbs, possesses diverse functions and is effective in eliminating a variety of cancer cell types, both in vitro and in vivo (15–19). Multiple investigations have demonstrated that cancer cell death induced by β-elemene occurs by the induction of apoptosis. Here, we show that β-elemene strongly inhibited the growth and proliferation of malignant brain tumor cells in vitro through significant induction of cancer cell apoptosis. The present study is consistent with previous reports from our group and others.
Malignant brain tumor is the most aggressive type of cancer in humans, and glioblastoma is among the most lethal forms of human cancer. As current treatments are unsuccessful, development of novel therapeutic compounds for this disease is imperative. In this study, we tested the cytotoxic efficacy of β-elemene in the three most commonly used human malignant brain tumor cell lines. The MTT assay showed that at low concentrations (10–40 μg/ml), β-elemene slightly promoted cell growth; however, at higher concentrations (>40 μg/ml), it exhibited considerable cytotoxicity towards all three tumor cell lines, and at 100 μg/ml or higher concentrations, β-elemene killed more than 90% of the cancer cells. These data indicate that β-elemene effectively inhibited human brain tumor cell growth and proliferation, although the cytotoxic responses of the three cell lines were slightly different. U-87MG cells were slightly less sensitive to β-elemene (IC50 of 88.6 μg/ml) compared with A172 cells (IC50 of 80.8 μg/ml) and CCF-STTG1 cells (IC50 of 82.8 μg/ml).
Alternative compounds with better efficacy and lower costs are always being sought by chemists and scientists. Thus, we compared the cytotoxic efficacy of β-elemene and its synthetic analogs Lr-1, Lr-2, and Lr-3 in human brain tumor cells. Compared with β-elemene, both Lr-1 and Lr-2 exhibited as good as, or better anticancer efficacy towards CCF-STTG1 and U-87MG cells, but not to A172 cells. Lr-3 was less potent than β-elemene. These results suggest that Lr-1 and Lr-2 are potential alternatives to β-elemene in treating patients with brain cancer, although both compounds have differential efficacy among different forms of brain tumor cells.
Apoptosis is the most common mechanism by which antitumor drugs affect cancer cells. In the present study, we used an annexin V assay to detect apoptosis as a possible mechanism of β-elemene action. Annexin V is a calcium-dependent phospholipid-binding protein with high affinity for phosphatidylserine, a plasma membrane phospholipid. One of the earliest features of apoptosis is the translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane, thereby exposing phosphatidylserine to the external environment. Annexin V binds to phosphatidylserine exposed on the cell surface and identifies cells at an early stage of apoptosis. In our experiment, the annexin V-FITC binding level was significantly increased in all three brain tumor cell lines after β-elemene treatment, indicating increased combined percentages of cells undergoing early-apoptosis, late-apoptosis and necrosis. This suggests that apoptosis is involved in the cell death process of brain cancer cells triggered by β-elemene.
DNA fragmentation, a form of degradation of nuclear DNA into nucleosomal units, is also a hallmark of apoptotic cell death. This phenomenon is characterized by the activation of endogenous endonucleases (caspase-activated DNAse, CAD) with subsequent cleavage of chromatin DNA into internucleosomal fragments. This effect can be used to detect apoptosis using several assays such as TUNEL and ELISA. In the present study, apoptotic DNA fragmentation significantly increased in β-elemene-treated cancer cells by both TUNEL staining and ELISA, again confirming β-elemene-induced apoptosis of human brain cancer cells.
The occurrence of apoptosis is dependent on the activation of a series of caspases belonging to a group of cysteine proteases. Caspases cleave selected cellular substrates after aspartic residues (30–33). The activation of caspases is tightly regulated by two apoptotic signaling pathways. The extrinsic pathway is mediated by the activation of a group of death receptors located at the cell surface and is associated with the activation of caspase-8. The intrinsic pathway, also called the mitochondrial pathway, is triggered by the release of specific proteins, including cytochrome c and SMAC/DIABLO, from the mitochondria (32). This pathway governs cell death by inducing the assembly of a functional apoptosome and by activating caspase-9 (34). Activated caspase-8 and -9 initiate a series of downstream events, including the activation of caspase-3 and -7, leading to the induction of apoptosis of cells. XIAP is the only cellular component that can dramatically suppress the enzymatic activity of caspases at both the initiation (caspase-9) and execution stages (caspase-3 and -7) of apoptosis, thereby potentiating resistance to apoptosis (35). In the present study, caspase-3/7/10 activity in brain cancer cells was significantly increased upon β-elemene treatment, in a time- and dose-dependent manner. The increased activity was blocked by a general caspase inhibitor, Z-VAD-FMK. Thus, β-elemene induced apoptosis of brain cancer cells by activating multiple caspases, all of which are critical for initiating and executing apoptosis.
The BCL-2 family of proteins contain both pro-apoptotic (e.g. BCL-xS, BAD, BAX, and BAK) and anti-apoptotic proteins (e.g. BCL-2, BCL-XL, MCL-1, A1, and BAG-1) (36–38). Cell fate is dependent on the dynamic balance between anti- and pro-apoptotic proteins, and a slight imbalance can either inhibit or promote cell death (36, 38). Our results showed that β-elemene up-regulated BAX expression and down-regulated BCL-2, BCL-XL, and XIAP expression in the three brain tumor cells lines, suggesting that the BCL-2 family of proteins are involved in the apoptotic signaling triggered by β-elemene in human brain cancer cells.
On the basis of these results, we conclude that: (i) β-elemene is effective in suppressing brain cancer cell growth and proliferation; (ii) the antitumor effect of β-elemene is strongly associated with the induction of apoptosis; (iii) the initiation and execution of apoptosis induced by β-elemene are governed by apoptotic signaling pathways; and (iv) the β-elemene analogs Lr-1 and Lr-2 have the same antitumor efficacy as β-elemene in brain cancer cells. Although the precise mechanisms of its action remain to be determined, the current research provides evidence, to our knowledge for the first time, that some synthetic β-elemene analogs present tremendous potential as alternatives for malignant brain tumor treatment and provide enormous hope for patients with brain cancer in the future.
Acknowledgments
This publication was made possible by grants from the Natural Science Foundation of Science & Technology Department of Guangxi Province (no. 0991294) and the Guangxi Scientific Research and Technological Development Program (no. 200901059), and by grants from the National Institutes of Health (no. P20RR16440-010003, P20RR16440-020003, P20RR16440-030003, P20RR16440-040003) and West Virginia University School of Medicine Research Grant (to Q. Q. Li).
References
- 1.Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura Lima A, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F, Rao G. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clinical article. J Neurosurg. 2011;114:576–584. doi: 10.3171/2010.6.JNS091326. [DOI] [PubMed] [Google Scholar]
- 2.Ahsan H, Neugut AI, Bruce JN. Trends in incidence of primary malignant brain tumors in USA, 1981–1990. Int J Epidemiol. 1995;24:1078–1085. doi: 10.1093/ije/24.6.1078. [DOI] [PubMed] [Google Scholar]
- 3.Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro Oncol. 2001;3:141–151. doi: 10.1093/neuonc/3.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 6.Zada G, Bond AE, Wang YP, Giannotta SL, Deapen D. Incidence trends in the anatomic location of primary malignant brain tumors in the United States: 1992–2006. World Neurosurg. 2012;77:518–524. doi: 10.1016/j.wneu.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 7.Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: A review. J Neurooncol. 2011;104:639–646. doi: 10.1007/s11060-011-0565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amelio D, Lorentini S, Schwarz M, Amichetti M. Intensity-modulated radiation therapy in newly diagnosed glioblastoma: A systematic review on clinical and technical issues. Radiother Oncol. 2010;97:361–369. doi: 10.1016/j.radonc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell WL, Aristizabal SA. Treatment of glioblastoma multiforme. A review. Acta Radiol Ther Phys Biol. 1975;14:505–512. doi: 10.3109/02841867509132691. [DOI] [PubMed] [Google Scholar]
- 10.Jubelirer SJ. A review of the treatment and survival rates of 138 patients with glioblastoma multiforme. W V Med J. 1996;92:186–190. [PubMed] [Google Scholar]
- 11.Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O’Neill B, Faul C. A clinical review of treatment outcomes in glioblastoma multiforme – the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br J Radiol. 2012;85:729–733. doi: 10.1259/bjr/83796755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slatkin DN. Glioblastoma treatment. Science. 1994;265:1644–1645. doi: 10.1126/science.7993457. [DOI] [PubMed] [Google Scholar]
- 13.Dang YY, Li XC, Zhang QW, Li SP, Wang YT. Preparative isolation and purification of six volatile compounds from essential oil of Curcuma wenyujin using high-performance centrifugal partition chromatography. J Sep Sci. 2010;33:1658–1664. doi: 10.1002/jssc.200900453. [DOI] [PubMed] [Google Scholar]
- 14.Guo YT. Isolation and identification of elemene from the essential oil of Curcuma wenyujin. Zhong Yao Tong Bao. 1983;8:31–32. [PubMed] [Google Scholar]
- 15.Li QQ, Wang G, Huang F, Banda M, Reed E. Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. J Pharm Pharmacol. 2010;62:1018–1027. doi: 10.1111/j.2042-7158.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 16.Li QQ, Wang G, Reed E, Huang L, Cuff CF. Evaluation of cisplatin in combination with β-elemene as a regimen for prostate cancer chemotherapy. Basic Clin Pharmacol Toxicol. 2010;107:868–876. doi: 10.1111/j.1742-7843.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 17.Li QQ, Wang G, Zhang M, Cuff CF, Huang L, Reed E. β-Elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol Rep. 2009;22:161–170. doi: 10.3892/or_00000420. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wang G, Zhao J, Ding H, Cunningham C, Chen F, Flynn DC, Reed E, Li QQ. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol Life Sci. 2005;62:894–904. doi: 10.1007/s00018-005-5027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Li X, Huang F, Zhao J, Ding H, Cunningham C, Coad JE, Flynn DC, Reed E, Li QQ. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci. 2005;62:881–893. doi: 10.1007/s00018-005-5017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W, Liu K, Tang X. Preliminary study on the antitumor immuno-protective mechanism of β-elemene. Zhonghua Zhong Liu Za Zhi. 1999;21:405–408. [PubMed] [Google Scholar]
- 21.Zhang R, Tian A, Shi X, Yu H, Chen L. Downregulation of IL-17 and IFN-γ in the optic nerve by β-elemene in experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2010;10:738–743. doi: 10.1016/j.intimp.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zhang Z, Gao J, Xie J, Yang L, Hu S. Down-regulation effects of β-elemene on the levels of plasma endotoxin, serum TNF-α, and hepatic CD14 expression in rats with liver fibrosis. Front Med. 2011;5:101–105. doi: 10.1007/s11684-011-0111-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Li QQ, Zou B, Wang G, Li X, Kim JE, Cuff CF, Huang L, Reed E, Gardner K. In vitro combination characterization of the new anticancer plant drug β-elemene with taxanes against human lung carcinoma. Int J Oncol. 2007;31:241–252. [PubMed] [Google Scholar]
- 24.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: A review. Biochim Biophys Acta. 2011;1813:238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pore MM, Hiltermann TJ, Kruyt FA. Targeting apoptosis pathways in lung cancer. Cancer Lett. 2011;305:218–227. doi: 10.1016/j.canlet.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Signore M, Ricci-Vitiani L, De Maria R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2011;301:7–16. doi: 10.1016/j.canlet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson M, Zhivotovsky B. Caspases and cancer. Cell Death Differ. 2011;18:1441–1449. doi: 10.1038/cdd.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S. Mechanisms mediating caspase activation in cell death. Cell Death Differ. 1999;6:1060–1066. doi: 10.1038/sj.cdd.4400600. [DOI] [PubMed] [Google Scholar]
- 34.Fearnhead HO, Rodriguez J, Govek EE, Guo W, Kobayashi R, Hannon G, Lazebnik YA. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci USA. 1998;95:13664–13669. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann T, Strasser A, Jost PJ. FAS death receptor signalling: Roles of BID and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ola MS, Nawaz M, Ahsan H. Role of BCL-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 37.Martinou JC, Youle RJ. Mitochondria in apoptosis: BCL-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Yang Y, Xing D. BCL-2 and BCL-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]