Abstract
Tremendous progress has been made in understanding the genetics of hereditable pulmonary arterial hypertension (HPAH) since its description in the 1950s. Germline mutations in the gene coding bone morphogenetic receptor type 2 (BMPR2) are detectable in the majority of cases of HPAH, and in a small proportion of cases of idiopathic pulmonary arterial hypertension (IPAH). HPAH is an autosomal dominant disease characterized by reduced penetrance, variable expressivity, female predominance, and genetic anticipation. These characteristics suggest that endogenous and exogenous factors modify disease expression and areas of emphasis for future investigation. The variable clinical expression makes genetic counseling complex because the majority of carriers of a BMPR2 mutation will not be diagnosed with the disease. This issue will become increasingly important, as clinical testing for BMPR2 mutations is now available for the evaluation of patients and family members with HPAH and IPAH.
Keywords: Bone morphogenetic protein receptor 2, pulmonary arterial hypertension, genetics, modifiers, hereditary hemorrhagic telangiectasia
BACKGROUND
Pulmonary arterial hypertension (PAH) was first described by Dresdale et al in 1951 as a sporadic disease entity known as primary pulmonary hypertension (PPH).1 In 1954, Dresdale et al described the familial transmission of PPH in a kindred, thus providing the first known reports of both idiopathic PAH (IPAH) and familial PAH (FPAH).2 In the wake of years of genetic research, it is now recognized that PAH comes in many forms, including idiopathic (PAH), hereditable (HPAH), and associated with drug exposures (such as anorectic fenfluramine compounds) or other medical conditions.3
In the 30 years following the initial description of PAH in families, reports of additional families with HPAH appeared periodically in the literature, resulting in a total of 13 U.S. families reported by 1984. A follow-up analysis of these 13 families in 1984 revealed an additional eight new cases, as well as the description of a new family located in Tennessee. This fourteenth family contained the largest number of affected family members described to date, with six deaths due to pulmonary hypertension during two generations.4 Analysis of these 14 families showed vertical transmission with male to male transmission that proved an autosomal dominant mode of inheritance. It also became clear that a proportion of cases of pulmonary hypertension which appeared to occur as sporadic had a familial basis that was not obvious due to the reduced penetrance that allowed some carriers to remain unaffected yet able to silently pass their HPAH mutation to their offspring. In fact, the latest international classification scheme, which incorporates the label HPAH, does so at least in part to recognize the fact that up to 26% of cases previously thought to be idiopathic harbor identifiable mutations and therefore pose a hereditary risk to other family members. Finally, the notion of genetic anticipation, in which disease expression occurs at a younger age with each successive generation, was inferred from careful study of these family pedigrees.7
In the mid-to late 1980s, the National Institutes of Health (NIH) prospective registry of PPH provided the foundation for the clinical definition of PAH and facilitated interaction of participating investigators to collect and organize sufficient numbers of families to provide robust statistical power for a genomewide search for HPAH loci.8 Independently, two teams of investigators mapped the locus for the gene, named PPH1, for HPAH to chromosome 2q31–32 in 1997.9,10 Subsequently, both teams also demonstrated that germline mutations in the gene encoding bone morphogenetic protein receptor type-2 (BMPR2), a TGF-β superfamily of receptors member, was the gene responsible for the majority of cases of the autosomal dominant familial disease now known as HPAH.11,12 It is currently recognized that a small percentage of HPAH families (15 to 20%) have multiple affected individuals but do not have mutations identified in BMPR2 despite comprehensive testing. It is likely that mutations at one or more other loci are responsible, as demonstrated by HPAH associated with hereditary hemorrhagic telangiectasia (HHT) and mutations in activin-like kinase type 1 (ALK1) and endoglin (ENG).13,14
TRANSMISSION PATTERNS AND CLINICAL EXPRESSION OF PAH
Penetrance refers to the frequency with which a specific genotype results in the expression of a specific trait (phenotype) by the individual with that genotype. As with some other genetic diseases, such as Huntington disease, the penetrance of a BMPR2 mutation is reduced. Reduced penetrance of BMPR2 germline mutations can make establishing familial transmission difficult because generations of mutation-carrying individuals may not express disease.4,7 Thus only ∼20% of individuals with a known genetic mutation in BMPR2 will develop detectable PAH. In addition, expressivity of the disease (variations in the phenotype among those with a given genotype and disease penetrance), is variable. For example, onset can occur at any age, and although most die within 1 to 5 years of diagnosis without therapy some do survive many years. Finally, several other interesting findings characterize HPAH, including female predominance (≥2:1 female: male ratio) and genetic anticipation. All of these factors can be confounded in a highly mobile society such as ours, full family genealogical history is often impaired.15
Reduced Penetrance
Mutant BMPR2 alleles with reduced penetrance can segregate in HPAH kindreds, indicating that heterozygosity for a mutation is required but is not sufficient to precipitate clinical expression of HPAH in most cases. To date, the mechanisms that reduce penetrance are unknown but are likely to include additional genetic and/or environmental modifiers of disease expression.17
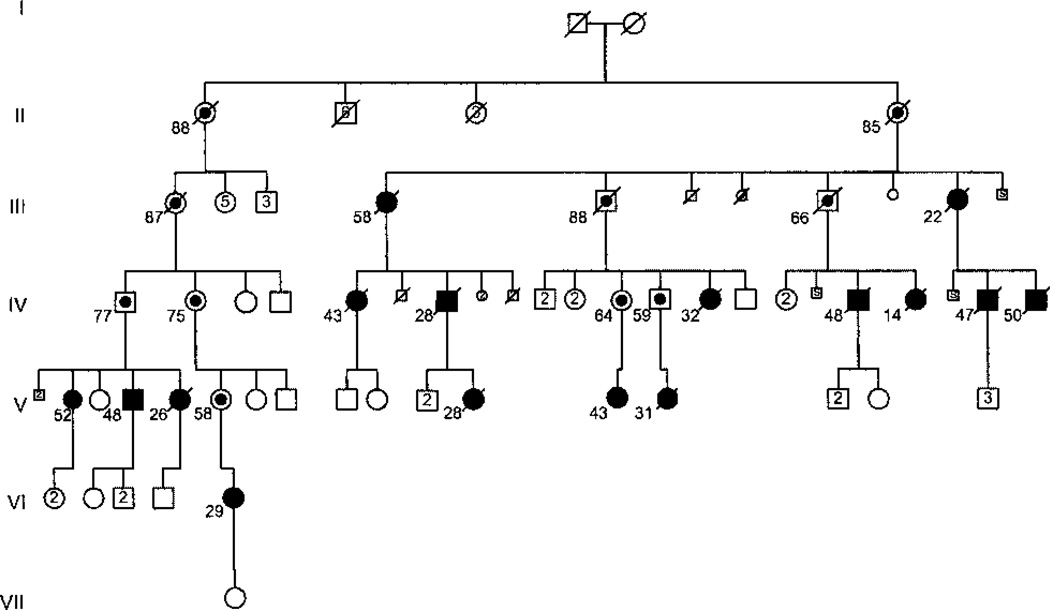
At Vanderbilt University, we follow a research cohort of 120 families with HPAH, each with reduced BMPR2 penetrance. Of 64 families with comprehensive genetic testing for germline BMPR2 mutations completed, 52 have a detectable mutation, whereas 12 do not. Of the 351 individuals formally diagnosed with PAH, 253 are females and 98 are males. The age at death spans the entire age spectrum, although a greater percentage of male deaths occur in childhood compared with percentage of female deaths in childhood. Although there are another 4085 bloodline family members at risk to develop HPAH, 18% of bloodline family members have been diagnosed with PAH to date. Of note, among the six most heavily affected families, 26% of first-degree relatives of patients with PAH have also been diagnosed with HPAH. Fig. 1 shows the family that has the second-largest number of affected subjects in our research registry (16 affected subjects). This family has a mutation in exon 9 of BMPR2 that causes haploinsufficiency (HI) due to the activation of the nonsense-mediated decay (NMD) pathway of RNA surveillance. Not surprisingly, this family’s pedigree demonstrates female predominance of the disease, as well as reduced disease penetrance and variable ages at disease onset.
Figure 1.
Pedigree of an extended kindred with heritable pulmonary arterial hypertension (HPAH) due to a BMPR2 mutation with reduced penetrance. This pedigree contains 16 total patients (11 females, 5 males) with HPAH, as well as 10 known heterozygotes (obligate carriers). Solid symbols represent individuals with disease. Circles represent women, squares represent men. Line through symbol represents death. Dot inside symbol represents obligate carrier of the BMPR2 mutation. Numbers below symbols represent age at death or current living age. Numbers inside symbols represent numbers of unaffected siblings of each gender. S inside symbol represents stillbirth. Diamond symbol represents sex unknown.
Of note, we continue to follow the fourteenth family described in our 1984 report, and it remains the largest known family with the disease.4 Twenty-eight family members have been diagnosed with HPAH overall, including 24 females and four males diagnosed at various ages between 9 and 70 years. Consistent with the reduced genetic penetrance of HPAH, this family contains numerous unaffected but at-risk individuals who have both a parent and progeny diagnosed with HPAH. These individuals must carry the germline BMPR2 mutation responsible for this disease, and they are at risk to develop the disease themselves.
A similar-sized research cohort at Columbia University, started by Drs. Robyn Barst and Jane Morse, is currently directed by Dr. Wendy Chung. The Columbia registry includes 106 families with 278 patients and also exhibits the characteristic reduced penetrance (personal communication). Together, the Vanderbilt and Columbia cohorts suggest that in North America there are at least 226 families consisting of at least 629 patients, along with several thousand family members at risk. At-risk family members who have not been genetically tested have an estimated risk of ∼1 in 10 to develop disease during their lifetime. This is due to the 50% chance they possess an HPAH-causing autosomal dominant allele (‘gene mutation’), and 20% chance that allele will be penetrant.
Skewed Gender Ratio
Since the 1950s, it has been known that ≥2 females are diagnosed for every male. In fact, aside from a BMPR2 mutation, gender is the most reliable determinant of HPAH, and understanding the mechanism by which gender modulates disease may one day improve diagnosis and therapy of PAH. Whether the female predominance reflects increased risk for females or decreased risk for males remains unclear. Suggestions of some hormonal influence (including exogenous ovarian hormone therapy) or effect increased by the presence of 46 XX, but decreased by 46 XY, chromosome complements have yet to be substantiated.18–21 Finally, it has been speculated that the gender discrepancy could result from increased male fetal losses—perhaps due to abnormalities of embryologic development.7
Variable Expressivity
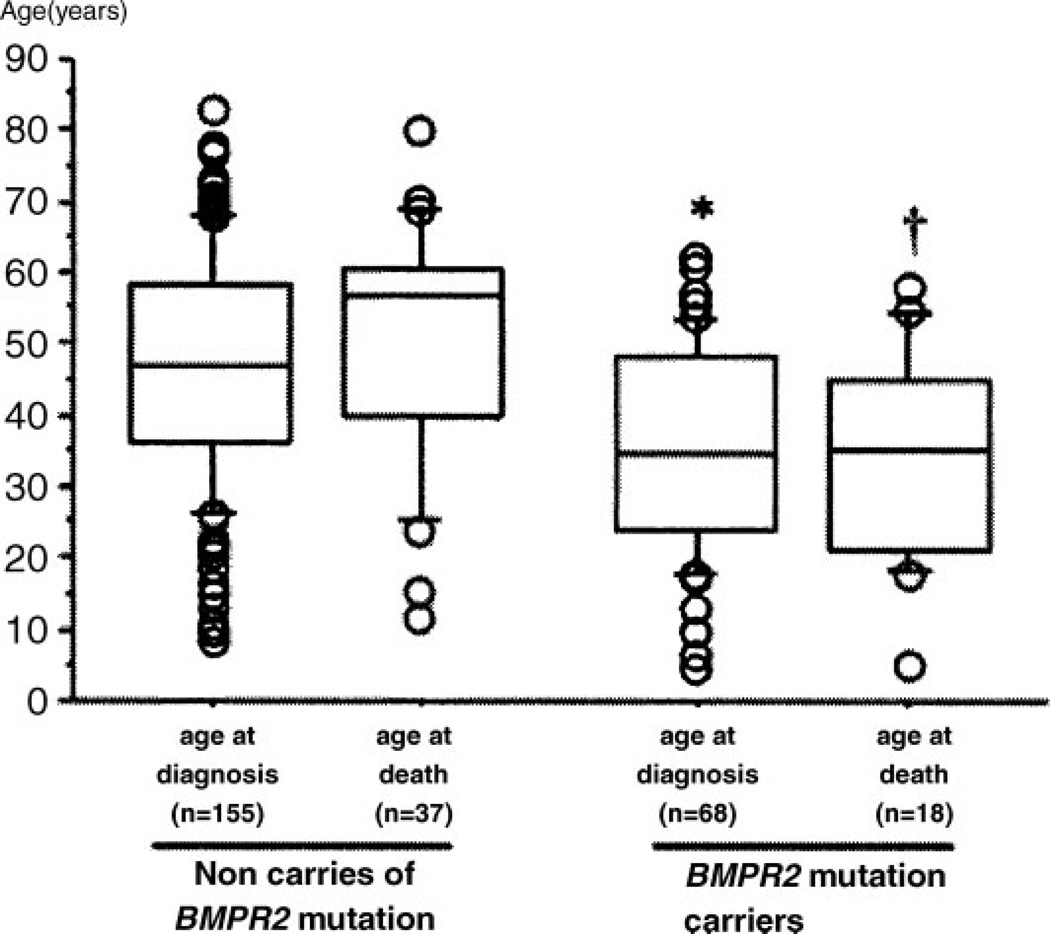
The variable age of onset of HPAH, which can affect individuals at any age from early childhood to late adulthood, reflects the variable expressivity of disease.22 Although there is a paucity of data convincingly outlining the impact of BMPR2 genotype upon clinical outcomes and therapeutic response in PAH, this is beginning to change. Recently, Sztrymf et al reported more severe disease among patients with a BMPR2 mutation. Although ascertainment bias of family members could account for the 10-year difference in age at diagnosis reported, the majority of subjects with a BMPR2 mutation (59%) had no known family history to prompt earlier clinical evaluation. In addition, the age at death was ∼15 years younger among BMPR2 mutation carriers (see Fig. 2). Finally, compared with patients without a mutation, BMPR2 mutation carriers had more severe hemodynamic compromise at diagnosis, including higher mean pulmonary artery pressure, lower cardiac index, and higher pulmonary vascular resistance. Interestingly, BMPR2 mutation status did not appear to impact survival.23
Figure 2.
Age at diagnosis and death from pulmonary arterial hypertension in a French series of BMPR2 mutation carriers and noncarriers. Age at diagnosis (P < 0.001) and age at death (p = 0.003) are lower in BMPR2 mutation carriers, as compared with noncarriers. Adapted from Sztrymf et al.23 Reproduced with permission of American Journal of Respiratory and Critical Care Medicine.
Although the study by Sztrymf et al did not demonstrate a survival difference based upon mutation status, there are attempts to use BMPR2 mutation type and status to select therapy in HPAH. Because a small proportion of PAH patients have good long-term response to calcium channel blockers, Elliott et al24 sought to compare the results of vasoreactivity testing according to BMPR2 mutation type.25,26 This retrospective study demonstrated that, compared with patients without a BMPR2 mutation, patients with nonsynonymous BMPR2 mutations were nearly nine times less likely to demonstrate vasoreactivity by standard testing [vasoreactivity in 3.7% of 27 patients with nonsynonymous BMPR2 variations versus 35% of 40 patients without mutation (p = 0.003)].24 Rosenzweig et al found similar results, as well as worse hemodynamic parameters consistent with those seen in the French report, in a cohort of adult and pediatric patients with PAH and a BMPR2 mutation.27
Genetic Anticipation
Several studies have shown a progressively earlier age of onset of HPAH in subsequent generations, a phenomenon known as genetic anticipation.7,28 Although it clearly occurs in some families, it is not uniformly seen and its significance is of considerable debate among investigators. Because its biological underpinnings in HPAH remain unproven, arguments remain that it may be a function of ascertainment bias. Trinucleotide repeat expansion (the basis of anticipation in many familial neurological diseases) and progressive telomere shortening (as seen in dyskeratosis congenita) do not appear to explain the younger age of onset reported in subsequent generations in HPAH.29,30
LOCUS HETEROGENEITY AND HPAH
Although the discovery that BMPR2 mutations constitute the genetic basis of the majority of cases of HPAH was a great step forward in the understanding of this disease, the genetic and molecular understanding is incomplete.31 For example, although it is the most frequently found mutation in HPAH, the BMPR2 gene is not the only locus at which a detectable mutation results in phenotypic expression of PAH.
With the cooperation of several large families with HPAH, microsatellite markers and linkage analysis focused the search for the gene ultimately identified as BMPR2 to chromosome 2, in the region of q31–33.9,32,33 Subsequently, a positional candidate gene approach identified BMPR2, which is a member of the transforming growth factor β (TGF-β) superfamily of receptors, as the responsible gene.15 Germline mutations in this large gene (∼192 kb), which resides on chromosome 2q33 and spans 13 exons, cause HPAH in most affected kindreds.11,12 The translation of a BMPR2 transcript generates a polypeptide, composed of four distinct functional domains, of 1038 amino acids in humans.34 The BMPR2 protein is a constitutively active serine-threonine receptor kinase whose downstream signaling has profound effects on developmental processes, including vasculogenesis.
Although at least two additional loci have been identified that can cause a PAH phenotype, these result in conjunction with a larger heritable disease known as hereditary hemorrhagic telangiectasia (HHT). HHT is a vascular dysplasia characterized by mucocutaneous telangiectasias, recurrent epistaxis, and gastrointestinal bleeding, as well as arteriovenous malformations of the pulmonary, hepatic, and cerebral circulations. Mutations in additional components of the TGF-β signaling superfamily receptor complex, activin receptor-like kinase 1 (ALK1) located on chromosome 12 and endoglin (ENG) on chromosome 9, contribute to the pathogenesis of HHT.35,36 Pulmonary hypertension is also known to occur in HHT, typically a secondary form related to a high cardiac output state. However, PAH that is clinically and histologically identical to HPAH and IPAH has recently been described in multiple unique kindreds, with HTT associated with detectable mutations in ALK1, implicating ALK1 as an additional locus for HPAH.37,38 Interestingly, an ENG germline mutation was recently described in a patient with HHT and PAH that was associated with dexfenfluramine exposure, a genetic–environmental interaction which has also been described in BMPR2 mutation carriers.39 As has also been described for BMPR2 mutation carriers, investigators recently reported a father and his child with an ENG mutation resulting in abnormal transcript splicing causing markedly reduced levels, suggesting HI leading to disease expression; whereas he himself was unaffected, the man’s child was affected with PAH as a youth.40
The locus heterogeneity identified in genes that all encode members of the TGF-β signaling pathway in patients with HHT and PAH suggests that defects in this pathway can precipitate pulmonary vascular disease. Although no direct interaction between the gene products of the BMPR2, ALK1, or ENG genes is known, and these receptors do not appear to share activating ligands, the receptors all signal intracellularly via the Smad family of coactivators.41,42 Although the exact mechanisms have yet to be elucidated, it is evident that variations at different genetic loci that signal via the TGFβ super-family of receptors can result in a similar phenotypic expression, and that a better understanding of this signaling will improve understanding of HPAH.
THE BMP SIGNALING PATHWAY
BMPR2 is ubiquitously expressed and highly conserved throughout nature as a receptor for a family of cytokines known as bone morphogenetic proteins (BMPs). As members of the TGF-β superfamily of receptors, BMPs play a crucial role in the regulation of mammalian development. They are regulators of embryonic lung morphogenesis as well as bone and cartilage development.43 Although genetic studies strongly implicate the TGF-β superfamily in the regulation of pulmonary vascular cell growth and differentiation, the precise molecular mechanisms involved are unclear.
It is clear that heterodimerization of the serine/ threonine transmembrane kinases, BMPR1 and BMPR2, is critical to BMPR2 signaling. Four functional domains comprise BMPR2: ligand binding, kinase, transmembrane, and cytoplasmic domains. Activation of the heterodimeric BMPR2/BMPR1 receptor complex leads to phosphorylation of a series of cytoplasmic mediators, which include the Smad family. Following phosphorylation, Smad proteins 1, 5, and 8 complex with Smad 4 for translocation into the nucleus to regulate target gene transcription in concert with specific nuclear repressors and cofactors.41 In this manner, the Smad signaling pathway appears to participate in the inhibition of cell growth and the induction of apoptosis.44 It is postulated that BMPR2 mutations eliminate a critical growth regulatory function in pulmonary vascular cells by disrupting Smad activation.45
Other substrates are affected by BMPR2/ BMPR1 receptor activation, including mitogen-activated protein kinases (MAPKs), NH2-terminal kinase, and others.41 Unopposed mitogen-activated protein kinase (MAPK) function has been associated with BMPR2 mutations. For instance, Yang et al found that BMPR2 kinase domain mutations result in down-regulation of Smad signaling in pulmonary artery smooth muscle cells (PASMCs), with resultant loss of antiproliferative effect. The resulting imbalance between Smad and MAPK function may lead to proproliferative and antiapoptotic effects that promote the development of PAH.46
The BMPs and TGF-β superfamily play critical roles in a diverse array of cellular processes beyond the BMPR2 pathway. Vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) are two growth factors that have been implicated in PAH pathogenesis. They are believed to interact with BMPs and perhaps with the BMPR2 pathway. It is known that plexiform lesions in PAH have elevated levels of VEGF expression. Whether VEGF acts to promote or inhibit apoptosis and remodeling in the setting of PAH is unknown.47,48 It does appear, however, that at least one BMP (BMP-9) diminishes VEGF activity.49
PDGF is a potent mitogen and pulmonary vascular smooth muscle cell chemoattractant, which may contribute to pulmonary vasculature remodeling in PAH.50 Elevated levels of PDGF have been found in the lungs of individuals with PAH, whereas BMPR2 activity has been shown to dampen PDGF-associated PASMC proliferation. It has been suggested that insufficient BMPR2 activity results in a loss of PDGF restraint, which may be rescued by peroxisome proliferator-activated receptor λ (PPARλ) activation.
PREVALENCE OF BMPR2 MUTATIONS IN HERITABLE PAH
Despite locus heterogeneity, BMPR2 mutations are responsible for PAH in the vast majority of HPAH, as well as some cases of PAH without family history. Germline BMPR2 mutations appear to cause PAH regardless of ethnic group.52–54 A variety of testing methodologies have been used to detect BMPR2 mutations, including sequencing of genomic DNA, Southern blot analysis, high-performance liquid chromatography, and melting curve analysis. The majority of mutations encode frameshift, nonsense, or splice site donor/acceptance site mutations.55 Multiplex ligation-dependent probe amplification (MLPA) analysis of genomic DNA with confirmation by real-time polymerase chain reaction (PCR) has expanded investigators’ ability to detect BMPR2 mutations.56 It is now generally accepted that ∼70% of families with documented PAH in one or more members have a detectable mutation in BMPR2 in affected individuals.3 This agreement further supports the primary role of BMPR2 in the central pathogenesis of disease in HPAH.34
BMPR2 MUTATIONS IN IDIOPATHIC PAH
Given the clinical and pathologic similarity to HPAH, it is not surprising that mutations in BMPR2 cause some cases of IPAH. These mutations may be either inherited low-penetrance alleles or de novo.34 Although the detection rates vary in part due to the availability of detailed family histories, mutations are typically detected in ∼10% of patients (6 to 40%).6,34,57,58 To date, only 6.8% (3/44) IPAH patients had a detectable BMPR2 mutation by direct sequencing and MLPA testing in our center (unpublished data, December 2008). This low percentage may be due to more complete family histories, which make the number of subjects with true IPAH lower. Of note, although the precise rate of BMPR2 mutations in the general population is unknown, it is extremely low.15
ALLELIC HETEROGENEITY IN HPAH DUE TO BMPR2 MUTATIONS
Although it is no surprise that not all BMPR2 mutations result in identical clinical presentations given the variations in clinical expression noted earlier, the functional impact of BMPR2 mutations has been incompletely investigated to date, with variable consequences for signaling activity reported.59,60
Different types of BMPR2 mutations, such as those that activate the NMD pathway versus those that do not, can differentially impact BMP pathway signaling. NMD is a mechanism of RNA surveillance used by the cell to destroy RNA transcripts from nonsense mutations that would otherwise lead to the production of aberrant truncated proteins.61 NMD modifies the phenotype of mutations because NMD activation prevents the production of protein products from mutant transcripts that could have a dominant-negative (DN) effect. The expected result of NMD destruction of mutant transcripts is an HI effect resulting from lower levels of normal protein (supplied by the remaining normal allele), which presumably causes a less severe bv phenotype.61,62 Thus NMD eliminates transcripts encoding truncated proteins that could be harmful. In contrast, mutations that do not activate the NMD mechanism are potentially DN because in some cases they can result in stable mutant proteins with deleterious effects that impair or completely block the beneficial activity of the remaining normal allele.34 Thus NMD status of BMPR2 mutations may profoundly influence the phenotype produced by different mutations in this autosomal dominant disease.61,63
Complete disruption of BMP signaling may lead to absence of critical mechanisms in the antiproliferative and differentiation mechanisms in the pulmonary vasculature. HI has been generally accepted as the predominant molecular mechanism by which a BMPR2 mutation predisposes to PAH.52,64 Consistent with this, ∼50 to 60% of the reported pathogenic mutations should result in a premature truncation codon (PTC), which, if it resides in certain exons will activate the NMD pathway.52 In addition, various deletions within exons 2 to 13 have been reported, which lead to nonfunctional peptides.56,57 Although it is clear that alleles resulting in HI effects cause PAH, it is unclear what percentage of BMPR2 mutations that result in disease have DN effects on BMP signaling.59,61 In vitro functional studies have shown DN effects on BMP signaling due to mutations or truncations in the kinase domain of BMPR2.46 Although some data suggest that DN mutations are more detrimental than HI mutations, whether reproducible phenotypic differences will emerge is unknown.65
Recent work by Hamid et al suggests that, although characterization of the mutation by NMD status is important, it is not the only component of BMPR2 allelic variation that correlates genotype with phenotype. In fact, the level of production of BMPR2 transcript by the remaining wild-type allele, particularly in the setting of HI mutations due to NMD activation, may be a critical modifier of disease expression. Studying lymphoblastoid cell lines derived from BMPR2 mutation carriers with four different HI mutations, real-time PCR assays were used to determine wild-type and mutated BMPR2 transcript levels. As expected, HI mutant alleles contribute very little to the total BMPR2 transcript levels (0 to 2.5%), so that the wild-type BMPR2 allele is the major determinant of total transcript amount. Interestingly, HPAH patients had significantly lower wild-type BMPR2 transcript levels compared with unaffected mutation carriers with the same HI mutation. Because the BMPR2 receptor forms a heteromeric complex with BMPR1a and BMRP1b, deficiency of the normal BMPR2 receptor could affect the stoichiometric balance on the cell surface, leading to decreased signaling through the receptor and a greater predisposition to disease penetrance. This finding that variations in the amount of normal BMPR2 transcripts can affect penetrance supports the concept that, at least among HI mutations, the extent of transcript production by the wild-type BMPR2 allele may be a critical modifier of disease penetrance (see Fig. 3).66
Figure 3.
Wild-type BMPR2 transcript levels, as measured by relative real-time polymerase chain reaction (PCR) analysis, in affected and unaffected BMPR2 mutation carriers. This study includes four distinct families with four different types of BMPR2 mutations predicted to have a haploinsufficient effect due to the activation of the nonsense-mediated decay pathway. Note the lower relative BMPR2 expression among affected carriers, as compared with unaffected carriers. Adapted from Hamid et al.66 Reproduced with permission of John Wiley & Sons, Inc.
Reduced wild-type transcripts may explain Atkinson et al’s discovery that upon immunohistochemical staining of lung tissue from HPAH patients there is a near complete absence of BMPR2 protein in those heterozygous for the mutation.67 That is, given the presence of one mutated germline allele and one normal germline allele, one would expect to see ∼50% the amount of protein produced by the wild-type allele unless the mutation had a DN effect—unless affected HI mutation carriers are those with particularly reduced wild-type BMPR2 transcript levels. An alternative model is a lung-specific alteration of the wild-type BMPR2 allele by somatic mutation, similar to the loss of a second tumor suppressor gene in the “two-hit” model of cancer development. However, a search for evidence of microsatellite instability with somatic loss of function in the remaining wild-type BMPR2 allele has not found this two-hit process to be a likely cause.17
BMPR2 MUTATIONS AND ADDITIONAL DISEASE MODIFIERS
Reduced penetrance implies that a mutation of the BMPR2 gene is required but insufficient alone for phenotypic expression. Although Hamid et al have shown that variation in levels generated by the wild-type BMPR2 allele can modify disease, it is likely that additional endogenous and exogenous modifiers also mediate the clinical expression of HPAH.66 The most obvious endogenous factors are genetic in origin, especially those involving biologically relevant candidate genes. Several such genes have been investigated and implicated, but findings have not been convincingly replicated.65,68–73 It is unknown whether these might be genes whose action promotes clinical expression of a BMPR2 mutation in those who develop PAH, or whether these are protective modifiers that prevent disease in the mutation carriers who never develop disease.
Common genetic variations (CVs) (also known as polymorphisms) in the serotonin (5-hydroxytryp-tamine, 5-HT) pathway, or CVs in the serotonin transporter (SERT) gene, have been suggested to play a role in HPAH. Serotonin is a cellular mitogen and stimulates PASMC proliferation through a signaling pathway mediated via SERT.74 Internalization of 5-HT by SERT leads to downstream activation of the MAPK cascade, in part via the production of reactive oxygen species, which ultimately acts to stimulate transcription of genes to drive cellular proliferation. PASMCs respond strongly to these stimulatory effects in vitro.75,76 Thus upregulation of the MAPK cascade as an effect of abnormal serotonin signaling has garnered attention as a potential antagonist to the growth inhibitory effects of BMP signaling pathways and possibly confers susceptibility to PAH expression.
After studies implicated this pathway in humans by identifying increased plasma serotonin levels in 16 patients with PAH,77 Eddahibi et al found increased growth of PASMC in culture from patients with PAH compared with controls when stimulated by serotonin or serum, and attributed these mitogenic effects to increased expression of SERT.78 Furthermore, the authors proposed a clinical link to the molecular cause for SERT overexpression by finding variations in the SERT gene promoter that associate with severe PAH. Homozygosity for a long promoter variant (L-allelic variant) was present in ∼65% of patients (LL genotype individuals) with PAH but only 27% of control subjects.78 This suggested that CVs that affect SERT expression may modulate the penetrance of HPAH.
Machado et al further examined the role of polymorphic variation within the SERT gene to modify PAH in a case-control study. In contrast to Eddahibi et al, they found no difference in frequency of SERT gene alleles among any of the groups studied, suggesting that this SERT polymorphism was not likely to contribute to phenotypic expression of PAH in subjects with or without BMPR2 mutation. Likewise, genetic variation of the SERT promoter gene locus did not correlate with differences in age of onset of disease. Nor did such variation differ by gender, the only known risk factor for development of PAH.71
Willers et al also studied the role of polymorphisms in the SERT gene, specifically the L allele, in a cohort of patients with IPAH and HPAH compared with controls. The results were comparable to those of Machado et al in showing no difference in SERT genotype distribution between groups. However, on subset analysis, HPAH patients homozygous for the L allele (LL genotype) had an earlier age at diagnosis than other HPAH patients without this genotype, and they concluded that unclear interactions between BMPR2 mutations and polymorphisms in the SERT gene affect disease expression.72
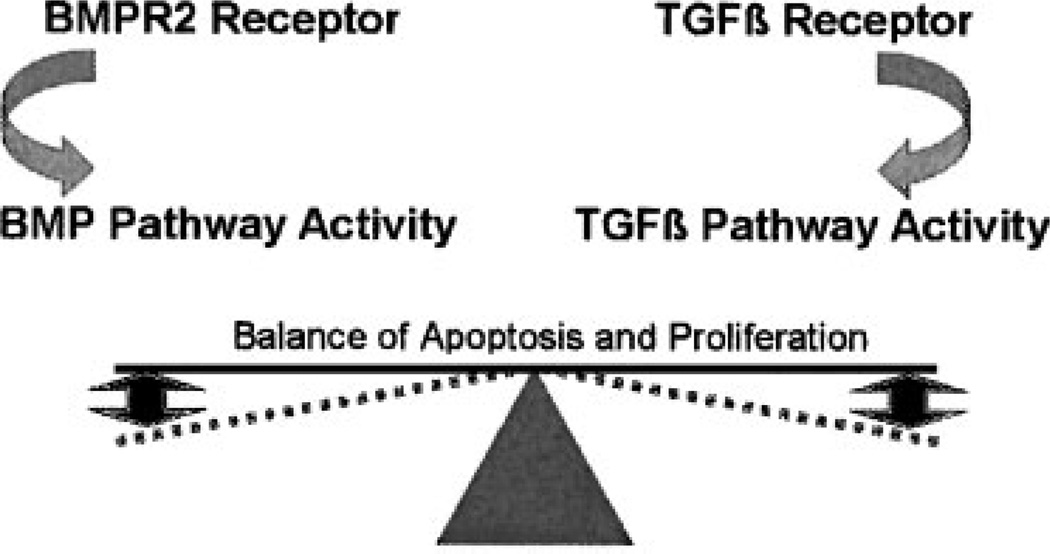
The balance of signaling among the various members of the TGFβ pathway of receptors, including BMPR2 and TGFβ receptor signaling, may also modify disease expression.13 It has been suggested that a reciprocal relationship exists between pathways composed of members of this superfamily of receptors, with the perturbation of BMPR2 signaling by a genetic mutation detrimental to this balance (see Fig. 4).79 Interestingly, TGFβ signaling appears enhanced in various forms of pulmonary hypertension, although BMPR2 mutations may alter the cellular response to this activity.80 Zaiman et al recently found reduced markers of pulmonary vascular remodeling and prevention of disease onset following inhibition of the TGFβ type 1 receptor using the rat monocrotaline model of pulmonary hypertension.81
Figure 4.
A delicate balance between BMPR2 pathway and TGFβ pathway signaling modulates proproliferative and apop-totic forces. A BMPR2 mutation may perturb this balance, resulting in disease expression.
Consistent with Zaiman et al’s work and with genetic modifier studies of lung disease in cystic fibrosis, in a cohort of individuals with varying types of BMPR2 mutations, we found that those subjects with genetic polymorphisms in the gene TGFβ1 predicted to increase TGFβ pathway activity were more likely to be penetrant.65 Although biologically plausible, this finding requires replication and validation.
BMPR2 AND ALTERNATIVE CAUSES OF PULMONARY HYPERTENSION
Mutations in BMPR2 have not been consistently found in other causes of pulmonary hypertension. However, pulmonary veno-occlusive disease (PVOD), a rare form of pulmonary hypertension in which the vascular changes also affect small pulmonary veins and venules, has been linked to mutations in BMPR2. This has been confirmed in at least three independent studies.57,82,83
The detection of BMPR2 mutations in PVOD emphasizes that clinical heterogeneity can result from a BMPR2 mutation. This clinical heterogeneity arises from the ability of different allelic mutations at a single locus to produce different disease phenotypes. Further, PVOD and PAH may well represent different ends of the same spectra of disease, with phenotype influenced by genetic and/or environmental modifiers.82
PAH related to certain drug exposures is well known. Specifically, PAH has been reported in conjunction with exposure to appetite suppressants, such as fenfluramine and dexfenfluramine.84,85 Although the exact mechanism of PAH promotion has not been elucidated, these drugs must serve as environmental triggers to facilitate disease expression, possibly in genetically susceptible individuals. Individual factors of susceptibility are particularly plausible given the low numbers of PAH in those exposed to fenfluramine (∼1 case per 10,000 people exposed).86 In 9% of unrelated patients with PAH associated with fenfluramine exposure, Humbert et al found distinct BMPR2 mutations. Interestingly, when compared with other patients with PAH associated with fenfluramine, patients heterozygous for a BMPR2 mutation expressed disease following a significantly shorter interval of exposure to fenfluramine.64 While rare, the association of drug exposure and BMPR2 mutations in PAH further emphasizes the importance of endogenous and exogenous factors in the expression of disease.
Roberts et al found BMPR2 variations in 6% of 106 children and adults with congenital heart disease, regardless of pulmonary vascular pressures, but no studies have been undertaken using the expanded genetic analyses currently available.87 Because the BMP pathway activity is known to be important in the embryologic development of the cardiovascular system, further studies are warranted.
GENETIC IMPLICATIONS FOR CLINICAL EVALUATION AND THERAPY
Information on the impact of BMPR2 genotype upon clinical outcomes and therapeutic response in PAH has steadily increased over the past several years. For instance, a small proportion of PAH patients have good long-term response to calcium channel blockers as predicted by vasoreactivity testing, so Elliott et al retrospectively compared the results of vasoreactivity testing with known BMPR2 mutations (specifically, nonsynonymous BMPR2 sequence variations).24 They found that patients with HPAH or IPAH with non-synonymous BMPR2 sequence variations were less likely to demonstrate vasoreactivity by standard testing.24 In a cohort of adult and pediatric patients with PAH and a BMPR2 mutation, Rosenzweig and colleagues found similar results, as well as worse hemodynamic parameters.27
Interestingly, Sztrymf et al reported that having certain BMPR2 mutations is associated with a more aggressive form of PAH. By examining their large registry of individuals with PAH in France, they found that 20% of patients without a family history of PAH had a detectable mutation in BMPR2, suggesting that hereditable disease may be more common than currently appreciated. They also found that subjects with a BMPR2 mutation had a more severe form of the disease, based on an earlier age at diagnosis and more severe hemodynamic abnormalities than those lacking a mutation. Although survival was comparable in both groups, patients with BMPR2 mutation were more likely to be treated with parenteral prostacyclin therapy or undergo lung transplantation.23
Additional studies are needed to enable correlation of genotype and phenotype because of the heterogeneous molecular basis of reduced penetrance seen in PAH.88 In addition, they provide clinical evidence that not all BMPR2 mutations result in equal effects. Therefore, as noted earlier, HI mutations may result in less severe disease than those resulting in DN effects. Also, kindred individuals with the same BMPR2 mutation may have different polymorphic alleles or environmental exposures that modify the penetrance and severity of their shared BMPR2 mutations. One day, patients and their physicians may be able to implement a management strategy that is based in part upon one’s genotype and environmental exposures.88
GENETIC TESTING AND SCREENING THOSE AT RISK
The issue of genetic testing on a clinical basis is challenging. As an autosomal dominant disease, the siblings or children of patients with HPAH, or of known heterozygotes for a BMPR2 mutation, have an overall risk of 50% to inherit the disease-causing allele. With reduced penetrance such that ∼20% of carriers will develop disease, this yields an estimated risk of 10% (50% × 20%) to express disease for each child of a BMPR2 mutation carrier. Thus the majority of firstdegree relatives (i.e., parent, sibling, or child) will be asymptomatic even though 50% will be carriers of a BMPR2 mutation.
A negative test result for the BMPR2 mutation for a family member of an affected BMPR2 carrier is reassuring. If negative, an individual PAH family member’s risk of disease falls from 1 in 10 (∼10%, noted above) to that of the population, which is ∼1 in 1,000,000 (100,000-fold risk reduction). However, if an asymptomatic individual at risk is found to carry the known familial BMPR2 mutation, because of reduced penetrance the risk increases only twofold (from ∼10 to ∼20%) to express clinical disease throughout their lifetime.
Genetic testing on a clinical basis for HPAH is currently available. It is most efficiently used for unaffected individuals with a known family history of PAH, or PAH patients. Laboratory evaluation for a BMPR2 mutation should start with full sequencing of all 13 exons, including splice junctions. Advanced methods, such as MLPA, may be needed to identify some mutations, which involve deletions or duplications missed by genomic sequencing.3 Also, cDNA sequencing may be required to detect some mutations found deep in introns that perturb splicing. Genetic testing should only be provided in concert with professional genetic counseling by experienced counselors available before and after test results are available.15,89 It is important to consider that a positive test for a BMPR2 mutation in a subject with IPAH converts the concept of disease from one of a “sporadic” finding to that of a heritable family disease; this situation can be terrifying for the family because kin are at risk to harbor the same mutation. Not surprisingly, the major reason subjects give for testing is to provide information to their children.90
BMPR2 mutations are usually family-specific, without a common recurring mutation type. Given the vast number of potential mutations in the large BMPR2 gene (∼300 are currently known), screening for a mutation is most efficient in an affected patient with IPAH or FPAH, so that the specific mutation in their family can be identified, if present. There is no rationale for clinical genetic testing of relatives of a PAH patient unless a mutation is identified in the patient sample.
Current screening recommendations for asymptomatic family members of patients with HPAH include a surveillance echocardiogram at 3- to 5-year intervals.89 Based upon studies of families with HPAH, investigators in Germany proposed a screening process utilizing estimation of systolic pulmonary artery pressure at rest and during exercise by echocardiography. Specifically, Gru¨nig et al reported an abnormal pulmonary artery pressure response to hypoxia during echocardiographic evaluation in individuals deemed at risk to develop PAH based upon a known BMPR2 mutation or a possible “risk haplotype.”91 The development of echocardiography and other advanced imaging modalities is critical to improve the earlier detection of PAH in at-risk individuals. However, the use of stress echocardiography as a screening tool for PAH has not been widely accepted and requires more extensive evaluation and independent confirmation. As such, it is not currently recommended for general use.
CONCLUSION AND FUTURE DIRECTIONS
Tremendous progress in the evaluation of PAH has been made in relation to the genetic underpinnings of this disease. The discovery that mutations in the BMPR2 gene underlie the majority of cases of HPAH and an important subset of cases of IPAH has energized the field and prompted significant progress in understanding the molecular basis of PAH. So, too, has the association of HPAH in HHT with genes that encode additional members of the TGFβ superfamily of receptors. However, much work is still needed to elucidate the cause(s) of the reduced penetrance and variable expressivity of BMPR2 mutations, including the identity and role of various genetic and environmental modifiers of disease expression. In addition, studies should strive to elucidate the role of gender, the strongest disease modifier known.
The identification of novel genes and proteins involved in disease pathogenesis and progression, such as those involved in the serotonin and TGF-β signaling pathways, is an area of intense study. The endogenous and exogenous influences that converge to promote pulmonary vasculature-specific cellular proliferation and vascular remodeling require further investigation. Hopefully, a greater understanding of the genetic and molecular mechanisms of PAH will lead to earlier diagnosis, advanced pharmacogenetics, and perhaps one day in the future, disease prevention.
ACKNOWLEDGMENTS
The authors thank the many patients and families who graciously contributed to this work, and Ms. Lisa Wheeler, whose service is invaluable as coordinator of the Vanderbilt Familial Pulmonary Arterial Hypertension study.
FUNDING
This work was funded by NIH PO1 HL072058, NIH K12 RR1 7697, and GCRC RR000095.
REFERENCES
- 1.Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension, I: Clinical and hemodynamic study. Am J Med. 1951;11:686–705. doi: 10.1016/0002-9343(51)90020-4. [DOI] [PubMed] [Google Scholar]
- 2.Dresdale DT, Michtom RJ, Schultz M. Recent studies in primary pulmonary hypertension, including pharmacodynamic observations on pulmonary vascular resistance. Bull N Y Acad Med. 1954;30:195–207. [PMC free article] [PubMed] [Google Scholar]
- 3.Machado R, Chung W, Eickelberg O, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. In press doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis. 1984;129:194–197. doi: 10.1164/arrd.1984.129.1.194. [DOI] [PubMed] [Google Scholar]
- 5.Thomas AQ, Gaddipati R, Newman JH, Loyd JE. Genetics of primary pulmonary hypertension. Clin Chest Med. 2001;22:477–491. doi: 10.1016/s0272-5231(05)70285-9. ix. [DOI] [PubMed] [Google Scholar]
- 6.Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, III, Newman JH. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet. 1997;15:277–280. doi: 10.1038/ng0397-277. [DOI] [PubMed] [Google Scholar]
- 10.Morse JH, Barst RJ. Detection of familial primary pulmonary hypertension by genetic testing. N Engl J Med. 1997;337:202–203. doi: 10.1056/NEJM199707173370315. [DOI] [PubMed] [Google Scholar]
- 11.Lane KB, Machado RD, Pauciulo MW, et al. The International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 12.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman JH, Phillips JA, III, Loyd JE. Narrative review: the enigma of pulmonary arterial hypertension: new insights from genetic studies. Ann Intern Med. 2008;148:278–283. doi: 10.7326/0003-4819-148-4-200802190-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sztrymf B, Yaı¨ci A, Girerd B, Humbert M. Genes and pulmonary arterial hypertension. Respiration. 2007;74:123–132. doi: 10.1159/000098818. [DOI] [PubMed] [Google Scholar]
- 15.Newman JH, Trembath RC, Morse JA, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol. 2004;43(12, Suppl S):33S–39S. doi: 10.1016/j.jacc.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 17.Machado RD, James V, Southwood M, et al. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation. 2005;111:607–613. doi: 10.1161/01.CIR.0000154543.07679.08. [DOI] [PubMed] [Google Scholar]
- 18.Irey NS, Manion WC, Taylor HB. Vascular lesions in women taking oral contraceptives. Arch Pathol. 1970;89:1–8. [PubMed] [Google Scholar]
- 19.Irey NS, Norris HJ. Intimal vascular lesions associated with female reproductive steroids. Arch Pathol. 1973;96:227–234. [PubMed] [Google Scholar]
- 20.Kleiger RE, Boxer M, Ingham RE, Harrison DC. Pulmonary hypertension in patients using oral contraceptives. A report of six cases. Chest. 1976;69:143–147. doi: 10.1378/chest.69.2.143. [DOI] [PubMed] [Google Scholar]
- 21.Morse JH, Horn EM, Barst RJ. Hormone replacement therapy: a possible risk factor in carriers of familial primary pulmonary hypertension. Chest. 1999;116:847. doi: 10.1378/chest.116.3.847. [DOI] [PubMed] [Google Scholar]
- 22.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 23.Sztrymf B, Coulet F, Girerd B, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 24.Elliott CG, Glissmeyer EW, Havlena GT, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113:2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 25.Montani D, Marcelin AG, Sitbon O, Calvez V, Simonneau G, Humbert M. Human herpes virus 8 in HIV and non-HIV infected patients with pulmonary arterial hypertension in France. AIDS. 2005;19:1239–1240. doi: 10.1097/01.aids.0000176230.94226.06. [DOI] [PubMed] [Google Scholar]
- 26.Archer SL, Michelakis ED. An evidence-based approach to the management of pulmonary arterial hypertension. Curr Opin Cardiol. 2006;21:385–392. doi: 10.1097/01.hco.0000231410.07426.9b. [DOI] [PubMed] [Google Scholar]
- 27.Rosenzweig EB, Morse JH, Knowles JA, et al. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27:668–674. doi: 10.1016/j.healun.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Sztrymf B, Yaici A, Jaïs X, Simonneau G, Sitbon O, Humbert M. Genetics of pulmonary arterial hypertension: recent data and practical applications [in French] Rev Mal Respir. 2005;22(5 Pt 1):796–805. doi: 10.1016/s0761-8425(05)85637-2. [DOI] [PubMed] [Google Scholar]
- 29.Pearson CE, Nichol Edamura K, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6:729–742. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- 30.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman JH. Pulmonary hypertension. Am J Respir Crit Care Med. 2005;172:1072–1077. doi: 10.1164/rccm.200505-684OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morse JH, Jones AC, Barst RJ, Hodge SE, Wilhelmsen KC, Nygaard TG. Familial primary pulmonary hypertension locus mapped to chromosome 2q31-q32. Chest. 1998;114(1, Suppl):57S–58S. doi: 10.1378/chest.114.1_supplement.57s. [DOI] [PubMed] [Google Scholar]
- 33.Morse JH, Jones AC, Barst RJ, Hodge SE, Wilhelmsen KC, Nygaard TG. Mapping of familial primary pulmonary hypertension locus (PPH1) to chromosome 2q31–q32. Circulation. 1997;95:2603–2606. doi: 10.1161/01.cir.95.12.2603. [DOI] [PubMed] [Google Scholar]
- 34.Machado RD, Aldred MA, James V, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 36.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 37.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 39.Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison RE, Berger R, Haworth SG, et al. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441. doi: 10.1161/01.CIR.0000153798.78540.87. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-L A, Sanz-Rodriguez F, Blanco FJ, Bernabéu C, Botella LM. Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-beta signaling pathway. Clin Med Res. 2006;4:66–78. doi: 10.3121/cmr.4.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Caestecker M, Meyrick B. Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir Res. 2001;2:193–197. doi: 10.1186/rr57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derynck R, Zhang YE. Smad-dependent and Smadindependent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 45.Davies RJ, Morrell NW. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest. 2008;134:1271–1277. doi: 10.1378/chest.08-1341. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Long L, Southwood M, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 47.Perros F, Dorfmüller P, Humbert M. Current insights on the pathogenesis of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2005;26:355–364. doi: 10.1055/s-2005-916149. [DOI] [PubMed] [Google Scholar]
- 48.Said SI. Mediators and modulators of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L547–L548. doi: 10.1152/ajplung.00546.2005. [DOI] [PubMed] [Google Scholar]
- 49.Scharpfenecker M, van Dinther M, Liu Z, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 50.Eddahibi S, Humbert M, Sediame S, et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension: effect of prostacyclin therapy. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1493–1499. doi: 10.1164/ajrccm.162.4.2003124. [DOI] [PubMed] [Google Scholar]
- 51.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptorgamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 52.Machado RD, Pauciulo MW, Thomson JR, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koehler R, Grünig E, Pauciulo MW, et al. Low frequency of BMPR2 mutations in a German cohort of patients with sporadic idiopathic pulmonary arterial hypertension. J Med Genet. 2004;41:e127. doi: 10.1136/jmg.2004.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Hum Mutat. 2004;23:632. doi: 10.1002/humu.9251. [DOI] [PubMed] [Google Scholar]
- 55.Elliott CG. Genetics of pulmonary arterial hypertension: current and future implications. Semin Respir Crit Care Med. 2005;26:365–371. doi: 10.1055/s-2005-916150. [DOI] [PubMed] [Google Scholar]
- 56.Cogan JD, Pauciulo MW, Batchman AP, et al. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aldred MA, Vijayakrishnan J, James V, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara M, Yagi H, Matsuoka R, et al. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ J. 2008;72:127–133. doi: 10.1253/circj.72.127. [DOI] [PubMed] [Google Scholar]
- 59.Rudarakanchana N, Flanagan JA, Chen H, et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- 60.Nishihara A, Watabe T, Imamura T, Miyazono K. Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension. Mol Biol Cell. 2002;13:3055–3063. doi: 10.1091/mbc.E02-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khajavi M, Inoue K, Lupski JR. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet. 2006;14:1074–1081. doi: 10.1038/sj.ejhg.5201649. [DOI] [PubMed] [Google Scholar]
- 62.Noensie EN, Dietz HC. A strategy for disease gene identification through nonsense-mediated mRNA decay inhibition. Nat Biotechnol. 2001;19:434–439. doi: 10.1038/88099. [DOI] [PubMed] [Google Scholar]
- 63.Kuzmiak HA, Maquat LE. Applying nonsense-mediated mRNA decay research to the clinic: progress and challenges. Trends Mol Med. 2006;12:306–316. doi: 10.1016/j.molmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Humbert M, Deng Z, Simonneau G, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- 65.Phillips JA, III, Poling JS, Phillips CA, et al. Synergistic heterozygosity for TGFbeta1 SNPs and BMPR2 mutations modulates the age at diagnosis and penetrance of familial pulmonary arterial hypertension. Genet Med. 2008;10:359–365. doi: 10.1097/GIM.0b013e318172dcdf. [DOI] [PubMed] [Google Scholar]
- 66.Hamid R, Cogan JD, Austin ED, et al. Penetrance of pulmonary arterial hypertension is modulated by the expression of normal BMPR2 allele. Hum Mutat. 2009;30:649–654. doi: 10.1002/humu.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 68.Abraham WT, Raynolds MV, Badesch DB, et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst. 2003;4:27–30. doi: 10.3317/jraas.2003.003. [DOI] [PubMed] [Google Scholar]
- 69.Hoeper MM, Tacacs A, Stellmacher U, Lichtinghagen R. Lack of association between angiotensin converting enzyme (ACE) genotype, serum ACE activity, and haemodynamics in patients with primary pulmonary hypertension. Heart. 2003;89:445–446. doi: 10.1136/heart.89.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koehler R, Olschewski H, Hoeper M, Janssen B, Grünig E. Serotonin transporter gene polymorphism in a cohort of German patients with idiopathic pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. Chest. 2005;128(6, Suppl):619S. doi: 10.1378/chest.128.6_suppl.619S. [DOI] [PubMed] [Google Scholar]
- 71.Machado RD, Koehler R, Glissmeyer E, et al. Genetic association of the serotonin transporter in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:793–797. doi: 10.1164/rccm.200509-1365OC. [DOI] [PubMed] [Google Scholar]
- 72.Willers ED, Newman JH, Loyd JE, et al. Serotonin transporter polymorphisms in familial and idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;173:798–802. doi: 10.1164/rccm.200509-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Remillard CV, Tigno DD, Platoshyn O, et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007;292:C1837–C1853. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- 74.Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinaseinduced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- 76.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol. 1999;277(2 Pt 1):L282–L291. doi: 10.1152/ajplung.1999.277.2.L282. [DOI] [PubMed] [Google Scholar]
- 77.Hervé P, Launay JM, Scrobohaci ML, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 78.Eddahibi S, Humbert M, Fadel E, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3:680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 80.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 81.Zaiman AL, Podowski M, Medicherla S, et al. Role of TGF-{beta}/ALK5 kinase in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:896–905. doi: 10.1164/rccm.200707-1083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Runo JR, Vnencak-Jones CL, Prince M, et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med. 2003;167:889–894. doi: 10.1164/rccm.200208-861OC. [DOI] [PubMed] [Google Scholar]
- 83.Montani D, Achouh L, Dorfmüller P, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine (Baltimore) 2008;87:220–233. doi: 10.1097/MD.0b013e31818193bb. [DOI] [PubMed] [Google Scholar]
- 84.Simonneau G, Galie` N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(12, Suppl S):5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 85.Abenhaim L, Moride Y, Brenot F, et al. International Primary Pulmonary Hypertension Study Group. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 86.Sztrymf B, Yaïci A, Jaïs X, Sitbon O, Simonneau G, Humbert M. Idiopathic pulmonary hypertension: what did we learn from genes? Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(Suppl 1):S91–S100. [PubMed] [Google Scholar]
- 87.Roberts KE, McElroy JJ, Wong WP, et al. BMPR2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24:371–374. doi: 10.1183/09031936.04.00018604. [DOI] [PubMed] [Google Scholar]
- 88.Rubin LJ. BMPR2 mutation and outcome in pulmonary arterial hypertension: clinical relevance to physicians and patients. Am J Respir Crit Care Med. 2008;177:1300–1301. doi: 10.1164/rccm.200804-495ED. [DOI] [PubMed] [Google Scholar]
- 89.McGoon M, Gutterman D, Steen V, et al. American College of Chest Physicians. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1, Suppl):14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 90.Jones DL, Sandberg JC, Rosenthal MJ, Saunders RC, Hannig VL, Clayton EW. What patients and their relatives think about testing for BMPR2. J Genet Couns. 2008;17:452–458. doi: 10.1007/s10897-008-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gru¨nig E, Dehnert C, Mereles D, et al. Enhanced hypoxic pulmonary vasoconstriction in families of adults or children with idiopathic pulmonary arterial hypertension. Chest. 2005;128(6, Suppl):630S–633S. doi: 10.1378/chest.128.6_suppl.630S-a. [DOI] [PubMed] [Google Scholar]