Abstract
Bone mass is dependent on osteoblast proliferation, differentiation and life-span of osteoblasts. Parathyroid hormone (PTH) controls osteoblast cell cycle regulatory proteins and suppresses mature osteoblasts apoptosis. Intermittent administration of PTH increases bone mass but the mechanism of action are complex and incompletely understood. Cell Cycle and Apoptosis Regulatory Protein (CARP)-1 (aka CCAR1) is a novel transducer of signaling by diverse agents including cell growth and differentiation factors. To gain further insight into the molecular mechanism, we investigated involvement of CARP-1 in PTH signaling in osteoblasts. Immunostaining studies revealed presence of CARP-1 in osteoblasts and osteocytes, while a minimal to absent levels were noted in the chondrocytes of femora from 10-12-week old mice. Treatment of 7-day differentiated MC3T3-E1 clone-4 (MC-4) mouse osteoblastic cells and primary calvarial osteoblasts with PTH for 30 min to 5 hr followed by Western blot analysis showed 2-3 fold down-regulation of CARP-1 protein expression in a dose- and time-dependent manner compared to the respective vehicle treated control cells. H-89, a Protein Kinase A (PKA) inhibitor, suppressed PTH action on CARP-1 protein expression indicating PKA-dependent mechanism. PMA, a Protein Kinase C (PKC) agonist, mimicked PTH action, and the PKC inhibitor, GF109203×, partially blocked PTH-dependent downregulation of CARP-1, implying involvement of PKC. U0126, a Mitogen-Activated Protein Kinase (MAPK) Kinase (MEK) inhibitor, failed to interfere with CARP-1 suppression by PTH. In contrast, SB203580, p38 inhibitor, attenuated PTH down-regulation of CARP-1 suggesting that PTH utilized an Extracellular Signal Regulated Kinase (ERK)-independent but p38 dependent pathway to regulate CARP-1 protein expression in osteoblasts. Immunofluorescence staining of differentiated osteoblasts further revealed nuclear to cytoplasmic translocation of CARP-1 protein following PTH treatment. Collectively, our studies identified CARP-1 for the first time in osteoblasts and suggest its potential role in PTH signaling and bone anabolic action.
Keywords: Osteoblast, Differentiation, CARP-1, PTH
1. INTRODUCTION
Osteoblasts are bone forming cells that synthesize and mineralize the skeleton [1]. Bone mass is dependent on proliferation, differentiation and life-span of mature osteoblasts. Parathyroid hormone (PTH), secreted from parathyroid glands, is involved in calcium homeostasis, and is a critical mediator of skeletal development and remodeling [2]. Daily injection of PTH increases bone mass and reduces fracture incidence in osteoporotic patients [3]. PTH mediates multiple signals that coordinate distinct cellular functions in bone including osteoblast proliferation, differentiation and apoptosis [4,5,6,7]. While PTH is critical for maintenance of bone homeostasis, the intracellular mechanisms of PTH receptor-1 (PTHR1) signaling remains unclear and continued to be explored. The therapeutic use of PTH is limited by the principal side effects of disruption of calcium homeostasis [8] and possible bone cancer concerns [9]. Therefore, elucidating the molecular mechanisms underlying the anabolic action of PTH is essential for understanding the pathophysiology of bone loss, optimizing patient care and yielding novel therapeutic strategies to promote bone formation.
Cell Cycle and Apoptosis Regulatory Protein (CARP)-1 (aka CCAR1) is a novel transducer of cell growth and apoptosis signaling by diverse agents including cell growth and differentiation factors [10]. CARP-1 was previously characterized as a peri-nuclear protein that functions to regulate chemotherapy-dependent apoptosis signaling in breast cancer cells [11], or demonstrated as a nuclear protein following UV-C irradiation in mouse embryonic fibroblasts [12]. Recent studies revealed CARP-1 interaction with cell cycle regulatory Anaphase-Promoting Complex/Cyclosome (APC/C) subunit APC-2 that regulates cell growth and apoptosis [13]. CARP-1 binding with APC-2 causes G2M cell cycle arrest. Depletion of CARP-1, however, interferes with agonist dependent cell growth inhibition [13]. The fact that PTH prevents osteoblast apoptosis [6] and induces growth arrest in differentiated osteoblasts by modulating cell cycle associated proteins [14,15,16] suggests that CARP-1 could be important in the PTH regulation of osteoblast growth and differentiation. We tested this hypothesis and revealed that CARP-1 is expressed in osteoblasts and osteocytes, and involved in PTH/PTHR1 regulation of osteoblast growth and differentiation.
2. MATERIALS AND METHODS
2.1. Experimental animals
The experiments in this study were performed with 10-12 week old male or female 129J/C57BL6 mice, fed with rodent chow (Lab diet, Bentwood, MD). All animals were maintained in facilities operated by Wayne State University, and all animal experimental procedures were approved by the Institutional Animal Care and Use Committee for the Use and Care of Animals.
2.2. Antibodies and reagents
Generation of anti CARP-1 (α2) rabbit polyclonal antibodies was described previously [11]. Anti Glyceraldehyde 3-phospho Dehydrogenase (GAPDH) was from Sigma (St. Louis, MO). Secondary antibody HRP conjugates to rabbit or mouse immunoglobulins were obtained from GE Healthcare Life Sciences (Pittsburg, PA). Tissue culture minimum essential medium alpha (αMEM) and fetal bovine serum were from Invitrogen (Carlsbad, CA, USA). Human PTH (PTH1-34) was purchased from Bachem (Torrance, CA). U0126, MEK inhibitor, was from Promega (Madison, WI, USA) and SB203580, phospho-p38 inhibitor, was obtained from EMD Biosciences (Darmstadt, Germany). H-89 was obtained from EMD Biosciences (San Diego, CA, USA), PMA and GF109203× were from Calbiochem (San Diego, CA, USA). Collagenase A was from Roche Diagnostics (Indianapolis, IN). Trypsin was obtained from Life Technologies (Gaithersburg, MD, USA).
2.3. Primary osteoblast cell culture
Primary osteoblasts were isolated from calvaria (skull) by serial digestion [14]. Briefly, calvaria were dissected, isolated and subjected to sequential digestions in collagenase A (2 mg/ml) and trypsin (0.25%) for 20, 40, and 90 min. Cells from the third digest were rinsed, counted and plated in primary culture medium containing αMEM, 10% FBS, 100 U/ml penicillin and 1 μg/ml streptomycin. Primary cultures were used without passage.
2.4. MC3T3-E1 subclone 4 (MC-4) cell culture, differentiation and treatments
MC-4 cells with high osteoblast differentiation potential were maintained and passaged every 4-5 days as previously described [14,15]. Briefly, cells were cultured in αMEM containing 100 units/ml penicillin and streptomycin and 10% fetal bovine serum. Cells were plated at 40-50,000 cells/cm2 and differentiation was induced by the addition of ascorbic acid (50 μg/ml) for 7 days [14]. The culture medium was changed every other day. Cells were subsequently treated with vehicle or PTH at 5-400 nM for 30-300 min as indicated. For some experiments cells were pretreated with U0126 (20 μM) for 2 hr, SB203580 (10 μM) for 2 hr, H-89 (20 μM) for 30 min or GF109203× (1μM) for 30 min before the addition of PTH (100 nM). In additional experiments the PKC agonist PMA (0.1μM) was added to the cells for 2h without PTH treatment [14,15]. The cell layers were harvested [14], and subsequent experiments were performed.
2.5. Histology and immunostaining
For immunostaining of histological sections, the femora of at least five mice were harvested, fixed with 4% paraformaldehyde for 24 hours, decalcified with 10% EDTA (pH 6.5) in PBS, dehydrated, infiltrated and paraffin-embedded. MC3T3-E1 cells or primary osteoblasts were plated either on eight-well tissue culture chamber slides (Ibidi LLC, Verona, WI) or on poly L-lysine (Sigma) treated coverslips in 6-well plates. Immunohistochemistry on paraffin sections or immunocytochemistry of cultured cells were performed essentially as described [10] using the CARP-1 primary antibodies in conjunction with Vecstatin Universal Quick Kit (Vector Laboratories, Burlingame, CA) and AEC substrate (BioGenex, Fremont, CA).
2.6. Immunofluorescence microscopy and imaging
MC3T3-E1 cells were seeded on coverslips as above and allowed to attach overnight. Cells were differentiated for 7-12 days and treated with vehicle or 100nM PTH for 2 hours. At the end of the treatment the cells were fixed in chilled methanol and immunostaining was performed utilizing CARP-1 primary antibodies as above. For immunofluorescence studies Alexa Fluor 568 labeled goat anti rabbit IgG (Invitrogen) was used. After the final rinse in PBS the cells were mounted on slides using DAPI Mounting Medium (Vector Laboratories, Burlingame, CA). Immunostained specimens were examined with a Nikon Eclipse Ti fluorescent microscope with Sfluor 40× oil DIC H N2 objective, a Cool SNAP HQ 2 digital camera (Roper Scientific), and NIS Element AR3.2 imaging software (Nikon). To evaluate expression of CARP-1, red fluorescence signal from at least 3-4 separate fields/treatment were acquired for differentiated cells in 3 independent experiments. Corresponding DAPI signal was utilized to select nuclei and visualize subcellular distribution of CARP-1 with or without PTH treatment.
2.7. SDS-PAGE and Western analysis
SDS-PAGE and Western analysis were performed as described previously [14,15]. Cells were washed twice with cold PBS, scraped and lysed for 30 min at 4°C following sonication with RIPA buffer containing protease inhibitors (Sigma). Cell lysates were cleared by centrifugation at 14,000 × g for 30 min. SDS-PAGE was performed in 10-12% polyacrylamide with equal protein loads. Pre-stained molecular weight standards were run in parallel lanes. The proteins were transferred to PVDF membrane (Bio-Rad laboratories, Inc., Hercules, CA) in buffer containing 25 mM Tris-HCL, 192 mM glycine, 20% v/v methanol, 0.01% SDS (pH 8.5). Residual protein binding sites on the membrane were blocked by incubation for 1 hr to overnight in TBST buffer (20 mM Tris-HCL, pH7.6, 137 mM NaCl, 0.5% Tween-20) containing 5% nonfat dry milk. The membrane was then incubated with primary antibody for 1 hour to overnight. After washing with TBST, secondary antibody (anti IgG conjugated with horseradish peroxidase) was added and incubated for 20-60 min. The proteins were visualized by autoradiography using an enhanced chemiluminescence (ECL) detection system Super-Signal West Femto (Fisher Scientific, Pittsburg, PA).
2.8. Densitometry and statistical analyses
The protein band intensities on ECL Western autoradiograms (all with exposures within the linear range of the film) were quantitated using Scion software (Frederick, MD). Results were analyzed using unpaired two-tailed Student’s t test, with the Instat 2.1 biostatistics program (GraphPad Software, San Diego, CA) and data expressed as mean ± SEM. p values less than 0.05 were considered statistically significant.
3. RESULTS AND DISCUSSION
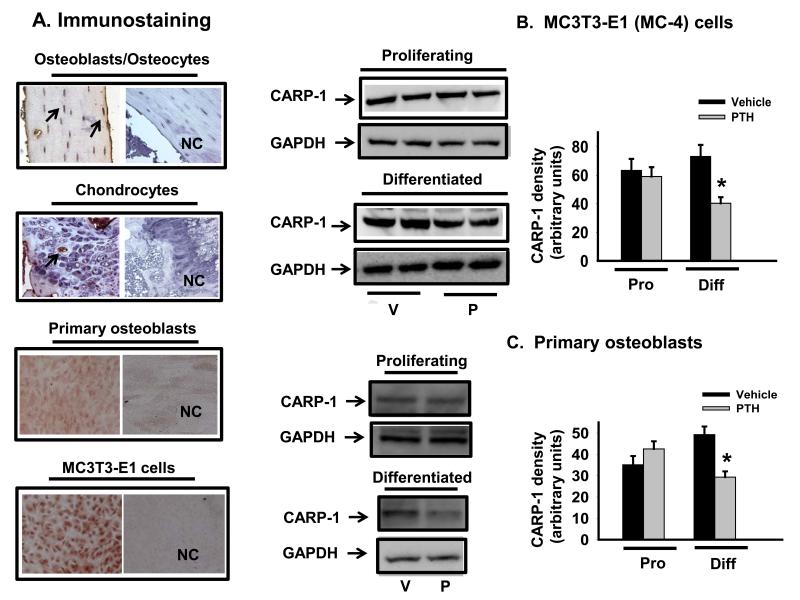
PTH has both anabolic and catabolic action in bone, but the downstream mediators of the intracellular signaling pathways are not well defined relative to specific actions. Current views of the anabolic action of PTH indicate that it acts on osteoblasts to promote their proliferation or differentiation, while inhibiting osteoblast and osteocyte apoptosis [14,15,17]. Despite the reported role of CARP-1 as a key intracellular transducer of apoptosis and cell proliferation of normal as well as cancer cells in response to different signals [11,18,19], the presence of CARP-1 in bone cells and involvement in PTH signaling has not been described so far in the known literature. As a first step we conducted immunostaining for the presence of CARP-1 in murine osteoblasts by utilizing adult mice femora, primary calvarial osteoblasts and MC-4 osteoblastic cells. Results showed CARP-1 was highly expressed in vivo in osteocytes/osteoblasts (Fig. 1A, top row), primary calvarial osteoblasts (Fig.1A, third row) and MC-4 cells (Fig.1A, bottom row). However, the presence of this protein was absent or insignificant in chondrocytes (Fig.1A, second row). To test our hypothesis that CARP-1 plays a key role in PTH signal transduction pathway and serves as a regulator of osteoblast growth and differentiation, we next investigated PTH action on CARP-1 expression in osteoblasts.
Figure 1. CARP-1 expression and response to PTH in differentiated osteoblasts.
(A) Representative immunostainings for CARP-1 protein expression are shown in osteocytes/osteoblasts in mouse femur (top row), growth plate chondrocytes (second row), differentiated primary calvarial osteoblasts (third row) and MC3TC-E1 osteoblastic cells (bottom row). NC, negative control. (B) MC3T3-E1 cells and (C) primary calvarial osteoblasts were induced to differentiate with ascorbic acid for 7 days and treated with 100nM PTH (P) or vehicle (V). Total cellular protein were harvested from proliferating or differentiated cells and subjected to SDS PAGE. Western blot analyses were performed with anti CARP-1 antibody. GAPDH was used as a protein loading control. Densitometric analyses were performed, normalized with GAPDH protein and plotted. Representative data (mean ± SEM) from at least three to four independent experiments are shown. *, p<0.05 vs V; Pro, proliferating; Diff, differentiated.
Our laboratory previously reported that PTH regulation in osteoblasts is maturation stage specific [14,15,20,21]. PTH targets cell cycle machinery and is either proproliferative or antiproliferative in immature cells and in more mature osteoblasts respectively [14,15]. It is interesting to note that CARP-1 also functions in a biphasic manner. It is a signaling mediator leading to apoptosis [11,18] as well as proliferation [19]. To examine whether PTH regulated CARP-1 expression, proliferating or 7-10 day differentiated MC-4 and primary calvarial osteoblasts were treated with 100 nM PTH for 2 hours followed by Western blot analysis. As shown in Figure 1, the levels of CARP-1 protein were downregulated (2-3 folds) in differentiated MC-4 (Fig.1B) and primary calvarial osteoblasts (Fig. 1C). In contrast, no significant differences of CARP-1 protein expression were found in the proliferating cells when compared to vehicle treated controls.
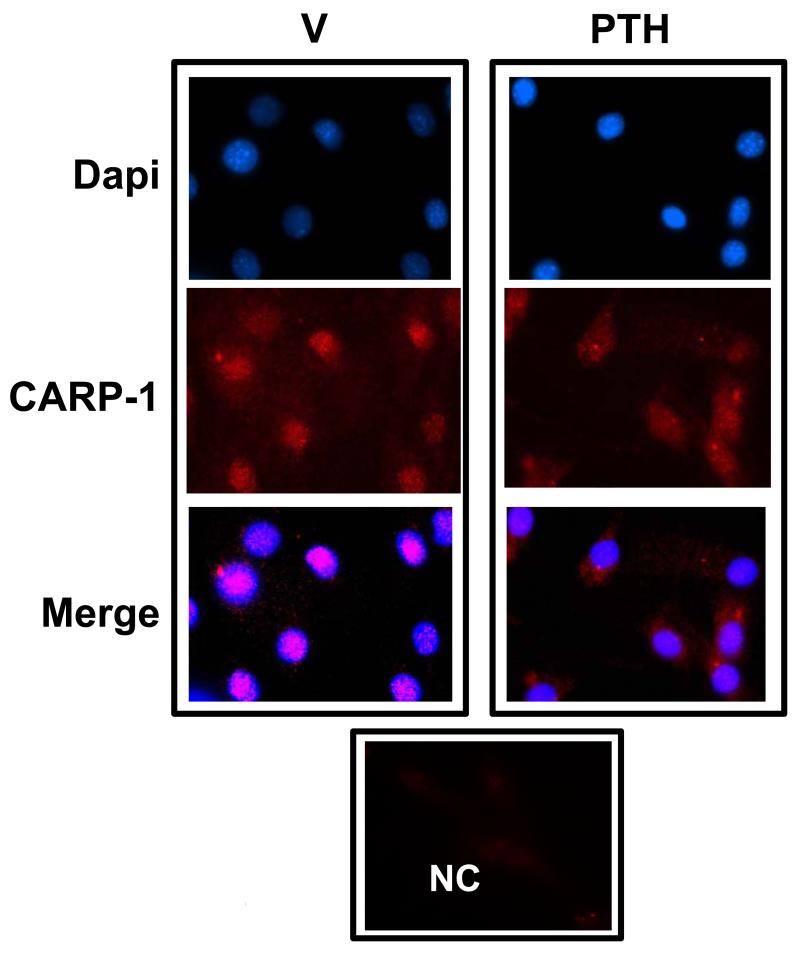
Earlier studies showed peri-nuclear or nuclear localization of CARP-1 depending on the cell type [11,12]. Performing immunofluorescence imaging CARP-1 was ubiquitously found in 10-12 day differentiated MC-4 osteoblastic cells and was present in the nuclei (Fig. 2, left column). Following PTH induction CARP-1 fluorescent signal appeared in the cytoplasm (Fig. 2, right column) when compared with its signal in untreated cells. Together our data in Figures 1 and 2 demonstrate that PTH modulates CARP-1 protein expression and subcellular distribution with osteoblast differentiation. The significance of CARP-1 translocation by PTH is not known at the present time. The requirement of CARP-1 for target gene activation by Wnt and β-catenin and cancer cell growth has been suggested [22]. The Wnt canonical signaling pathway relies on the cytosolic stabilization of β-catenin. The signaling of β-catenin, required for canonical Wnt pathway, has also been suggested to play an important role in bone physiology, bone mass accrual [23,24] and essential for osteoblast commitment and survival [25]. Apoptosis in differentiated osteoblasts and in progenitor cells may be β-catenin-dependent and-independent [26]. PTH promotes β-catenin activation in UMR, MC3T3-E1, and SAOS cells [27,28,29,30]. Evidence for the interaction of PTH and β-catenin pathway in regulating bone anabolic action has been known [24,31]. Although it is not yet clear to what extent the effect of Wnt/ β-catenin signaling on osteoblast apoptosis contributes to the overall impact of this pathway on bone formation [32], considering the current understanding that cell apoptosis plays a critical role during skeletal development and bone mass maintenance, it is likely that CARP-1 mediates β-catenin signaling, apoptosis and PTH bone anabolic action. The mechanisms underlying PTH-dependent CARP-1 regulation and the extent CARP-1 regulates β-catenin and apoptosis signaling are subjects of our ongoing investigations.
Figure 2. Immunofluorescence staining of CARP-1 translocation from the nuclear to the cytoplasm in differentiated osteoblasts.
Left column: Treatment with vehicle shows nuclear localization of CARP-1 protein in differentiated MC3T3-E1 cells. Right column: Treatment with 100nM PTH for 2 hours translocates CARP-1 in the cytoplasm. NC, negative control.
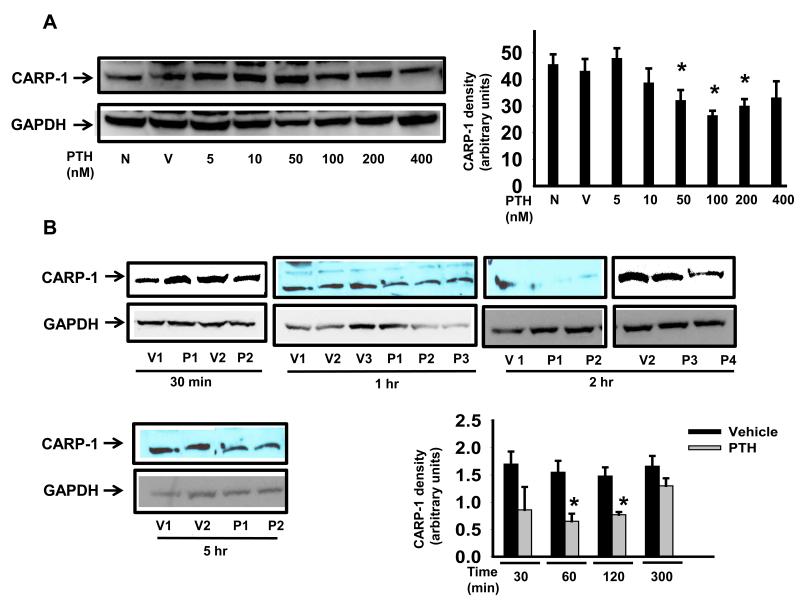
To further characterize the actions of PTH in osteoblasts and to establish the optimal dose and time point of CARP-1 inhibition, Western blot analyses of the total cellular extracts from 7-day differentiated MC-4 cells with and without PTH treatment were performed. Figure 3A shows that a 2 hour treatment of minimum 50 nM PTH inhibited CARP-1 protein levels. Next we determined the optimal time for PTH action on CARP-1 in MC-4 cells. Differentiated MC-4 cells were treated with 100 nM PTH for 30 mins to 5 hours. The concentration (100 nM) of PTH was chosen on the basis of our previously published reports. As shown in Figure 3B, 1 to 2 hours of PTH exposure decreased CARP-1 level by 1.5-2 folds. Although CARP-1 levels were reduced following 30 min PTH treatment, the reduction however was not found to be statistically significant. The level of CARP-1 started to increase at 5 hour indicating progressive recovery or desensitization of CARP-1 expression to the PTH effect.
Figure 3. Dose dependence and time course analysis of CARP-1 protein expression in response to PTH in differentiated osteoblasts.
MC3T3-E1 cells were induced to differentiate with ascorbic acid for 7 days and treated with (A) various concentrations of PTH (P) or vehicle (V) for 2 hours, (B) with 100 nM P or V for 30 mins to 5 hours. Total cellular protein were harvested and subjected to SDS PAGE. Western blot analyses were performed, normalized and plotted as in Fig.1. Representative data (mean ± SEM) from at least three to four independent experiments are shown. *, p<0.05 vs no treatment (N) or V.
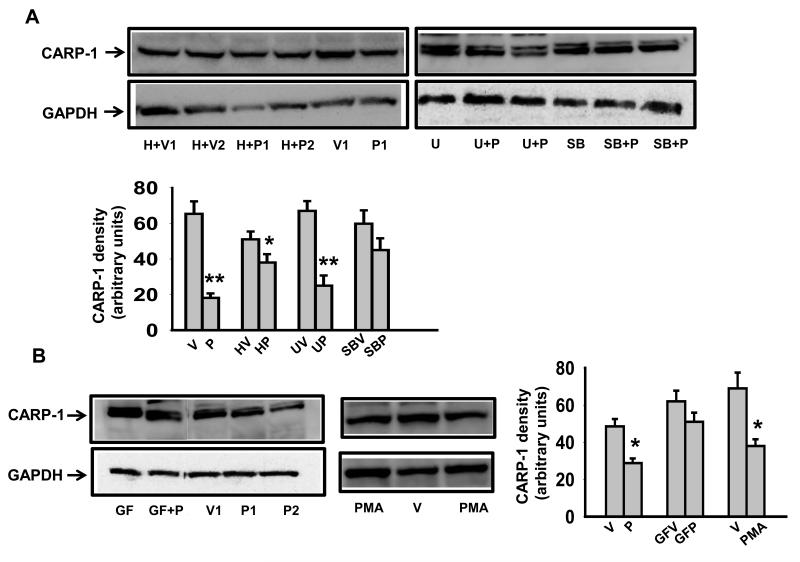
PTH is known to signal through PKA, PKC and MAPK pathways (for a review [5]). To gain insight into the signaling events that link PTH to CARP-1 and osteoblast differentiation, several known inhibitors of PTH signaling pathways were used. As expected, the treatment of 7-day differentiated MC-4 cells alone with PTH downregulated CARP-1 protein. This effect was partially antagonized by pretreating the cells with PKA inhibitor, H89 (Fig.4A). Although not significant, there was a trend of diminished CARP-1 expression by H89 treatment alone (Fig.4A). In human breast cancer cell line treatment with H89 resulted in apoptosis that involved enhanced CARP-1 expression [10]. It was shown that in these cells CARP-1 binds signal transducer TAZ, a ligand for 14-3-3 protein [33]. Overexpression of TAZ inhibits apoptosis by CARP-1 [10]. It would be interesting to evaluate the extent of interaction of TAZ and CARP-1 occurring in differentiated osteoblasts. We next examined whether PTH effect on CARP-1 involved MAPK pathway. Pretreatment of cells with U0126, which inhibits MEK, a kinase that lies upstream of ERK-MAPK, did not hinder PTH effect of CARP-1 downregulation (Fig.4A). In contrast, SB203580, a P-p38-MAPK inhibitor, blocked PTH effect (Fig.4A). Likewise, GF109203×, which inhibits PKC, suppressed PTH action on CARP-1 (Fig.4B). To further investigate the role of PKC, activation of PKC pathway and CARP-1 expression in differentiated MC-4 cells was examined. As shown by Western blot analysis in Figure 4B, treatment with PMA, a PKC agonist, decreased CARP-1 protein level compared with vehicle in a manner that was similar to the effect of PTH on CARP-1 in these cells, thus providing further evidence that PKC signaling was also involved. Taken together, these results suggest that PKA, PKC and P-p38 MAPK, but not P-ERK-MAPK are likely contributors to PTH-dependent CARP-1 downregulation in osteoblastic cells.
Figure 4. Downregulation of CARP-1 by PTH is PKA, PKC and P-p38 dependent.
MC3T3-E1 cells were induced to differentiate with ascorbic acid for 7 days and were either pretreated with (A) the PKA inhibitor H-89 (H), the MEK inhibitor U0126 (U), the P-p38 inhibitor (SB), and (B) the PKC inhibitor (GF) followed by treatment with PTH (P) or vehicle (V), or treated with (B) the PKC agonist PMA alone as described in the Materials and Methods. Total cellular protein were harvested and subjected to SDS PAGE. Western blot analyses were performed, normalized and plotted as in Fig.1. Representative data (mean ± SEM) from three to four independent experiments are shown. *, p<0.05, **, p< 0.01 vs V.
Our previous studies have shown that PTH downregulates cyclin D1, P-p38 to control osteoblast growth and mineralization [14,20]. Although CARP-1 expression causes suppression of cell cycle regulatory genes such as c-Myc and cyclin B1, while inducing cyclin-dependent kinase inhibitor p21 WWF1/CIP1 leading to apoptosis in breast cancer cells [11], it is unclear whether any of these cell cycle regulatory genes or additional genes are downstream effectors of PTH/CARP-1 signaling in osteoblasts. Since CARP-1 is also a co-activator of p53 and transduces inhibitory effects of DNA damage, it is possible that CARP-1 functions in bone to regulate osteoblast growth and differentiation by modulating cell cycle machinery in a p53-dependent manner [34].
In summary, our current study demonstrates that CARP-1 is abundant in osteoblasts and is a target of PTH, an active regulator of bone remodeling. We found that CARP-1 is differentially regulated by PTH during osteoblast proliferation and differentiation. Furthermore we noted that PTH promoted translocation of CARP-1 from the nucleus to the cytoplasm during the differentiation process. These results provide a basis for further investigations of mechanisms of action of CARP-1 in osteoblasts that could be crucial in regulation of bone tissue remodeling and repair.
Highlights.
CARP-1 is identified for the first time in bone cells.
PTH downregulates CARP-1 expression in differentiated osteoblasts.
PTH displaces CARP-1 from nucleus to the cytoplasm in differentiated osteoblasts.
Downregulation of CARP-1 by PTH involves PKA, PKC and P-p38 MAPK pathways.
ACKNOWLEDGEMENTS
This work was supported by funding from WSU OVPR and NIH DK087848 to NSD; and the Medical Research Services of the Department of Veteran Affairs Merit Review grant to AKR.
Abbreviations
- CARP-1
Cell Cycle and Apoptosis Regulatory Protein -1
- PTH
Parathyroid Hormone
- PTHR1
PTH receptor-1
- MC-4
MC3T3-E1 clone 4
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- MAPK
Mitogen-Activated Protein Kinase
- MEK
MAPK Kinase
- ERK
Extracellular Signal Regulated Kinase
- PMA
phorbol-12-myristate-13 acetate
- EDTA
ethylenediamine tetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lian JB, Stein GS, Aubin JE. Bone formation: maturation and functional activities of osteoblast lineage cells. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. American Society for Bone and Mineral Research; Washington: 2003. pp. 13–28. [Google Scholar]
- [2].Hock JM, Fitzpatrick LA, Bilezikian JP. Actions of Parathyroid Hormone. In: Bilezikian JP, Raisz LG, Rodan GA, Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Principles of Bone Biology. 2nd ed. 2nd ed. Academic Press; Academic Press; San Diego: San Diego: 2002. pp. 463–482. [Google Scholar]
- [3].Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- [4].Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang YH, Liu Y, Rowe DW. Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab. 2007;292:E594–603. doi: 10.1152/ajpendo.00216.2006. [DOI] [PubMed] [Google Scholar]
- [8].Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, Wong M, Marcus R. Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab. 2007;92:3535–3541. doi: 10.1210/jc.2006-2439. [DOI] [PubMed] [Google Scholar]
- [9].Pietrogrande L. Update on the efficacy, safety, and adherence to treatment of full length parathyroid hormone, PTH (1-84), in the treatment of postmenopausal osteoporosis. Int J Womens Health. 2010;1:193–203. doi: 10.2147/ijwh.s4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang Y, Puliyappadamba VT, Zhang L, Wu W, Wali A, Yaffe MB, Fontana JA, Rishi AK. A novel mechanism of cell growth regulation by Cell Cycle and Apoptosis Regulatory Protein (CARP)-1. J Mol Signal. 2010;5:7. doi: 10.1186/1750-2187-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rishi AK, Zhang L, Boyanapalli M, Wali A, Mohammad RM, Yu Y, Fontana JA, Hatfield JS, Dawson MI, Majumdar AP, Reichert U. Identification and characterization of a cell cycle and apoptosis regulatory protein-1 as a novel mediator of apoptosis signaling by retinoid CD437. J Biol Chem. 2003;278:33422–33435. doi: 10.1074/jbc.M303173200. [DOI] [PubMed] [Google Scholar]
- [12].Hervy M, Hoffman LM, Jensen CC, Smith M, Beckerle MC. The LIM Protein Zyxin Binds CARP-1 and Promotes Apoptosis. Genes Cancer. 2010;1:506–515. doi: 10.1177/1947601910376192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Puliyappadamba VT, Wu W, Bevis D, Zhang L, Polin L, Kilkuskie R, Finley RL, Jr., Larsen SD, Levi E, Miller FR, Wali A, Rishi AK. Antagonists of anaphase-promoting complex (APC)-2-cell cycle and apoptosis regulatory protein (CARP)-1 interaction are novel regulators of cell growth and apoptosis. J Biol Chem. 2011;286:38000–38017. doi: 10.1074/jbc.M111.222398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Datta NS, Chen C, Berry JE, McCauley LK. PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J Bone Miner Res. 2005;20:1051–1064. doi: 10.1359/JBMR.050106. [DOI] [PubMed] [Google Scholar]
- [15].Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK. Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res. 2007;22:951–964. doi: 10.1359/jbmr.070328. [DOI] [PubMed] [Google Scholar]
- [16].Datta NS, Kolailat R, Fite A, Pettway G, Abou-Samra AB. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal. 2010;22:457–466. doi: 10.1016/j.cellsig.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rishi AK, Zhang L, Yu Y, Jiang Y, Nautiyal J, Wali A, Fontana JA, Levi E, Majumdar AP. Cell cycle- and apoptosis-regulatory protein-1 is involved in apoptosis signaling by epidermal growth factor receptor. J Biol Chem. 2006;281:13188–13198. doi: 10.1074/jbc.M512279200. [DOI] [PubMed] [Google Scholar]
- [19].Kim JH, Yang CK, Heo K, Roeder RG, An W, Stallcup MR. CCAR1, a key regulator of mediator complex recruitment to nuclear receptor transcription complexes. Mol Cell. 2008;31:510–519. doi: 10.1016/j.molcel.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahalingam CD, Sampathi BR, Sharma S, Datta T, Das V, Abou-Samra AB, Datta NS. MKP1-dependent PTH modulation of bone matrix mineralization in female mice is osteoblast maturation stage specific and involves P-ERK and P-p38 MAPKs. J Endocrinol. 2013;216:315–329. doi: 10.1530/JOE-12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mahalingam CD, Datta T, Patil RV, Kreider J, Bonfil RD, Kirkwood KL, Goldstein SA, Abou-Samra AB, Datta NS. Mitogen activated protein kinase phosphatase-1 regulates bone mass, osteoblast gene expression, and responsiveness to parathyroid hormone. J Endocrinol. 2011;211:145–156. doi: 10.1530/JOE-11-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ou CY, Kim JH, Yang CK, Stallcup MR. Requirement of cell cycle and apoptosis regulator 1 for target gene activation by Wnt and beta-catenin and for anchorage-independent growth of human colon carcinoma cells. J Biol Chem. 2009;284:20629–20637. doi: 10.1074/jbc.M109.014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rawadi G, Roman-Roman S. Wnt signalling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets. 2005;9:1063–1077. doi: 10.1517/14728222.9.5.1063. [DOI] [PubMed] [Google Scholar]
- [24].Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- [26].Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- [27].Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005;95:1178–1190. doi: 10.1002/jcb.20506. [DOI] [PubMed] [Google Scholar]
- [28].Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K. Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147:2583–2590. doi: 10.1210/en.2005-1627. [DOI] [PubMed] [Google Scholar]
- [29].Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Suzuki A, Ozono K, Kubota T, Kondou H, Tachikawa K, Michigami T. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem. 2008;104:304–317. doi: 10.1002/jcb.21626. [DOI] [PubMed] [Google Scholar]
- [31].Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci U S A. 2012;109:15048–15053. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- [33].Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anantharaman V, Aravind L. Analysis of DBC1 and its homologs suggests a potential mechanism for regulation of sirtuin domain deacetylases by NAD metabolites. Cell Cycle. 2008;7:1467–1472. doi: 10.4161/cc.7.10.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]