Abstract
Apart from the degree of stenosis, the morphology of carotid atherosclerotic plaques and presence of neovascularization are important factors that may help to evaluate the risk and ‘vulnerability’ of plaques and may also influence the choice of treatment. In this article, we aim to describe the techniques and imaging findings on CTA, high resolution MRI and contrast enhanced ultrasound in the evaluation of carotid atherosclerotic plaques. We also discuss a few representative cases from our institute with the related clinical implications.
Keywords: Carotid, magnetic resonance imaging, stenosis, ultrasound, vulnerable plaque
Introduction
Stroke is one of the most important causes of death and the greatest cause of disability all over the world. Approximately 20-30% of the infarcts can be related to carotid artery stenosis.[1,2] The severity of stenosis is a standard parameter in the evaluation of risk, and it is one of the most important criteria that may influence the choice of treatment. However, it is possible that even a low-grade stenosis in the carotid arteries can lead to the development of cerebrovascular events. Hence, it may be important to look beyond the degree of stenosis and determine wall and plaque morphology. Patients with “vulnerable” plaques (plaque ulceration, composition, and neovascularization) are more prone to developing future neurovascular events. Neovascularization is an important factor contributing to vulnerability of atherosclerotic plaque. In this article, we aim to describe the techniques and imaging findings in the evaluation of carotid atherosclerotic plaques. We also discuss a few representative cases from our institute with the related clinical implications.
Current Role of Imaging in Evaluation of Carotid Atherosclerotic Plaques
The degree of stenosis has been associated with symptoms in various studies. This was traditionally measured on carotid angiography. Large randomized trials such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET)[3] and the European Carotid Surgery Trial [ECST][4] have shown the benefit of carotid endarterectomy (CEA) for recently symptomatic patients with severe (>70%) stenosis. The role of CEA in asymptomatic individuals is also still much debated in light of the results of the Asymptomatic Carotid Atherosclerosis Study [ACAS][5] and the Asymptomatic Carotid Surgery Trial [ACST].[6] Various interpretations of these studies have led to discrepant recommendations of optimal treatment strategies.
However, currently, the role of imaging in carotid atherosclerotic disease is mainly to determine the percentage of stenosis, whether by digital subtraction angiogram (DSA) or by noninvasive techniques like ultrasound/color Doppler, CT angiography (CTA) or MR angiography.[7]
Why Evaluating Luminal Stenosis Alone is Insufficient?
Measurement of only the luminal stenosis may give incomplete information about the plaque burden. The diseased adventitial boundary can compensate for a large carotid plaque by outward expansion of the vessel wall. Atheroma initially forms eccentrically with compensatory dilatation of the lumen such that there is no luminal stenosis. Once atheroma occupies roughly 40% of the area circumscribed by the internal elastic lamina (IEL), luminal encroachment begins. This phenomenon of vessel remodeling was first described by Glagov et al.[8] In addition, luminograms give no information regarding the plaque “vulnerability.”
“Vulnerable” plaques are atherosclerotic plaques that have a high likelihood to cause thrombotic complications such as myocardial infarction or stroke. Plaques that tend to progress rapidly are also considered to be vulnerable.[9] The concept of “vulnerable plaque” was first introduced for coronary arteries[10] and later expanded to the carotid circulation.
Pathology of Carotid Atherosclerosis
Atherosclerosis occurs near the branch ostia, bifurcations, and bends, suggesting that flow dynamics plays an important role in its induction.[11] Atherosclerotic plaque tends to occur at regions where the flow velocity and shear stress are reduced.[12] The flow properties of the carotid bifurcation contributes to the initiation of atherosclerotic plaque. This plaque passes through various stages of evolution.[13,14]
The early lesions consist of two distinct nonatherosclerotic intimal lesions, intimal thickening, and intimal xanthoma (“fatty streak”). The precursor lesion in most progressive lesions is pathologic intimal thickening, which is a transition between early lesions of atherosclerosis and the more advanced fibroatheroma. The fibrous cap (FC) atheroma is the first advanced lesion of atherosclerosis. It is composed of a lipid-rich necrotic core encapsulated by fibrous tissue. The origin and development of the necrotic core occurs by invasion of macrophages and their apoptosis. The FC atheroma may result in significant luminal narrowing and is prone to complications of surface disruption, thrombosis, and calcification. Fibrocalcific plaques are characterized by thick fibrous plaques overlying extensive accumulation of calcium in the intima close to the media or lumen.
This lesion may develop intraplaque hemorrhage from the disruption of the intraplaque vasa vasorum. A thin FC (less than 165 μm)[15] may be prone to plaque rupture with erosion/ulceration which leads to thrombus formation with subsequent embolization and cerebral ischemic events.[16] Infiltration of inflammatory cells to the surface of carotid plaques is a critical step in promoting plaque rupture and the resultant embolization or carotid occlusion.[17]
The histologic hallmarks of vulnerable plaques include a larger lipid core (>40% of total lesion area), a thinner FC, many inflammatory cells,[18,19] and neoangiogenesis.[20]
T lymphocytes may be central to plaque instability. T cells can induce macrophages to secrete matrix metalloproteinases (MMPs) via stimulation of CD40 (causing weakening of the tensile strength of the plaque) and in addition, through production of interleukin-1, can promote smooth muscle cell apoptosis.[21]
Vasa vasorum are physiological structures that provide nourishment to the vessel wall and play an important role in the pathogenesis of atherosclerosis. They are normally present in the adventitia. The work of Koester[22] in 1876 and Winternitz[23] in 1938 first implicated them in the pathophysiology of atherosclerosis. Various histopathologic studies have correlated plaque neovascularization and histological patterns, such as higher immature microvessel density, subsequent intraplaque hemorrhage, and FC rupture, with features of plaque vulnerability associated with clinically symptomatic disease.[24,25,26,27,28] Thus, plaque characterization and neovascularization could be an important adjunct for stroke risk stratification in patients with carotid artery stenosis.
Techniques for Imaging Atherosclerotic Plaques
Imaging methods have the potential to be used as a screening tool for the presence of atherosclerosis and the degree of stenosis.[7] Imaging also helps to distinguish stable from vulnerable plaques and ultimately to distinguish patients with low versus those with high risk of cardiovascular complications.
The DSA represents the traditional and gold standard method for assessing the severity of luminal narrowing in the carotid artery.[29] The luminal diameter along the length of the artery can be accurately measured in a two-dimensional plane.
However, DSA does not demonstrate plaque morphology, with the exception of luminal ulceration, and the degree of stenosis may not necessarily predict the vulnerability. It is labor, time, and material intensive. Only two-dimensional images are provided. In addition, it is not without risks.[30]
Noninvasive methods commonly used in imaging patients with atherosclerosis include B-mode ultrasonography (US) and Doppler, intravascular US, conventional angiography, computed tomography (CT), and magnetic resonance imaging (MRI).
Carotid Ultrasound Examination
Ultrasound provides a fast, noninvasive technique to assess the carotid bifurcation. It can provide local diameter and wall thickness measurements as well as flow characteristics, including waveforms, gradient, and peak velocities. However, it is operator dependent and has limited field of view.
The calculation of internal carotid artery stenosis using duplex ultrasonography is based on standardized velocity criteria.[31,32,33,34] It is likely to remain the first-line imaging modality in this context. However, before performing carotid endarterectomy, a second imaging modality is used for confirming the percentage of stenosis.[29]
Apart from the degree of stenosis, echogenicity on ultrasound has been shown to predict ipsilateral ischemic stroke; patients with echolucent plaques are at increased risk compared to those with echorich plaques[35,36] [Figure 1]. However, hazard ratios for development of stroke that are associated with differing echogenicity scores are not sufficiently great to warrant translation into clinical practice. Furthermore, studies investigating the use of gray-scale median (GSM) in selection for carotid stenting have shown conflicting results.[37,38] The Imaging in Carotid Angioplasty and Risk of Stroke study[37] revealed that high echolucency increases the risk of stroke. Subsequently, Reiter and colleagues[38] showed no such relationship between plaque echolucency and stroke risk with carotid angioplasty and stenting (CAS).
Figure 1 (A, B).
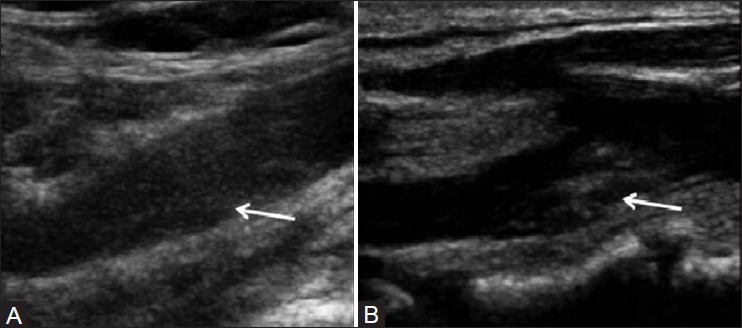
Evaluation of echogenicity of plaque on B mode USG: Hypoechoic plaque (arrow) (A) hyperechoic plaque (arrow) (B)
Contrast-Enhanced Ultrasound
Carotid contrast ultrasound imaging is an emerging technique for identifying plaque angiogenesis.[39,40] Increased enhancement on CEUS is associated with greater neovascularization at histology.[41,42,43] Studies have confirmed the association of contrast enhancement on ultrasound with clinical symptoms. There is correlation between the degree of plaque enhancement with contrast agent microbubbles and clinical symptoms.[44,45] Coli et al.,[41] have found that echolucent plaques have higher degree of contrast enhancement.
Ultrasound Contrast Medium
Microbubble-based contrast agents are injected intravenously to enhance ultrasound scans, and are composed of 1-10-mm-diameter albumin or lipid shells filled with air or high molecular weight gas.[46] Modern ultrasonic methods of distinguishing microbubble from native tissue rely on the fact that ultrasound waves cause microbubbles to compress and expand, whereas the tissue is virtually incompressible. The resonance frequency for microbubbles is within the range of frequencies used for clinical ultrasound. Sound waves of very low energy, therefore, will return detectable signal from microbubbles, provided the transmitted wave is around the microbubble resonance frequency. Native tissue, however, will not respond to such low-energy waves[47] [Figure 2].
Figure 2.
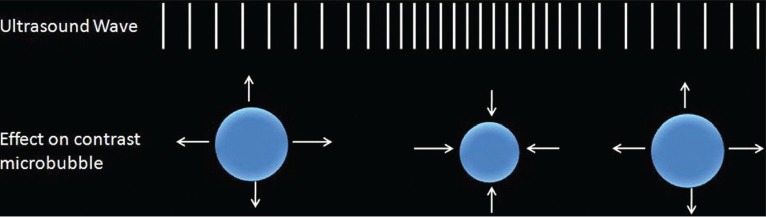
Mechanism of action of contrast-enhanced ultrasound
Technique of CEUS
The contrast (e.g. Sonovue, Bracco, Milan) is reconstituted as per instructions given by the contrast agent vendor. A short (1.5 ml) bolus is injected into an antecubital vein (20-gauge Venflon), promptly followed by a 5-ml saline flush. Low mechanical index (0.4-1.4) technique in the contrast mode is used for CEUS. Linear array probe (7-15 MHz) is used.[47]
Analysis of CEUS
Ultrasound contrast agent (UCA) microbubbles are visualized few seconds after the bolus injection as a hyperechoic dynamic flow in the vessel lumen, providing an enhanced visualization of the carotid intima–media complex and a better identification of the plaque surface and the degree of stenosis. Few seconds after the contrast agent detection in the carotid lumen, the dynamic distribution of the UCA inside the plaque, seen as moving microbubbles on real-time scan, allows the visualization of the plaque vascularization.[41,47]
MRI Plaque Imaging
MRI has great potential to enable assessment of plaque burden/volume and characterization of atherosclerotic plaque composition and morphology,[48,49] and thus to help assess plaque vulnerability. MRI is well suited for plaque imaging because it is noninvasive, has excellent contrast resolution, does not involve ionizing radiation, enables visualization of the vessel lumen and wall, and can be repeated serially to track progression or regression. Multiple pulse sequences are recommended to fully characterize plaque morphology and composition.[48,50] Black blood sequences with double inversion recovery preparatory pulses cause suppression of the signal of flowing blood. These can be T1, T2, and PD weighted. Bright blood sequences include time of flight (TOF) sequences. Naghavi et al.,[51] have given five major and five minor criteria of vulnerable plaques.
MRI can detect and also quantify all major carotid plaque components including fibrous tissue, calcium, and lipid, which can be identified using signal intensity variation from different weightings.[50] Many studies have favorably compared findings on in vivo MRI with histopathology.[49,52,53] The presence of intraplaque hemorrhage/intraluminal thrombus can also be studied.[54,55,56,57] The state of the FC can be described as being (a) intact and thick, (b) intact and thin, or (c) ruptured, based on TOF source images.[58] The FC can also be seen on T2-weighted images and with short inversion time inversion recovery (STIR) sequences as a hyperintensity located between the dark lumen and the plaque.[59] The presence of a thin or ruptured FC, as seen on MRI scans, was highly associated with recent transient ischemic attack (TIA) or stroke.[60]
Wasserman et al.,[61] showed that the wash-in kinetics of gadolinium into the FC and lipid core may be correlated with the degree of neovascularization. Other techniques for detecting plaque inflammation include the use of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles.[62]
Patient Preparation
MRI plaque imaging is performed as a separate study to maximize patient co-operation (not performed as a part of a brain or MR angiogram study). Prior to the MRI study, the carotid bifurcation on the affected side is marked using B-mode ultrasound. The patients are counseled regarding the importance of lying still and avoiding swallowing. Appropriate positioning is performed with a head holder and coils are positioned at the level of carotid bifurcation marking.
Hardware Considerations for MRI
For carotid imaging with a 1.5-T MRI scanner, echo-planar capabilities are advantageous because the gradient amplifiers and higher slew rates supported by these systems allow shorter echo times and echo spacing (in fast SE sequences). Imaging at higher field strengths (3 T) has increased signal-to-noise ratio that can be traded off for increased resolution or time reduction.[63]
The carotid arteries are superficial structures whose length is greater than their distance from the surface. This configuration is well suited for the use of phased-array surface coils. A dedicated phased-array coil assembly [Figure 3] with overall dimensions of 6.4 × 10.8 cm is used (carotid coils, Machnet, Eelde, the Netherlands; http://www.machnet.nl/) to increase the signal-to-noise ratio. The carotid bifurcation is used as an internal landmark to reproducibly prescribe slice locations for serial studies.
Figure 3.
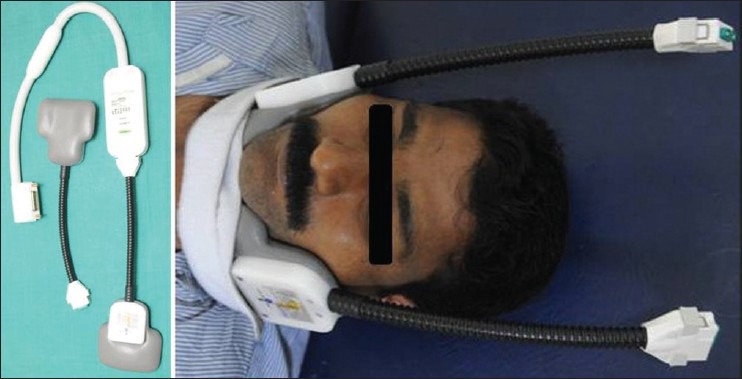
Carotid surface coils
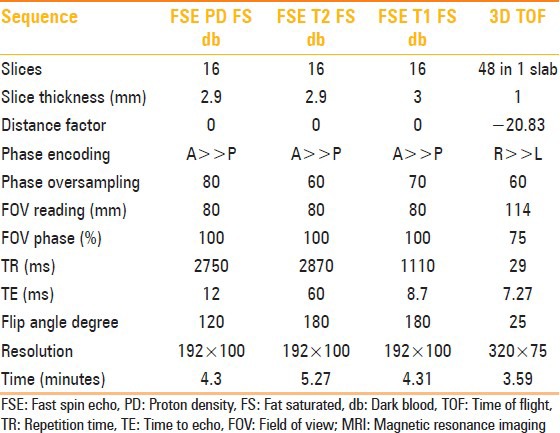
Parameters used for the various sequences are given in Table 1.
Table 1.
Sequence parameters for MRI
The signal intensity of the main plaque component is compared to the signal intensity of the ipsilateral sternocleidomastoid muscle for each image sequence. The various components of the plaques have been classified.[55] This is detailed in Table 2. The scheme for evaluating MRI images is given in Figure 4.
Table 2.
MRI characteristics of various plaque components
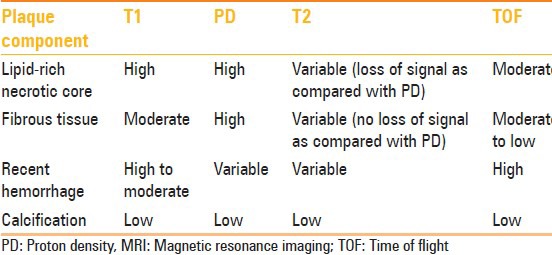
Figure 4.
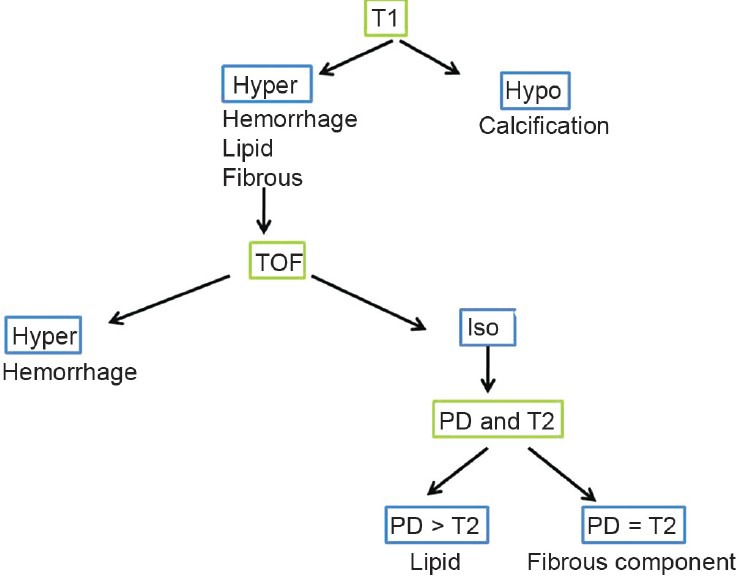
Scheme for evaluating MRI images
CTA in the Evaluation of Carotid Arteries
The use of CT in assessing carotid artery stenosis has increased steadily, especially with the development of multislice helical CT scanners that enable fast and accurate vessel imaging with minimal discomfort to the patient by peripheral intravenous injection of a contrast agent. High-resolution images of the luminal volume of arteries can be imaged from the aortic arch to the circle of Willis. The sensitivity and specificity of CTA for carotid artery stenosis are similar to those of DSA.[64] Limitations of CTA include ionizing radiation doses and ballooning artifacts of heavy calcification.
Apart from studying the degree of luminal stenosis and plaque volume, CTA is also useful for evaluating the distal portions of extra- and intracranial vessels and cerebral parenchymal abnormalities. CTA source images also give information regarding the plaque composition and surface irregularities.[65] A recent study demonstrated the feasibility of using dual-source CT compared with histopathologic specimens after CEA to evaluate carotid plaque composition.[66] Plaque surface ulceration is well evaluated on CTA and is associated with symptoms.[67,68,69]
In a recently published study, plaque relative lipid volume (using attenuation of <60 HU) was associated with the presence of ulceration.[70] There is a positive association between fatty plaque type and symptoms and between >70% stenosis and symptoms and an inverse association between calcified plaques and symptoms.[71]
Analysis of CTA
Measurements to quantify the degree of stenosis are made by selection of a plane perpendicular to the lumen centerline using the NASCET criteria.
Ulcerations are classified as types 1-4 based on the location of the ulcer neck and ulcer orientation. Type 1 is an ulcer that points out perpendicular to the lumen, Type 2 has a narrow neck and points out proximally and distally, Type 3 has an ulcer neck proximally and points out distally, and Type 4 has an ulcer neck distally and points out proximally.[72]
Based on the density, carotid plaques are classified into three different groups[73]: Fatty (soft) plaques with a density value < 50 HU, mixed (intermediate) plaque with a density value between 50 and 119 HU, and calcified plaque defined as a plaque with a density value > 120 HU. Window level and window center are generally set at 700 HU and 200 HU, respectively, for optimum visualization of the vascular structures.
Representative case vignettes have been illustrated in Figures 5-11.
Figure 5 (A-F).
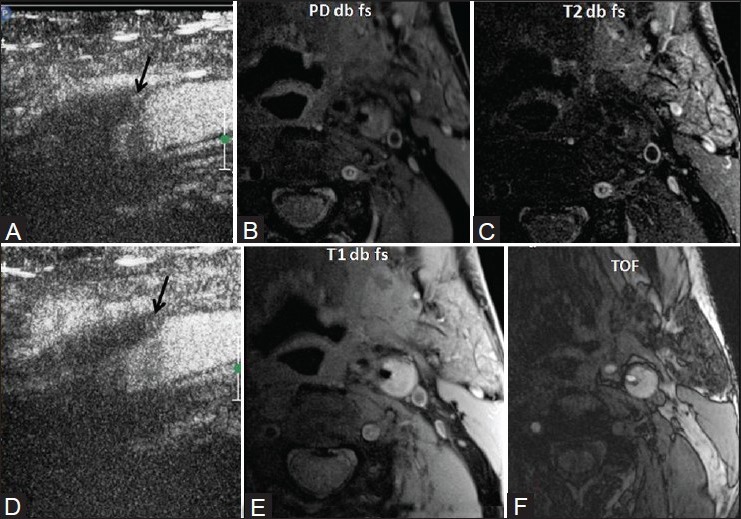
M/71 years with left middle cerebral artery (MCA) stroke. Left internal carotid artery (ICA) 80% stenosis. On CEUS, moving microbubbles are seen on the shoulder of the plaque (black arrows) (A, B) On MRI plaque imaging, it appears isointense on PD (C) hypointense on T2-weighted imaging (T2WI) with small hyperintense component (D) and hyperintense on T1-weighted imaging (T1WI) (E) and TOF s/o fibro-fatty plaque with a small hemorrhagic component (F) Surface ulceration is noted suggestive of plaque vulnerability
Figure 11 (A-F).
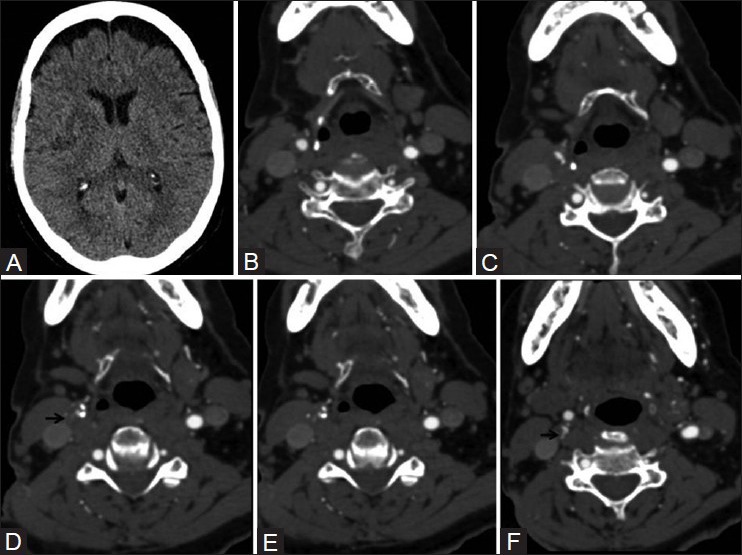
Ulcerated plaque on CTA. A 72-year-old lady with right MCA infarct (A) Sequential CTA images (B – F) show two ulcerations in right carotid bifurcation plaque, Type 3 proximally (arrow in D) and Type 1 distally (arrow in F)
Figure 6 (A-M).
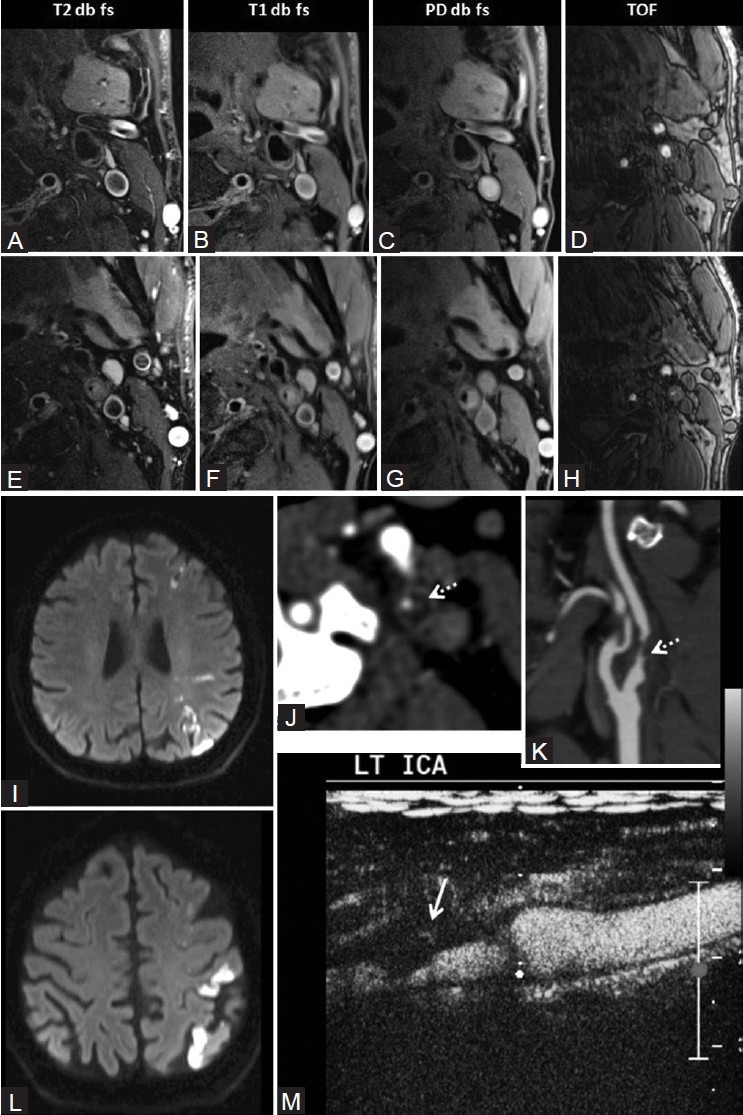
(A-E) On MRI plaque imaging, plaque appears isointense on PD and hypointense to isointense on T2WI. It is mildly hyperintense on T1WI and isointense on TOF images s/o fibro-fatty plaque. Surface ulceration is present. On MRI, plaque appears isointense on PD (H) hypointense on T2WI (F) mildly hyperintense on T1WI (G) and isointense on TOF (I) suggestive of fibro-fatty plaque. Fibrous cap is seen, but it is discontinuous at places suggestive of surface ulceration. Ultrasound and MRI findings are suggestive of plaque vulnerability. M/67 years with left MCA and MCA-anterior cerebral artery (ACA) watershed territory stroke on diffusion-weighted imaging of the brain (I, J) On CTA, left ICA 80% stenosis. Plaque has attenuation of 37.6 HU (K) Type III ulceration is present (dotted white arrows) (L) On CEUS, plaque neovascularization is seen with surface irregularity (M)
Figure 7 (A-G).
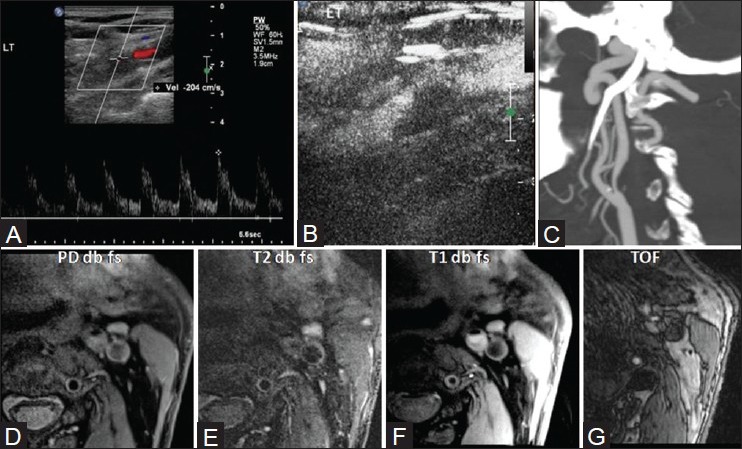
M/80 years with four episodes of left MCA TIAs. Color Doppler ultrasound shows left ICA 80% stenosis with peak systolic velocity of 204 cm/s (A). No neovascularization is seen on CEUS (B). On CTA, plaque is hypoattenuating and surface shows a single Type III ulceration (C). On MRI, plaque appeared isointense on PD (D), hypointense on T2WI (E), mildly hyperintense on T1WI (F), and isointense on TOF (G), suggestive of lipid core. Fibrous cap was well visualized
Figure 8 (A-G).
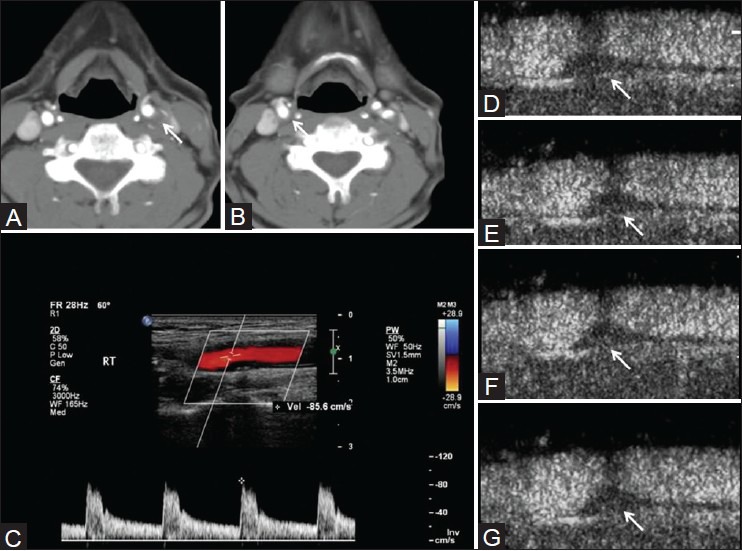
M/72 years with left hemiparesis. Right ICA 50% stenosis, left ICA 70% stenosis on CTA (A, B) Peak systolic velocity of 85.6 cm/s on the right side (C) CEUS shows moving microbubbles in the plaque (images at different time frames D-G). These features are suggestive of plaque neovascularization and vulnerability
Figure 9 (A-G).
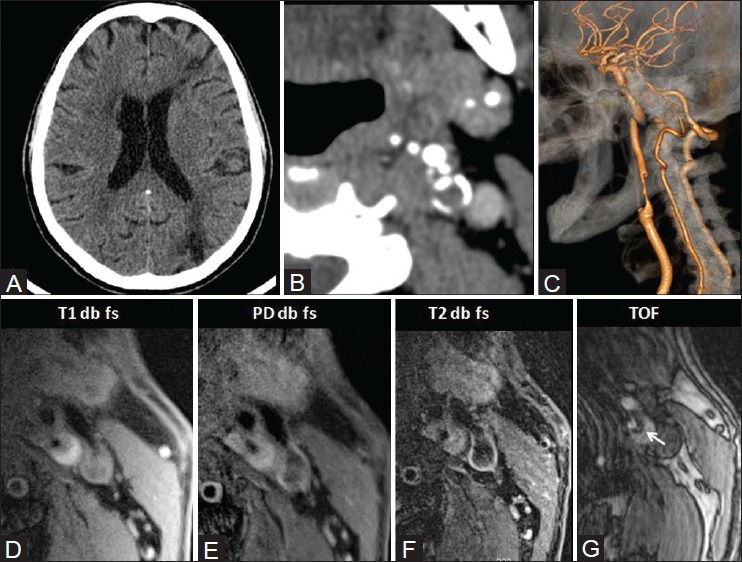
M/75 years with left carotid plaque. CT shows left ACA-MCA and MCA-posterior carotid artery (PCA) watershed territory infarcts (A) On CTA source images, the attenuation is 31 HU. Peripheral calcification is present (B) On reconstructed volume-rendered images, there is 80% stenosis (C) On MRI, plaque is hyperintense on all pulse sequences, suggestive of recent hemorrhage (D-G) Surface ulceration is present (arrow in TOF; G) suggestive of plaque vulnerability
Figure 10 (A, B).
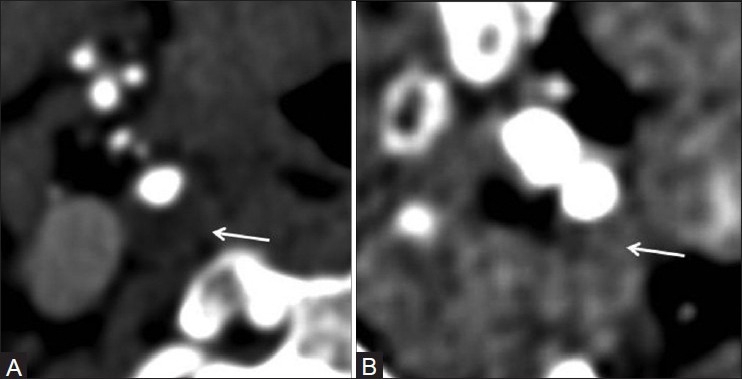
Attenuation of plaques on CTA source images: Hypoattenuating 4.8 HU (A) hyperattenuating 66.1 HU (B)
Other techniques for detecting plaque inflammation include thermography (measurement of plaque temperature), contrast-enhanced (CE) MRI, fluorodeoxyglucose positron emission tomography, and immunoscintigraphy.[51]
Conclusions
Different modalities of imaging provide supplementary information for the characterization of carotid plaques. The echogenicity of plaques on B mode ultrasound is the basic technique for characterizing plaques. CEUS, apart from better characterization of the stenosis and surface characteristics, can also depict the plaque neovascularization. Because CEUS agents are safe and commercially available, they can be used in the clinical setting to help in risk stratification of carotid atherosclerotic disease. CTA is a noninvasive technique for evaluation of luminal stenosis, and unlike ultrasound, it is not operator dependent. It can also provide information about the composition of plaque based on attenuation and also the surface ulceration. It is the “gold standard” for detecting calcification of plaques. Plaque composition, and thus vulnerability, can also be studied by MRI. Together, all these techniques provide complete information about the plaque characteristics and may be considered in planning further management of patients with carotid atherosclerotic plaques.
Acknowledgements
We would like to express our gratitude to the technologists in the Department of Imaging Sciences and Interventional Radiology for performing the MRI and CT Angiography scans.
Footnotes
Source of Support: The contrast enhanced ultrasound scans were funded by a student grant from the institute – project number 6074
Conflict of Interest: None declared.
References
- 1.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003;362:1211–24. doi: 10.1016/S0140-6736(03)14544-8. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger J. Diagnosis and prevention of atherosclerotic cerebral infarction. CNS Spectr. 2005;10:553–64. doi: 10.1017/s1092852900010208. [DOI] [PubMed] [Google Scholar]
- 3.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 4.MRC European Carotid Surgery Trial: Interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1991;337:1235–43. [PubMed] [Google Scholar]
- 5.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 6.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomized controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 7.Brobeck BR, Forero NP, Romero JM. Practical noninvasive neurovascular imaging of the neck arteries in patients with stroke, transient ischemic attack, and suspected arterial disease that may lead to ischemia, infarction, or flow abnormalities. Semin Ultrasound CT MR. 2006;27:177–93. doi: 10.1053/j.sult.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 9.Saam T, Cai J, Ma L, Cai Y, Ferguson M, Polissar N, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240:464–72. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann JM, Davies MJ. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996;94:928–31. doi: 10.1161/01.cir.94.5.928. [DOI] [PubMed] [Google Scholar]
- 11.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–31. [PubMed] [Google Scholar]
- 12.Lee D, Chiu JJ. Intimal thickening under shear in a carotid bifurcation-a numerical study. J Biomech. 1996;29:1–11. doi: 10.1016/0021-9290(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 13.Yazdani SK, Vorpahl M, Ladich E, Virmani R. Pathology and vulnerability of atherosclerotic plaque: Identification, treatment options, and individual patient differences for prevention of stroke. current treatment options in cardiovascular medicine. Curr Treat Options Cardiovasc Med. 2010;12:297–314. doi: 10.1007/s11936-010-0074-9. [DOI] [PubMed] [Google Scholar]
- 14.In: Chakfé N, Durand B, Kretz JG, editors. Strasbourg: Europrot, Strasbourg, France; 2007. New Technologies in Vascular Biomaterials: Fundamentals About Stents II; pp. 11–23. [Google Scholar]
- 15.Mauriello A, Sangiorgi GM, Virmani R, Trimarchi S, Holmes DR, Jr, Kolodgie FD, et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis. 2009;208:572–80. doi: 10.1016/j.atherosclerosis.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Avril G, Batt M, Guidoin R, Marois M, Hassen-Khodja R, Daune B, et al. Carotid endarterectomy plaques: Correlations of clinical and anatomic findings. Ann Vasc Surg. 1991;5:50–4. doi: 10.1007/BF02021778. [DOI] [PubMed] [Google Scholar]
- 17.Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–81. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 18.Kullo IJ, Edwards WD, Schwartz RS. Vulnerable plaque: Pathobiology and clinical implications. Ann Intern Med. 1998;129:1050–60. doi: 10.7326/0003-4819-129-12-199812150-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, et al. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285–92. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 21.Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes. Circ Res. 1997;81:448–54. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 22.Köester W. Endarteriitis and arteriitis. Berl Klin Wochenschr. 1876;12:454–5. [Google Scholar]
- 23.Winternitz MC, Thomas RM, LeCompte PM. Springfield: Charles C Thomas (Sage Publications Ltd); 1938. The Biology of Arteriosclerosis; p. 80. [Google Scholar]
- 24.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004;110:2032–8. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 25.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients. Early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–50. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy MJ, Loftus IM, Thompson MM, Jones L, London NJ, Bell PR, et al. Angiogenesis andbthe atherosclerotic carotid plaque: An association between symptomatology and plaque morphology. J Vasc Surg. 1999;30:261–8. doi: 10.1016/s0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- 27.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–9. doi: 10.1016/j.jvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 28.Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny T. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 2001;88:945–50. doi: 10.1046/j.0007-1323.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- 29.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry. 1993;56:967–72. doi: 10.1136/jnnp.56.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: Gray-scale and Doppler US diagnosis society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–6. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu PS, Allan PL. Ultrasound assessment of internal carotid artery stenosis. Clin Radiol. 1997;52:654–8. doi: 10.1016/s0009-9260(97)80027-x. [DOI] [PubMed] [Google Scholar]
- 33.Filis KA, Arko FR, Johnson BL, Pipinos II, Harris EJ, Olcott Ct, et al. Duplex ultrasound criteria for defining the severity of carotid stenosis. Ann Vasc Surg. 2002;16:413–21. doi: 10.1007/s10016-001-0175-8. [DOI] [PubMed] [Google Scholar]
- 34.Oates CP, Naylor AR, Hartshorne T, Charles SM, Fail T, Humphries K, et al. Joint recommendations for reporting carotid ultrasound investigations in the United Kingdom. Eur J Vasc Endovasc Surg. 2009;37:251–61. doi: 10.1016/j.ejvs.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104:68–73. doi: 10.1161/hc2601.091704. [DOI] [PubMed] [Google Scholar]
- 36.Polak JF, Shemanski L, O’Leary DH, Lefkowitz D, Price TR, Savage PJ, et al. Hypoechoic plaque at US of the carotid artery: An independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular health study. Radiology. 1998;208:649–54. doi: 10.1148/radiology.208.3.9722841. [DOI] [PubMed] [Google Scholar]
- 37.Biasi GM, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: The Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–62. doi: 10.1161/01.CIR.0000138103.91187.E3. [DOI] [PubMed] [Google Scholar]
- 38.Reiter M, Bucek RA, Effenberger I, Boltuch J, Lang W, Ahmadi R, et al. Plaque echolucency is not associated with the risk of stroke in carotid stenting. Stroke. 2006;37:2378–80. doi: 10.1161/01.STR.0000237087.86583.c8. [DOI] [PubMed] [Google Scholar]
- 39.Vicenzini E, Giannoni MF, Puccinelli F, Ricciardi MC, Altieri M, Di Piero V, et al. Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke. 2007;38:2841–3. doi: 10.1161/STROKEAHA.107.487918. [DOI] [PubMed] [Google Scholar]
- 40.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41:41–7. doi: 10.1161/STROKEAHA.109.560342. [DOI] [PubMed] [Google Scholar]
- 41.Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta MM, Melisurgo G, Mauriello A, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52:223–30. doi: 10.1016/j.jacc.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 42.Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularisation: A new surrogate marker of atherosclerosis? Vasc Med. 2007;12:291–8. doi: 10.1177/1358863X07083363. [DOI] [PubMed] [Google Scholar]
- 43.Giannoni MF, Vicenzini E, Citone M, Ricciardi MC, Irace L, Laurito A, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: A pilot study. Eur J Vasc Endovasc Surg. 2009;37:722–7. doi: 10.1016/j.ejvs.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 44.Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251:583–9. doi: 10.1148/radiol.2512081829. [DOI] [PubMed] [Google Scholar]
- 45.Owen DR, Shalhoub J, Miller S, Gauthier T, Doryforou O, Davies AH, et al. Inflammation within carotid atherosclerotic plaque: Assessment with late-phase contrast-enhanced US. Radiology. 2010;255:638–44. doi: 10.1148/radiol.10091365. [DOI] [PubMed] [Google Scholar]
- 46.Cosgrove D. Ultrasound contrast agents: An overview. Eur J Radiol. 2006;60:324–30. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Shalhoub J, Owen DR, Gauthier T, Monaco C, Leen EL, Davies AH. The use of contrast enhanced ultrasound in carotid arterial disease. Eur J Vasc Endovasc Surg. 2010;39:381–7. doi: 10.1016/j.ejvs.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–6. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 49.Altaf N, Goode SD, Beech A, Gladman JR, Morgan PS, Mac Sweeney ST, et al. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011;258:538–45. doi: 10.1148/radiol.10100198. [DOI] [PubMed] [Google Scholar]
- 50.Zhao XQ, Yuan C, Hatsukami TS, Frechette EH, Kang XJ, Maravilla KR, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: A case-control study. Arterioscler Thromb Vasc Biol. 2001;21:1623–9. doi: 10.1161/hq1001.098463. [DOI] [PubMed] [Google Scholar]
- 51.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies-Part I. Circulation. 2003;108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 52.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 53.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 54.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: A high-resolution MRI study. Stroke. 2004;35:1079–84. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 55.Kampschulte A, Ferguson MS, Kerwin WS, Polissar NL, Chu B, Saam T, et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation. 2004;110:3239–44. doi: 10.1161/01.CIR.0000147287.23741.9A. [DOI] [PubMed] [Google Scholar]
- 56.Ota H, Yarnykh VL, Ferguson MS, Underhill HR, Demarco JK, Zhu DC, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: Comparison of the diagnostic performance of three T1-weighted sequences. Radiology. 2010;254:551–63. doi: 10.1148/radiol.09090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altaf N, Beech A, Goode SD, Gladman JR, Moody AR, Auer DP, et al. Carotid intraplaque hemorrhage detected by magnetic resonance imaging predicts embolization during carotid endarterectomy. J Vasc Surg. 2007;46:31–6. doi: 10.1016/j.jvs.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 58.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–64. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 59.Serfaty JM, Chaabane L, Tabib A, Chevallier JM, Briguet A, Douek PC. Atherosclerotic plaques: Classification and characterization with T2-weighted high-spatial-resolution MR imaging-an in vitro study. Radiology. 2001;219:403–10. doi: 10.1148/radiology.219.2.r01ma15403. [DOI] [PubMed] [Google Scholar]
- 60.Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, Davis JW, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–5. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 61.Wasserman BA, Smith WI, Trout HH, 3rd, Cannon RO, 3rd, Balaban RS, Arai AE. Carotid artery atherosclerosis: In vivo morphologic characterization with gadolinium-enhanced doubleoblique MR imaging-initial results. Radiology. 2002;223:566–73. doi: 10.1148/radiol.2232010659. [DOI] [PubMed] [Google Scholar]
- 62.Trivedi RA, U-King-Im JM, Graves MJ, Cross JJ, Horsley J, Goddard MJ, et al. In vivo detection of macrophages in human carotid atheroma: Temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–5. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 63.Yarnykh VL, Terashima M, Hayes CE, Shimakawa A, Takaya N, Nguyen PK, et al. Multicontrast black-blood MRI of carotid arteries: Comparison between 1.5 and 3 tesla magnetic field strengths. J Magn Reson Imaging. 2006;23:691–8. doi: 10.1002/jmri.20562. [DOI] [PubMed] [Google Scholar]
- 64.Anderson GB, Ashforth R, Steinke DE, Ferdinandy R, Findlay JM. CT angiography for the detection and characterization of carotid artery bifurcation disease. Stroke. 2000;31:2168–74. doi: 10.1161/01.str.31.9.2168. [DOI] [PubMed] [Google Scholar]
- 65.Randoux B, Marro B, Koskas F, Duyme M, Sahel M, Zouaoui A, et al. Carotid artery stenosis: Prospective comparison of CT, threedimensional gadolinium-enhanced MR, and conventional angiography. Radiology. 2001;220:179–85. doi: 10.1148/radiology.220.1.r01jl35179. [DOI] [PubMed] [Google Scholar]
- 66.Das M, Braunschweig T, Mühlenbruch G, Mahnken AH, Krings T, Langer S, et al. Carotid plaque analysis: Comparison of dual-source computed tomography (CT) findings and histopathological correlation. Eur J Vasc Endovasc Surg. 2009;38:14–9. doi: 10.1016/j.ejvs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Lovett JK, Rothwell PM. Site of carotid plaque ulceration in relation to direction of blood flow: An angiographic and pathological study. Cerebrovasc Dis. 2003;16:369–75. doi: 10.1159/000072559. [DOI] [PubMed] [Google Scholar]
- 68.Homburg PJ, Rozie S, van Gils MJ, van den Bouwhuijsen QJ, Niessen WJ, Dippel DW, et al. Association between carotid artery plaque ulceration and plaque composition evaluated with multidetector CT angiography. Stroke. 2011;42:367–72. doi: 10.1161/STROKEAHA.110.597369. [DOI] [PubMed] [Google Scholar]
- 69.de Weert TT, Cretier S, Groen HC, Homburg P, Cakir H, Wentzel JJ, et al. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke. 2009;40:1334–40. doi: 10.1161/STROKEAHA.108.538439. [DOI] [PubMed] [Google Scholar]
- 70.Saba L, Sanfilippo R, Sannia S, Anzidei M, Montisci R, Mallarini G, et al. Association between carotid artery plaque volume, composition, and ulceration: A retrospective assessment with MDCT. AJR Am J Roentgenol. 2012;199:151–6. doi: 10.2214/AJR.11.6955. [DOI] [PubMed] [Google Scholar]
- 71.Saba L, Montisci R, Sanfilippo R, Mallarini G. Multidetector row CT of the brain and carotid artery: A correlative analysis. Clin Radiol. 2009;64:767–78. doi: 10.1016/j.crad.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Lovett JK, Gallagher PJ, Hands LJ, Walton J, Rothwell PM. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation. 2004;110:2190–7. doi: 10.1161/01.CIR.0000144307.82502.32. [DOI] [PubMed] [Google Scholar]
- 73.Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, et al. Noninvasive detection and evaluation of the atherosclerotic plaque with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430–5. doi: 10.1016/s0735-1097(01)01115-9. [DOI] [PubMed] [Google Scholar]


