Abstract
Flow compensation, a gradient pulse used for artifact reduction, often used to suppress cerebrospinal fluid (CSF) flow artifacts in spinal magnetic resonance imaging (MRI), can be switched off to make the CSF flow voids within syrinx (syringomyelia) and within aqueduct [normal pressure hydrocephalus (NPH)] more obvious (thus confirming CSF flow). It is a simple method which does not require much time or expertise.
Keywords: Flow compensation, CSF flow, syringomyelia, normal pressure hydrocephalus
Introduction
Detection of cerebrospinal fluid (CSF) flow within syrinx and within aqueduct [normal pressure hydrocephalus (NPH)] has prognostic significance: Syringes[1] with positive flow void sign respond to syrinx–subarachnoid shunting; NPH[2,3] with positive flow void sign responds to ventriculoperitoneal shunting.
T2-weighted images showing flow voids[4,5,6,7,8] is the usual imaging evidence of CSF flow.
We tried to see whether switching off the flow compensation (flow comp off) makes the CSF flow voids within syrinx (syringomyelia) and within aqueduct (NPH) more obvious (to confirm CSF flow).
Technique
Institutional review board approval was waived. Flow compensation[9,10] used for artifact reduction (in T2-weighted imaging) was switched off.
Results
In these illustrative cases, the following observations were made:
Faint flow voids were seen within cervical and thoracic syrinx on sagittal T2-weighted [Figure 1A and B] and axial T2-weighted [Figure 1C and E] images. Switching off the flow compensation [Figure 1D and F] made the flow voids more obvious
Faint flow voids were seen within aqueduct on sagittal T2-weighted [Figure 2A] image. Switching off the flow compensation [Figure 2B] made the flow voids more obvious.
Figure 1 (A-F).
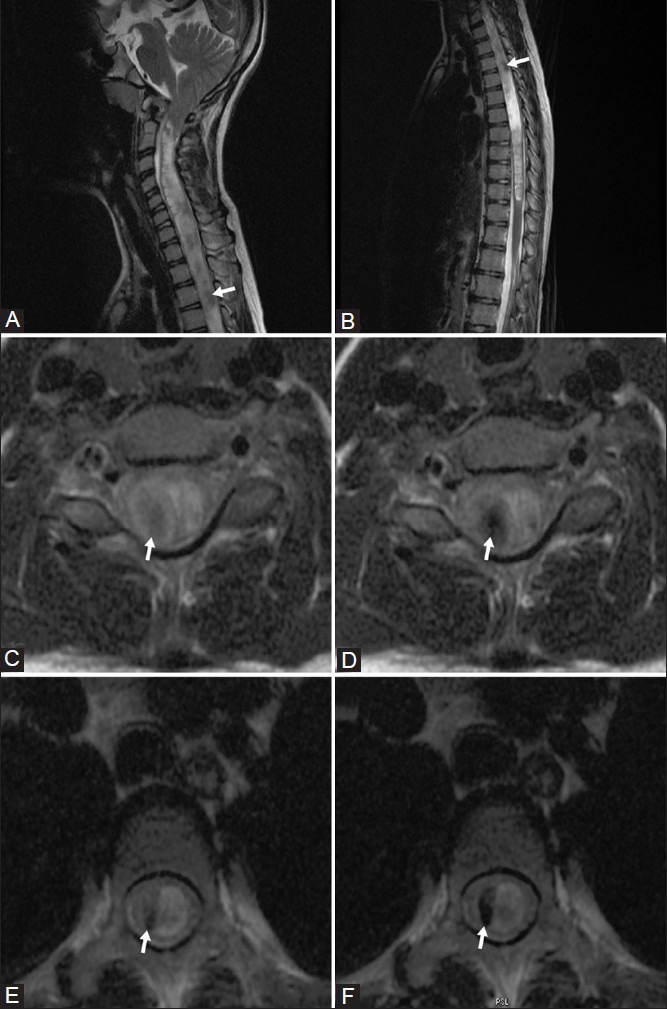
Sagittal T2-weighted images showing syrinx in cervical (A) and thoracic (B) cord; faint flow voids (arrows) are seen within the syrinx. (C, D): Axial T2-weighted images of cervical cord with (c) and without (d) flow compensation; flow voids (arrows) are more obvious in D (without flow compensation). (E, F): Axial T2-weighted images of thoracic cord with (E) and without (f) flow compensation; flow voids (arrows) are more obvious in F (without flow compensation)
Figure 2 (A, B).
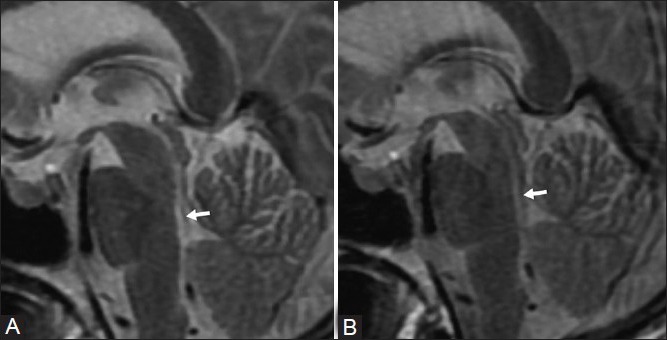
Sagittal T2-weighted image shows faint flow voids (arrow). (b) Flow voids (arrow) are more obvious (after switching off the flow compensation)
Discussion
Flow compensation (FC in GE/Philips)/Gradient Motion Rephasing (GMR in Siemens)/Motion Artifact Suppression Technique[9] (MAST in Piker) or Gradient Moment Nulling (GMN)[10] is a gradient pulse used for artifact reduction, often used to suppress flow artifacts in magnetic resonance imaging (MRI) of head, abdomen, chest and spine. FC is based on the principle of even-echo rephasing. Extra gradient pulses (called gradient lobes) are added to produce the even-echo rephasing effect on the first echo (thus eliminating first-echo dephasing). Rephasing is attained without having to use a double-echo sequence.
For gradient echo acquisitions, the relative strengths of gradient lobes are in a ratio of 1:2:1. This type of FC only corrects for first-order (i.e., constant velocity) flow. Correction of higher-order motion (like acceleration or jerk) requires additional gradient lobes, which lengthen the cycle, thus lengthening TR and minimum TE (thus reducing the number of slices). It is possible to apply FC along each and all of the three (x, y and z) co-ordinates.
Flow compensation (FC) suppresses CSF flow artifacts in spinal MRI imaging. FC is used with T2-weighted spin echo (SE) [not compatible with fast spin-echo (FSE) sequences] and gradient echo acquisitions; but not with T1-weighted spinal imaging as it increases the signal intensity of CSF and may decrease contrast between CSF and spinal cord/cauda equina. In contrast to presaturation, GMN cannot suppress artifacts due to pulsatile blood flow in spin-echo images.
Syringomyelia is a fluid-filled cavity or syrinx within the spinal cord, formed by CSF dissecting into surrounding white matter; hence, it is not lined by ependyma. Hydromyelia is dilatation of the central canal by CSF, lined by ependymal cells. It is very difficult to separate the two by imaging or histopathology; hence, the term syringohydromyelia is frequently used.
Syringomyelia may be idiopathic or occur as a complication of trauma, meningitis, hemorrhage, tumor or arachnoiditis. It may be associated with scoliosis, Arnold–Chiari malformation (a congenital abnormality where lower part of cerebellum protrudes into cervical canal), or other craniovertebral junction abnormalities. Post-traumatic syringomyelia is a relatively infrequent, but potentially devastating complication that can occur anytime (1 month-45 years) following traumatic spinal cord injury. Rostral or caudal cyst extension may be caused by the turbulence of CSF flow or a “one-way-valve” phenomenon that allows CSF into, but not out of, the cyst cavity.
Syringomyelia usually presents in third or fourth decades of life (mean age of 30 years) and usually involves the cervical cord. It may extend into medulla, producing a syringobulbia. Clinical presentations include sensory, motor and autonomic symptoms, painless ulcers of hands, neurogenic arthropathies (Charcot's joints of shoulder, elbow or wrist). There is a marked lack of correlation between cavity size and severity of clinical symptoms. CSF fluid analysis is usually not performed due to risk of herniation. Surgical treatments include suboccipital and cervical decompression, laminectomy and syringotomy (dorsolateral myelotomy), fourth/terminal ventriculostomy, and neuroendoscopic surgery.
Plain radiographs and computed tomography (CT) scan may demonstrate associated craniovertebral anomalies. Myelography (performed in special situations where MRI cannot be performed) may show widening of cord and complete subarachnoid block. MRI is the imaging modality of choice; real-time ultrasonography may be feasible in young children and thin patients. Sonography may also be used intraoperatively after laminectomy to visualize syrinx cavities and septations.
Flow voids[4] noted on T2-weighted images of syrinx indicate CSF flow; these are better seen on T2 images without flow compensation. Dephasing of moving protons explains the signal loss (flow voids).
Intra-syrinx flow was demonstrated to be pulsatile.[11] Phase-contrast flow studies[12] which measured velocities in syrinx (cyst) and pericystic subarachnoid spaces (PCSS), showed higher systolic and diastolic cyst velocities in large cysts and in patients with poor clinical status. Postoperatively, decrease of systolic and diastolic cyst velocities and parallel increase of systolic PCSS velocities were noted. Diastolic cyst velocities correlated with preoperative clinical status of patients and postoperative satisfactory foraminal enlargement (based on visibility of cisterna magna). These studies are time consuming and require some expertise.
Constructive Interference in Steady State (CISS), used in syrinx imaging[13] to detect subarachnoid webs in syrinx, shows less flow voids than T2 images; so it is not useful in detecting flow within syrinx.
Switching off the flow compensation (flow comp off) makes the flow voids more obvious and helps confirm CSF flow within syrinx. It is a simple method which does not require much time or expertise.
Normal pressure hydrocephalus (NPH) is a disease that presents clinically with gait apraxia, dementia, and incontinence and radiologically as chronic communicating hydrocephalus. A potentially treatable cause of dementia, it may account for as many as 10% of cases of dementia. As the name suggests, mean CSF opening pressure in patients with NPH is within the normal range (<18 cm H2O or 13 mm Hg with the patient in lateral decubitus position). Since first described in 1965, NPH and its treatment by surgical (CSF) shunting have been the focus of much investigation. Shunts may be placed from lateral ventricles to peritoneal cavity or superior vena cava.
Diagnostic imaging in NPH shows ventricular enlargement without sulcal widening (venticulosulcal disproportion), generally more in frontal and temporal horns of lateral ventricle. The corpus callosum may be bowed upward and the cerebral gyri flattened against the inner table of the skull. Periventricular white matter hyperintensities are often present.
CSF production (~500 ml/day) is mainly in choroid plexus of lateral ventricles. From there, CSF flows through third ventricle, cerebral aqueduct, fourth ventricle, and foraminae of Lushka and Magendi. Traditional teaching that CSF is absorbed by arachnoid villi and superior sagittal sinus has been challenged by studies suggesting a role for extracranial lymphatics.[14] Velocity of CSF flow is expected to be maximum in the narrowest part of ventricular system (the aqueduct). This aqueductal flow is pulsatile: Antegrade (caudal- into 4th ventricle) during cardiac systole and retrograde (rostral- into 3rd ventricle) during cardiac diastole.
Increased aqueductal CSF flow void has been noted in MR images of patients with communicating hydrocephalus.[5,6,7,8] This signal loss occurs more often in NPH than in healthy individuals, but not in acute communicating hydrocephalus or atrophy. This was confirmed by measurement of mean pixel intensities in aqueduct.[6] In patients clinically diagnosed as NPH, extent of flow voids on proton density-weighted, non–flow-compensated conventional spin-echo images highly correlated with favorable response to CSF diversion.[2,3] Later studies disapproved of the CSF flow void as they used flow-compensated conventional spin-echo or fast spin echo[15,16] (which is intrinsically flow compensated due to the multiple pairs of 180° pulses) techniques.
Increased peak velocity through aqueduct can be quantified[17] with cine phase-contrast MRI, and has been of proved value in selecting patients who are likely to respond to ventriculoperitoneal shunt surgery.[18,19,20,21] Patients with aqueductal CSF stroke volume (ACSV) >42 μl were more likely to respond to surgery. The positive predictive value[17] of response to shunt surgery for ACSV >42 μl was 100%, while for ACSV <42 μl, it was 50%.
The value 42 μl is machine dependent,[22,23] and may vary with machine manufacturers and with the different softwares for calculation. So, it is recommended[23] that normal baseline values be calculated in each center performing CSF flow studies by studying elderly patients without symptoms of NPH. Hyperdynamic CSF flow (which supports a diagnosis of shunt-responsive NPH) is to be declared when ACSV is twice the normal value. Other factors that may indicate shunt responsiveness include duration of presurgical symptoms (<6 months appears favorable), onset of gait disturbance before dementia, temporary pre-shunt symptom relief from a CSF tap test (removal of ~40 ml CSF through lumbar puncture) and absence of significant cerebral vascular disease.
Some institutions followed the following protocol:[24] in patients with symptoms of NPH, routine MR scan using conventional (not fast) spin echo is performed. If prominent aqueductal CSF flow void is detected, patient is considered for shunt surgery; if not, quantitative phase-contrast flow study is done. If ACSV is >42 μl, patient is considered for surgery.
MRI without flow compensation is thus a good technique of confirming flow within aqueduct and predicting response to shunt surgery.
The simple process of switching off the flow compensation (flow comp off) makes the flow voids more obvious and helps confirm CSF flow within syrinx (syringomyelia) and within aqueduct (NPH). It does not require much time or expertise; can be done in most MR scanners and can predict response to surgery.
Acknowledgement
Kesavadas Chandrasekhar and Bejoy Thomas are gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Koc K, Anik Y, Anik I, Cabuk B, Ceylan S. Chiari 1 malformation with syringomyelia: Correlation of phase-contrast cine MR imaging and outcome. Turk Neurosurg. 2007;17:183–92. [PubMed] [Google Scholar]
- 2.Bradley WG, Whittemore AR, Kortman KE, Watanabe AS, Homyak M, Teresi LM, et al. Marked cerebrospinal fluid void: Indicator of successful shunt in patients with suspected normal pressure hydrocephalus. Radiology. 1991;78:459–66. doi: 10.1148/radiology.178.2.1987609. [DOI] [PubMed] [Google Scholar]
- 3.Schroth G, Klose U. MRI of CSF flow in normal pressure hydrocephalus. Psychiatry Res. 1989;29:289–90. doi: 10.1016/0165-1781(89)90066-8. [DOI] [PubMed] [Google Scholar]
- 4.Sherman JL, Barkovich AJ, Citrin CM. The MR appearance of syringomyelia: New observations. AJR Am J Roentgenol. 1987;148:381–91. doi: 10.2214/ajr.148.2.381. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Mokri B, Laws ER, Jr, Houser OW, Baker HL, Jr, Petersen RC. MR findings in normal-pressure hydrocephalus: significance and comparison with other forms of dementia. J Comput Assist Tomogr. 1987;11:923–931. doi: 10.1097/00004728-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bradley WG, Kortman KE, Burgoyne B. Flowing cerebrospinal fluid in normal and hydrocephalic states: appearance on MR images. Radiology. 1986;159:611–616. doi: 10.1148/radiology.159.3.3704142. [DOI] [PubMed] [Google Scholar]
- 7.Sherman JL, Citrin CM, Gangarosa RE, Bowen BJ. The MR appearance of CSF flow in patients with ventriculomegaly. AJNR Am J Neuroradiol. 1986;7:1025–1031. [Google Scholar]
- 8.Quencer RM, Post MJD, Hinks RS. Cine MR in the evaluation of normal and abnormal CSF flow: intracranial and intraspinal studies. Neuroradiology. 1990;32:371–391. doi: 10.1007/BF00588471. [DOI] [PubMed] [Google Scholar]
- 9.Pattany PM, Phillips JJ, Chiu JC, Lipcamon JD, Duerk JL, McNally JM, et al. Motion artifact suppression technique (MAST) for MR imaging. J Comput Assist Tomogr. 1987;2:369–77. doi: 10.1097/00004728-198705000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ehman RL, Felmlee JP. Flow artifact reduction in MRI: A review of the roles of gradient moment nulling and spatial presaturation. Magn. Reson. Med. 1997;14:293–307. doi: 10.1002/mrm.1910140214. [DOI] [PubMed] [Google Scholar]
- 11.Enzmann DR, O’Donohue J, Rubin JB. CSF Pulsations within nonneoplastic spinal cord cysts. AJNR Am J Neuroradiol. 1987;8:517–525. doi: 10.2214/ajr.149.1.149. [DOI] [PubMed] [Google Scholar]
- 12.Brugieres P, Idy Peretti I, Iffenecker C, Parker F, et al. CSF flow measurement in syringomyelia. AJNR Am J Neuroradiol. 2000;21:1785–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Roser F, Ebner FH, Danz S, Riether F, Ritz R, Dietz K, et al. Three-dimensional constructive interference in steady-state magnetic resonance imaging in syringomyelia: Advantages over conventional imaging. J Neurosurg Spine. 2008;8:429–35. doi: 10.3171/SPI/2008/8/5/429. [DOI] [PubMed] [Google Scholar]
- 14.Johnston M, Papaiconomou C. Cerebrospinal fluid transport: A lymphatic perspective. News Physiol Sci. 2002;17:227–230. doi: 10.1152/nips.01400.2002. [DOI] [PubMed] [Google Scholar]
- 15.Hakim R, Black PM. Correlation between lumbo-ventricular perfusion and MRI-CSF flow studies in idiopathic normal pressure hydrocephalus. Surg Neurol. 1998;49:14–20. doi: 10.1016/s0090-3019(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 16.Krauss JK, Regel JP, Vach W, Jungling FD, Droste DW, Wakloo AK. Flow void of cerebrospinal fluid in idiopathic normal pressure hydrocephalus of the elderly: Can it predict outcome after shunting? Neurosurgery. 1997;40:67–73. doi: 10.1097/00006123-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Bradley WG, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal-pressure hydrocephalus: Evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198:523–9. doi: 10.1148/radiology.198.2.8596861. [DOI] [PubMed] [Google Scholar]
- 18.Egeler-Peerdeman SM, Barkhof F, Walchenbach R, Valk J. Cine phase-contrast MR imaging in normal pressure hydrocephalus patients: Relation to surgical outcome. Acta Neurochir Suppl (Wien) 1998;71:340–2. doi: 10.1007/978-3-7091-6475-4_98. [DOI] [PubMed] [Google Scholar]
- 19.Mase M, Yamada K, Banno T, Miyachi T, Ohara S, Matsumoto T. Quantitative analysis of CSF flow dynamics using MRI in normal pressure hydrocephalus. Acta Neurochir Suppl (Wien) 1998;71:350–3. doi: 10.1007/978-3-7091-6475-4_101. [DOI] [PubMed] [Google Scholar]
- 20.Kim DD, Choi JU, Huh R, Yun PH, Kim DI. Quantitative assessment of cerebrospinal fluid hydrodynamics using a phase-contrast cine MR image in hydrocephalus. Childs Nerv Syst. 1999;15:461–7. doi: 10.1007/s003810050440. [DOI] [PubMed] [Google Scholar]
- 21.Sharma AK, Gaikwad S, Gupta V, Garg A, Mishra NK. Measurement of peak CSF flow velocity at cerebral aqueduct, before and after lumbar CSF drainage, by use of phase-contrast MRI: Utility in the management of idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2008;110:363–8. doi: 10.1016/j.clineuro.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Scollatto A, Tenenbaum R, Bahl G, Celerini M, Salani B, Di Lorenzo N. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29:193–8. doi: 10.3174/ajnr.A0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley WG., Jr Idiopathic normal pressure hydrocephalus: New findings and thoughts on etiology. AJNR Am J Neuroradiol. 2008;29:1–3. doi: 10.3174/ajnr.A0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley WG. Normal pressure hydrocephalus: New concepts on etiology and diagnosis. AJNR Am J Neuroradiol. 2000;21:1586–90. [PMC free article] [PubMed] [Google Scholar]


