Abstract
Background
Subcutaneously infused insulin may interfere with the function of nearby glucose-sensing electrodes and vice versa. The prototype of the Combo-Set device (Medtronic) incorporates a subcutaneous insulin delivery catheter and continuous glucose monitoring (CGM) sensor assembled on the same platform and separated by 11 mm. We aim to evaluate Combo-Set’s insulin delivery and glucose-sensing functions.
Methods
Ten subjects with type 1 diabetes wore a Combo-Set and a Sof-Sensor inserted subcutaneously in contralateral abdominal areas connected to iPro recorders (Medtronic) for 53.25 ± 0.75 h (mean ± standard deviation). The Combo-Set delivered insulin diluent except during meal tests on days 1 and 3 when insulin lispro was delivered as a meal bolus and postmeal basal. Venous plasma samples were collected at the following time points from meal start: 0, 30, 60, 120, and 180 min for insulin measurements. The accuracy of the Combo-Set sensors was evaluated and compared with that of the Sof-Sensor, with each referenced against capillary glucose values (Contour Link Meter, Bayer).
Results
Accuracy of the Combo-Set sensor was comparable to that of the Sof-Sensor. Clarke error grid analysis showed that 97% of Combo-Set and 93% of Sof-Sensor values were in the A+B regions (p = .20, not significant). The Combo-Set showed the expected postbolus peak insulin time (67 ± 9 min, mean ± standard error). One “no delivery” alarm occurred during the 21 patient days of use.
Conclusions
A device providing for simultaneous adjacent placement of an insulin infusion catheter and a CGM sensor is feasible and functions within acceptable limits. The low “no delivery” alarm rate was similar to that of other infusion sets.
Keywords: accuracy, colocalization, continuous glucose monitoring, glucose sensing, insulin delivery
Introduction
All patients with type 1 diabetes mellitus (T1DM) require insulin, as do approximately 50% of patients with type 2 diabetes mellitus within 10 years of diagnosis.1 Continuous subcutaneous insulin infusion (CSII) delivers rapid-acting insulin subcutaneously, accurately, and flexibly via a subcutaneous catheter. The current-generation sensor-augmented pump (Paradigm Veo Pump, Medtronic, Northridge, CA) combines insulin delivery with real-time continuous glucose monitoring (RT-CGM).2 Glucose values and trend information are displayed on the screen of the pump in real time.3 The pump may also be programmed to stop insulin delivery in response to hypoglycemia detected by RT-CGM. Despite the utilization of these devices to both deliver insulin and process and display glucose readings, to date, glucose sensing and insulin delivery have employed separate insertion procedures at different anatomical sites.
This requirement for separate platforms and insertion processes for insulin delivery and RT-CGM, together with the associated increase in inconvenience and decrease in acceptability to the patient, may contribute to a decrease in patient compliance. This is relevant because, while RT-CGM has been shown to improve glycemic outcomes significantly in those with T1DM, this benefit is proportional to the percentage of time the sensor is worn.4–7 Combining a glucose sensor with an insulin delivery catheter could potentially improve patient acceptability and compliance because two important glycemic control functions—insulin delivery and continuous glucose measurements—will be executed by one device insertion procedure.
Lindpointner and coauthors8,9 have described a concentric microperfusion and microdialysis catheter combining insulin delivery and glucose measurement. However, other studies in dogs of a combination device incorporating a glucose-oxidase-based glucose sensing electrode into the wall of an insulin infusion catheter revealed interference with the sensor during both insulin and diluent (placebo) infusion (Sumona Adhya personal communication). Artifactual spikes in the glucose sensor trace were observed. This may possibly be due to interference by preservatives in the diluent, which include phenol/cresol10 found in all commercially available insulin preparations. Separate placement was needed to eliminate any possibility of interfering effects by these compounds, which could introduce unwanted bias to glucose measurements, thus affecting sensor accuracy. It has previously been estimated that a 0.25 ml bolus of insulin has an average subcutaneous radius of 7 mm.11 In light of these data, it was decided to develop a combination device incorporating a separate sensor and insulin catheter colocated on a single platform with a separation distance of 11 mm, sufficient to ensure that the sensor would be well outside any potential interference associated with insulin delivery.
The aim of this study was to collect performance data for a prototype insulin-delivery/glucose-sensing combination set (“Combo-Set”, Medtronic) over a 3-day period in subjects with T1DM. We wished to examine whether RT-CGM measurements were affected by the nearby infusion of insulin or its diluent and confirm that insulin infused via Combo-Sets was successfully delivered and absorbed.
Methods
The Combo-Set combines a discrete sensor and an insulin delivery catheter separated by a distance of 11 mm at the skin surface into a single platform (Figure 1). A single-center feasibility study was performed to collect data on the functionality of the Combo-Set. Approval from St. Vincent’s Hospital Human Research Ethics Committee was obtained, and written informed consent was provided by all subjects prior to entry into the study.
Figure 1.
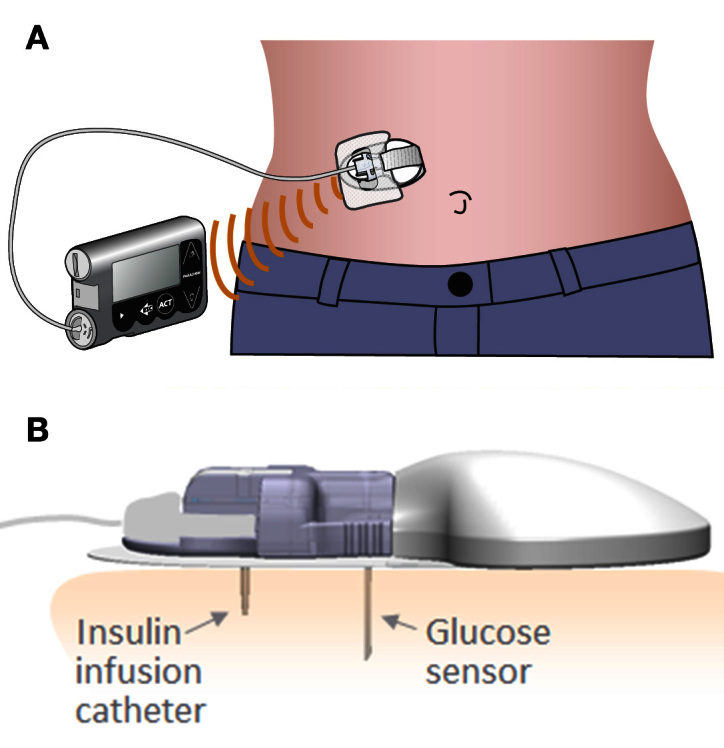
Combo-Set platform in situ. (B) Illustration of insulin delivery catheters and glucose sensor in situ
Subjects
Ten T1DM participants (2 male/8 female) aged [mean ± standard deviation (SD)] 47.4 ± 4.05 years with body mass index of 28.9 ± 5.0 kg/m2, diabetes duration of 22.1 ± 15.4 years, pump use for 8.4 ± 1.4 years, and hemoglobin A1c of 7.0% ± 0.8% were recruited. All were free of complications and managed with CSII therapy using a bolus calculator with established insulin-to-carbohydrate ratios and glucose correction factors. Individuals intolerant of tape adhesive, those with unresolved adverse skin conditions in the area of sensor or device placement, females who were pregnant or planning to conceive, and those with significant renal impairment (estimated glomerular filtration rate <50 ml/min) or with gastroparesis were excluded from the study.
Study Design
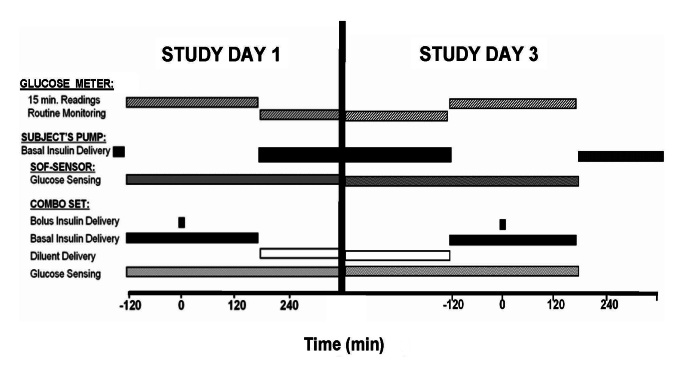
The study design is summarized in Figure 2. Subjects attended the clinical trial unit (CTU) on day 1 and day 3 of the study. The day 1 and day 3 studies described here were performed with the subjects semirecumbent.
Figure 2.
Schematic overview of the study protocol.
On day 1, a Combo-Set was inserted subcutaneously into the anterior abdominal wall using a dedicated insertion device and connected to a standard Paradigm Veo insulin pump, model MMT-754 or MMT-554. The reservoir was filled with insulin lispro (Lilly, Indianapolis, IN) and the line primed. The patient’s established insulin delivery parameters were entered into the study pump and linked to an investigational continuous glucose monitoring (CGM) sensor data gathering device that stored glucose data at 1 min intervals. The Combo-Set was compared with a sensor of a similar generation and design. This second companion CGM sensor (Sof-Sensor, Medtronic) was inserted subcutaneously into the opposite side of each subject’s abdomen and also attached to an identical investigational glucose sensor recorder. The CGM data were not provided to the subjects. The patient’s own insulin pump was then disconnected from their usual insulin delivery catheter, which was left in situ.
Following a 2 h observational period, a standardized test meal containing 65–70 g carbohydrate was consumed by each subject. An insulin bolus was administered immediately prior to the meal, as advised by the Bolus Wizard bolus estimation algorithm. Capillary glucose levels were measured using a Contour Link glucose meter (Bayer, Tarrytown, NY) at 15 min intervals for 2 h prior to the test-meal insulin bolus and every 15 min following the bolus for an additional 3 h. The Contour Link was chosen for its accuracy and interoperability with the insulin pump used in the study. Venous access was obtained and blood samples were drawn for glucose and insulin levels prior to and 30, 60, 120, and 180 min following the test meal bolus.
During the interval between the attendances on day 1 and day 3, subjects reverted to insulin delivery via their own pumps and standard insulin delivery sets. Prior to leaving the CTU following the test meal on day 1, the reservoir/delivery line in the study pump containing lispro was removed and replaced with a line containing insulin-free diluent (Lilly) that did not include insulin but was otherwise identical in composition to the lispro solution. Diluent was then infused according to each subject’s established insulin basal rate parameters. In addition, subjects were advised to administer boluses of diluent with meals and for corrections of elevated glucose levels during the interval between visits to mirror those concurrently administered by their own pumps delivering insulin. Subjects were instructed to continue to use the Contour Link glucose meters at home during the intervals between CTU visits.
On day 3, subjects returned to the CTU and the test meal protocol described earlier was repeated. At the conclusion of the study, 3 h following the second test meal, the Combo-Set and Sof-Sensor were removed and insertion sites inspected. Insulin delivery via the patient’s own insulin pump was resumed. Data were uploaded electronically from each of the glucose sensor recorders and the study pump for review. Following removal, all Combo-Sets were returned to Medtronic.
Biochemical Analysis
Plasma glucose concentrations were measured with a YSI glucose analyzer (YSI Life Sciences, Yellow Springs, OH), using the glucose oxidase method, having a coefficient of variance of 2.4% at 4.4 mmol/liter and 2.9% at 26 mmol/liter. Lispro insulin concentration was measured by radioimmunoassay (Lispro Insulin RIA kit, Millipore, Billerica, MA) in ethylenediaminetetraacetic acid plasma samples after polyethylene glycol extraction of antibodies. The interassay coefficients of variance are 5.7% at a lispro level of 11.2 mU/liter and 8.2% at 86 mU/liter. The assay is specific for lispro insulin and detects human proinsulin and human insulin at <0.05%.
Data Analysis
Descriptive statistics were used to assess the agreement between paired sensor glucose values and the reference glucose meter values for the Combo-Set and the Sof-Sensor, with an evaluation of their performance relative to each other and also study day 1 versus study day 3. Statistical calculations were performed using GraphPad Prism Version 5.0 (GraphPad Software, San Diego, CA). Data are reported as mean ± standard error of the mean or SD.
Results
All participants completed the study. The Combo-Set was well tolerated by all participants. There were no infections, skin reactions, or discomfort.
Insulin Delivery
Mean meal boluses of inpatient meal tests performed on days 1 and 3 of device insertion were of similar sizes (6.7 ± 0.7 and 7.2 ± 0.7 IU for meal test on days 1 and 3, respectively). There was one insulin delivery line occlusion unrelated to bolus delivery. Statistical analysis (paired t-test) demonstrated that there were no significant differences between total glucose excursions after meals 1 and 2, indicating comparable glucodynamic effectiveness of Combo-Sets on day 1 and day 3 of device wear [day 1 mean (min–max) 181.0 ± 32.4 mg/dl/h (range 75–329 mg/dl/h) and day 3 mean 135.5 ± 18.4 mg/dl/h (range 65–244 mg/dl/h); p = .1351].
Postmeal incremental lispro insulin area under the curve (0–180 min) was similar on day 1 and day 3 of the study [day 1 mean (SD) 5640.5 (657.7) mU/liter/min and day 3 mean 5303.1 (1008.6) mU/liter × min; p = not significant].
Glucose Sensing
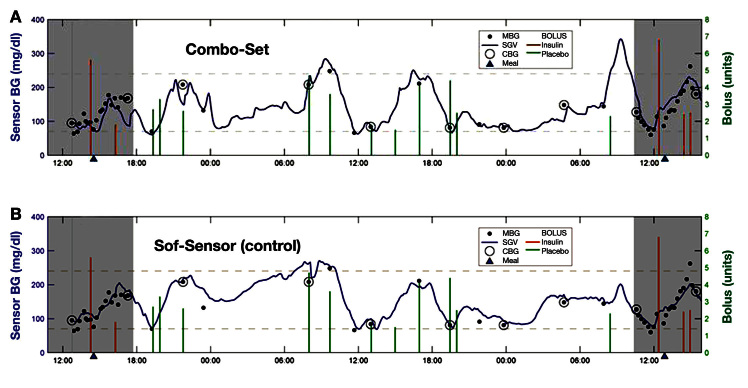
Review of the CGM traces from the Combo-Set sensor revealed no evidence of spike artifacts during insulin or diluent delivery (Figure 3A) and good agreement between the Combo-Set and contralateral Sof-Sensor (Figure 3B). Combo-Set sensor performance was evaluated based on a total of 471 paired blood glucose meter–sensor values generated by 10Combo-Set sensors worn in 10 subjects for (mean ± SD) 53.25 ± 0.75 h. Mean absolute relative difference (MARD; mean ± SD) values from Combo-Set sensors during insulin (n = 322 paired values) 17.2% ± 14.2% and diluent (n = 149 paired values) 16.4% ± 15.9% infusion were not significantly different (p = not significant). Sof-Sensor performance was evaluated based on a total of 481 paired (n = 332 for insulin; n = 149 for diluent) blood glucose meter–sensor values generated by 10 sensors worn in the same 10 subjects simultaneously. Clarke error grid percentages for the 20/20 range and MARD data are shown in Table 1 for Combo-Set and Sof-Sensor referenced against capillary blood glucose values. The data indicate that the glucose-sensing accuracy of the Combo-Set and control Sof-Sensor is comparable. Analysis of paired points from day 1 versus day 3 data does not suggest any degradation in performance with duration of use (Figure 4). The size of the study (10 patients) precluded the evaluation of any correlation between bolus size and sensor accuracy. YSI blood glucose measurements were compared with the Contour Link measurements with mean MARDs of 8.3% ± 0.7% (n = 65) for a set of YSI/meter blood glucose readings with identical time stamps.
Figure 3.
Combo-Set sensor versus (B) companion Sof-Sensor glucose tracings. BG, blood glucose; MBG, meter blood glucose; SGV, sensor glucose value; CBG, meter blood glucose reading used for calibration.
Table 1.
Mean and Median Absolute Relative Difference, Mean Absolute Relative Difference Range, Clarke A%, Clarke B%, Clarke A+B %, and 20/20 Range for Combo-Set and Sof-Sensor Referenced against Paired Capillary Blood Glucose Readings with a Glucose Meter (Contour Link Meter, Bayer)
| Combo-Set (n = 10) | Sof-Sensor (n = 10) | |
| Mean absolute relative difference | 17.0% | 18.9% |
| Median absolute relative difference | 13.5% | 13.3% |
| Mean absolute relative difference range | 11.5–36.4% | 9.8–36.9% |
| Clarke A | 71.5% | 65.7% |
| Clarke B | 25.3% | 27.4% |
| Clarke A+B | 96.8% | 93.1% |
| 20/20 range | 71.3% | 65.7% |
Figure 4.
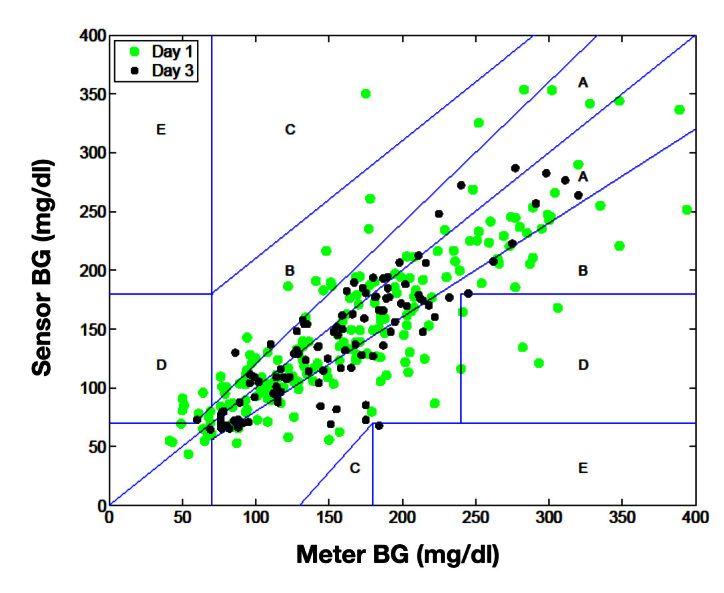
Clarke error grid for paired glucose readings obtained from Combo-Set sensor and capillary blood glucose readings with a glucose meter (Contour Link Meter, Bayer). Green dots, day 1; black dots, Day 3. BG, blood glucose
Discussion
This study in 10 patients with T1DM established on CSII therapy collected performance data, formally testing the feasibility of Combo-Set, a device that houses a subcutaneous insulin delivery catheter and a glucose sensor in a single platform. The device was well tolerated by all subjects, and this preliminary study indicates that neither insulin delivery nor glucose sensing modalities was compromised.
Exploratory animal studies by members of our research group suggested significant technological barriers associated with a single line combining insulin delivery and glucose-sensing functions (Sumona Adhya personal communication). In contrast, Lindpointner and coauthors,8,9 utilizing a single concentric microperfusion/microdialysis catheter inserted subcutaneously, have published data describing the potential viability of this approach. Interstitial fluid (ISF) glucose levels, though lower in insulin-exposed tissues, displayed a variance that was proportional and colinear with reference plasma glucose levels. While no artifact was reported, these studies did not use glucose-oxidase-based amperometric sensors. Also, ISF glucose was not measured continuously but assessed at 30 min intervals, and it is possible that the transient artifactual spikes may have been missed.
Linde and Philip11 have reported that a bolus of 0.25 ml of insulin (40 U/ml) has an average subcutaneous radius of 7 mm. In an approach similar to that taken with the Combo-Set, though utilizing a microdialysis catheter for glucose sensing, Hermanides and coauthors12 also employed a dual shaft device for ISF glucose sensing and insulin delivery. Their separation distance of 9 mm, being greater than 7 mm, may provide an explanation as to why, in contrast with Lindpointner and coauthors,9 they did not observe a reduction in ISF glucose relative to reference plasma glucose measurements. Combo-Set methodology, which utilizes capillary blood glucose measurements for calibration, would be expected to minimize the impact of insulin delivery in proximity to the sensor upon reported glucose measurements. No artifactual spikes were observed in the Combo-Set CGM traces. The 11 mm separation of the two shafts, being greater than the hypothesized 7 mm radius of the insulin pool, may be sufficient to avoid interference with our glucose-sensing electrode.
Performance of the sensor component of the Combo-Set, which incorporated technology identical to Sof-Sensor, closely matched that of the Sof-Sensor comparator. The Sof-Sensor represents an early generation sensor, and one would expect that a second-generation sensor such as Enlite (Medtronic) to have an improved performance profile, though, given that it also utilizes a glucose-oxidase-based approach, it too would have similar limitations to Sof-Sensor. An intravascular glucose sensor that operates on the principle of quenched fluorescence has been described.13 It remains to be seen whether such a sensor could be incorporated with a subcutaneous insulin delivery catheter into a single line.
No compromise in insulin delivery was observed in our study. In particular, no occlusion alarms occurred during insulin bolus administration, and this finding is expected to extend to the two other commercially available rapid-acting insulin analogs.
As health care professionals and researchers, it is our ultimate goal to maximize the physical and emotional wellbeing ofthose with T1DM while minimizing the burden associated with the management of a chronic life-long and potentially life-threatening disease affecting a predominantly young group of people. Real-time CGM has been shown to improve glycemia. This improvement is significantly related to the percentage of time the sensor is worn by the patient.4–7 However, there is an intellectual and physical burden associated with this technology that may explain a reduction in patient compliance over time, as has been reported by some investigators,14 which could erode positive glycemic outcomes. It could also impact quality of life and explain inconsistent related outcomes reported in previous studies.15–17
The wider adoption of CGM technology still faces challenges related to cost, reliability, comfort, ease of use, and integration with other technologies.18 A limitation specific to the Combo-Set itself is that there is a difference between the current recommendations for optimal duration for use of the insulin delivery catheter (3 days) and the glucose sensor (6 days). However, it should be recognized that the need to change the insulin delivery catheter does not mandate cessation of glucose sensing. The Combo-Set may be left in situ and continue to be used purely as a sensor with a new dedicated insulin catheter inserted. For those patients who use RT-CGM continuously, this would provide the option of a single platform for 50% of the time. Another potential related limitation may be that patients wearing the Combo-Set would be disinclined to perform an insulin-delivery line change every 3 days, leaving the insulin catheter in situ until it fails. However, these patients may well do the same with a dedicated insulin delivery catheter.
Despite the limitations described here, the Combo-Set may significantly improve patient acceptance and utilization of RT-CGM by combining two separate insertions into a single step and by reducing the patient’s body surface area encroached upon by the technology. This report details the findings of an initial feasibility study. The formal exploration of the influence that the Combo-Set, or an evolution of this platform, may have on patient compliance, glycemia, cost-effectiveness, quality of life, and other diabetes-related outcomes may form the basis for future research.
The ultimate goal for those developing the technology is a closed-loop system. A fully automated system will require a significant level of redundancy for reasons of safety. The Combo-Set may facilitate evolution toward a closed-loop system by combining sensing and delivery into a single device, therefore reducing the number of platforms required and the abdominal area utilized.
Conclusions
This report details the first feasibility study in humans of a colocated subcutaneous insulin delivery/CGM platform. There was no interference between the glucose sensing and insulin delivery functions. Comparison with a dedicated glucose sensor employing identical technology demonstrated durability of performance over 3 days. The combination of glucose-sensing and insulin-delivery modalities allowing for a single device insertion and a reduction in skin area occupied may increase RT-CGM acceptability and utilization and ultimately improve clinical outcomes in people living with T1DM.
Acknowledgments
We gratefully acknowledge the assistance of Ms. Jodie Horsburgh, Ms. Sue Kent, Ms. Catherine Peeler, Andrzej Januszewski, and all the patients who participated in the study. Sof-Sensor, iPro, Paradigm, Veo, Enlite, and Bolus Wizard are trademarks of Medtronic MiniMed Inc. Contour is a trademark of Bayer Diabetes Care Inc.
Glossary
- (CGM)
continuous glucose monitoring
- (CSII)
continuous subcutaneous insulin infusion
- (CTU)
clinical trial unit
- (ISF)
interstitial fluid
- (MARD)
mean absolute relative difference
- (RT-CGM)
real-time continuous glucose monitoring
- (SD)
standard deviation
- (T1DM)
type 1 diabetes mellitus
Funding
This work was funded by Medtronic Inc.
Disclosures
Sumona Adhya, Gayane Voskanyan, and John B. Welsh are full-time employees of Medtronic Inc. David N. O’Neal and Alicia J. Jenkins have received research funding and honoraria from Medtronic and Eli Lilly.
References
- 1.Turner RC, Cull CA, Frighi V, Holman RR, UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281(21):2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 2.Mastrototaro JJ. The MiniMed continuous glucose monitoring system. Diabetes Technol Ther. 2000;2(Suppl 1):S13–8. doi: 10.1089/15209150050214078. [DOI] [PubMed] [Google Scholar]
- 3.Mastrototaro JJ, Cooper KW, Soundararajan G, Sanders JB, Shah RV. Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Adv Ther. 2006;23(5):725–32. doi: 10.1007/BF02850312. [DOI] [PubMed] [Google Scholar]
- 4.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–83. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA, STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins AJ, Krishnamurthy B, Best JD, Cameron FJ, Colman PG, Farish S, Hamblin PS, O’Connell MA, Rodda C, Rowley K, Teede H, O’Neal DN. Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial. Diabetes Care. 2010;33(6):1242–8. doi: 10.2337/dc09-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindpointner S, Korsatko S, Köhler G, Köhler H, Schaller R, Kaidar R, Yodfat O, Schaupp L, Ellmerer M, Pieber TR, Regittnig W. Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes. Diabetes Care. 2010;33(3):595–601. doi: 10.2337/dc09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindpointner S, Korsatko S, Köhler G, Köhler H, Schaller R, Schaupp L, Ellmerer M, Pieber TR, Regittnig W. Glucose levels at the site of subcutaneous insulin administration and their relationship to plasma levels. Diabetes Care. 2010;33(4):833–8. doi: 10.2337/dc09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moatti-Sirat D, Velho G, Reach G. Evaluating in vitro and in vivo the interference of ascorbate and acetaminophen on glucose detection by a needle-type glucose sensor. Biosens Bioelectron. 1992;7(5):345–52. doi: 10.1016/0956-5663(92)85030-e. [DOI] [PubMed] [Google Scholar]
- 11.Linde B, Philip A. Massage-enhanced insulin absorption--increased distribution or dissociation of insulin? Diabetes Res. 1989;11(4):191–4. [PubMed] [Google Scholar]
- 12.Hermanides J, Wentholt IM, Hart AA, Hoekstra JB, DeVries JH. No apparent local effect of insulin on microdialysis continuous glucose- monitoring measurements. Diabetes Care. 2008;31(6):1120–2. doi: 10.2337/dc08-0145. [DOI] [PubMed] [Google Scholar]
- 13.Peyser T, Zisser H, Khan U, Jovanovič L, Bevier W, Romey M, Suri J, Strasma P, Tiaden S, Gamsey S. Use of a novel fluorescent glucose sensor in volunteer subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):687–93. doi: 10.1177/193229681100500323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Research in Children Network Study Group. Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, Beck R, Tamborlane W, Ruedy K. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10(2):91–6. doi: 10.1111/j.1399-5448.2008.00476.x. [DOI] [PubMed] [Google Scholar]
- 15.Rubin RR, Peyrot M. Treatment satisfaction and quality of life for an integrated continuous glucose monitoring/insulin pump system compared to self-monitoring plus an insulin pump. J Diabetes Sci Technol. 2009;3(6):1402–10. doi: 10.1177/193229680900300621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin RR, Peyrot M, STAR 3 Study Group Health-related quality of life and treatment satisfaction in the Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial. Diabetes Technol Ther. 2012;14(2):143–51. doi: 10.1089/dia.2011.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins AJ, Krishnamurthy B, Best JD, Cameron FJ, Colman PG, Hamblin PS, O’Connell MA, Rodda C, Teede H, O’Neal DN. An algorithm guiding patient responses to real-time-continuous glucose monitoring improves quality of life. Diabetes Technol Ther. 2011;13(2):105–9. doi: 10.1089/dia.2010.0139. [DOI] [PubMed] [Google Scholar]
- 18.Brauker J. Continuous glucose sensing: future technology developments. Diabetes Technol Ther. 2009;11(Suppl 1):S25–36. doi: 10.1089/dia.2008.0137. [DOI] [PubMed] [Google Scholar]