Abstract
Background
Blood glucose (BG) meters used for assisted monitoring of blood glucose (AMBG) require different attributes compared with meters designed for home use. These include safety considerations (i.e., minimized risk of blood-borne pathogen transmission), capability for testing multiple blood sample types, and enhanced performance specifications. The OneTouch® Verio™Pro+ BG meter is designed to incorporate all of these attributes.
Methods
Meter accuracy was assessed in clinical studies with arterial, venous, and capillary blood samples with a hematocrit range of 22.9–59.8%. The effect of interferents, including anticoagulants, on accuracy was evaluated. The meter disinfection protocol was validated, and instructions for use and user acceptance of the system were assessed.
Results
A total of 97% (549/566) of BG measures from all blood sample types and 95.5% (191/200) of arterial blood samples were within ±12 mg/dl or 12.5% of reference measurements. The system was unaffected by 4 anticoagulants and 57 of 59 endogenous and exogenous compounds; it was affected by 2 compounds: pralidoxime iodide and xylose. Bleach wipes were sufficient to disinfect the meter. Users felt that the meter's quality control (QC) prompts would help them to comply with regulatory requirements.
Conclusions
The meter provided accurate measurements of different blood samples over a wide hematocrit range and was not affected by 57 physiologic and therapeutic compounds. The QC prompts and specific infection-mitigating design further aid to make this meter system practical for AMBG in care facilities.
Keywords: assisted monitoring of blood glucose, bloodborne infection, diabetes, glucose, hepatitis, monitor, self-monitoring of blood glucose
Introduction
Most blood glucose (BG) meters are designed for self-monitoring of blood glucose (SMBG) by a single patient.1 When BG meters are used within institutional point-of-care testing (POCT) settings (e.g., clinics and hospitals) or assisted monitoring of blood glucose (AMBG) settings (i.e., nursing homes and long-term care homes), clinical usage changes from single-patient to multiple-patient use. Operated in this manner, BG meters require different attributes.1 These attributes include compatibility with disinfectants, validation for testing multiple blood sample types, availability of multiple levels of control solution, enhanced accuracy and performance specifications, lack of interference, and ease of use.
Assisted monitoring of blood glucose and POCT clinical settings require the use of control measures to prevent transmission of infectious bloodborne agents, such as hepatitis B virus (HBV). Several outbreaks reported in the United States and Europe have been attributed to poor infection control associated with AMBG.2–5 Thus the shift from SMBG to AMBG presents a “series of challenges for diabetes science and technology,” and requires the development of specific procedures and equipment.1 United States regulatory authorities such as the Centers for Disease Control and Prevention, the Food and Drug Administration (FDA), and the Centers for Medicare and Medicaid Services have taken steps to address these challenges by issuing recommendations and guidelines emphasizing safe AMBG practices.6–8
Blood glucose meters limited to testing capillary blood samples are not appropriate when venous or arterial BG sampling is clinically required.9,10 For example, BG determinations by finger stick can provide misleading data for patients with impaired peripheral perfusion, and venous or arterial blood better reflects true physiologic status. Therefore, BG meters that are used for POCT should ideally be capable of measuring BG in different sample types (i.e., arterial, venous, and capillary blood). Blood can also be the source of wide ranges of endogenous factors (e.g., hematocrit, cholesterol, and uric acid), especially in hospital or institutional settings, and exogenous factors [e.g., medications such as ascorbic acid and acetaminophen or those that contain additives like maltose (when administered parenterally) or lactose], which can interfere with the accuracy of BG meters.11,12 Therefore, there is a clinical need for POCT meters to provide accurate BG measurements when the blood sample contains a wide range of endogenous and exogenous compounds.
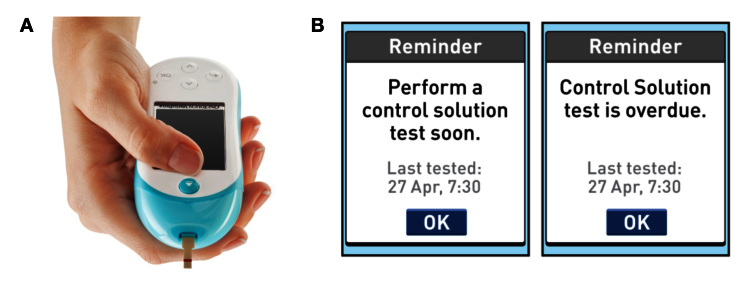
The OneTouch® Verio™Pro+ BG meter has been designed specifically for POCT and AMBG (Figure 1A). It uses OneTouch Verio test strips, the performance of which, in venous and capillary blood, has been described previously.13,14 This meter automatically detects when control solution has been applied, provides onscreen quality control (QC) prompts, and features a test strip ejector that is expected to reduce health care professional (HCP) contact with blood-filled test strips (Figure 1B). The meter has been designed to withstand disinfection in conformance with FDA guidance and comes with clear disinfection instructions.
Figure 1.
(A) The OneTouch VerioPro+ meter. (B) Examples of onscreen QC prompts.
The findings reported here include assessment of the accuracy of this meter when measuring BG in arterial, venous, and capillary blood over a wide range of hematocrit levels; evaluation of the effect of potential exogenous and endogenous compounds on meter readings; validation of the disinfection procedure for the meter; and evaluation of the instructions for use and user acceptance of the system by HCPs.
Methods
The clinical evaluation and accuracy studies were conducted at four study sites: three sites in the United Kingdom (Highland Diabetes Institute, Inverness; Royal Infirmary, Edinburgh; and Birmingham Heartlands Hospital, Birmingham) and one site in the United States (Scripps Whittier Diabetes Institute, La Jolla, CA). The study protocol had ethical committee approval from the National Health Service Scotland Research and Ethics Committee, United Kingdom, and the Internal Review Board of Scripps Whittier Diabetes Institute, La Jolla, CA. All participants gave their appropriate informed consent in compliance with the Declaration of Helsinki. Disinfection, interference, and technical performance tests were conducted at research and development facilities located at LifeScan Scotland (Inverness, UK).
Effect of Sample Type
Individuals with diabetes were enrolled in the U.K. sites for the testing of arterial, venous, and capillary blood; the U.S. site tested only residual arterial blood. Test strips were selected from three randomly assigned lots and tested with capillary, venous, or arterial blood samples. Each sample was tested for hematocrit and for reference plasma glucose with the YSI 2300 STAT PLUS™ glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH). Samples for arterial blood testing were obtained from an arterial line or arterial puncture (residual samples from an existing physician’s order for blood testing). The results were compared with the draft standards criteria proposed by the Clinical and Laboratory Standards Institute (CLSI) for hospital BG testing (i.e., within ±12 mg/dl of the corresponding reference results at BG concentrations <100 mg/dl and within ±12.5% of reference results at BG concentrations ≥100 mg/dl).15
Effect of Anticoagulants
The effect of anticoagulants was assessed according to CLSI guidance.16 Venous blood from donors with normal-range hematocrit levels (37–45%) was collected in tubes, each containing one of the following four anticoagulants: lithium heparin (control), sodium heparin, potassium ethylenediamine tetra-acetic acid (EDTA), and sodium citrate native. The blood was adjusted to one of four glucose levels (30 ± 5, 65 ± 5, 240 ± 12, or 560 ± 28 mg/dl) and one replicate was tested with five meters (i.e., 22 donors × 5 meters × 1 replicate; or n = 110 per anticoagulant and glucose concentration). The average bias to the YSI reference was calculated at each blood condition (across donor, meter, and replicate). Bias to YSI for sodium heparin, sodium citrate, or potassium EDTA was compared with bias with lithium heparin (control). In a similar fashion, sodium citrate and potassium EDTA were compared with sodium heparin. An anticoagulant was deemed noninterfering for testing venous blood with the system if the bias differences were within ±4 mg/dl at BG concentrations <80 mg/dl, and within ±5% at BG concentrations ≥80 mg/dl.
Effect of Interferents
Interferent testing was performed with 59 different substances, including endogenous substances commonly elevated in the blood of individuals with diabetes, endogenous substances known to have an interfering effect on some BG monitoring systems, and exogenous substances (medications or components of therapies) frequently used by individuals with diabetes. In line with CLSI recommendations, substances were tested using a paired-difference test and/or a dose-response protocol.17 For each strip lot and for the three lots combined, the average bias (or bias predicted by linear regression) difference between the claimed interferent level and the zero level had to be within ±6.04 mg/dl at BG levels <80 mg/dl, and within ±7.38% at BG levels ≥80 mg/dl. Reference values (high-normal endogenous and high therapeutic) were based on CLSI values unless otherwise stated.17–20
Disinfection
Hypochlorite wipes (0.55%, 5500 parts per million of sodium hypochlorite; Ultra Clorox Germicidal Bleach, EPA Reg. No. 67619-8) were evaluated as a disinfectant for the meter housing. Duck HBV (a surrogate for human HBV) was applied to three lots of the three housing materials of the system. Acceptability was defined as complete inactivation of the virus from the test material and demonstration that the disinfection was effective.7
User Acceptance
Health care professional feedback was provided through Design Science (Philadelphia, PA). In total, 38 HCPs were recruited (10 registered nurses, 15 certified nursing assistants, 7 licensed practical nurses, 5 POC coordinators, and 1 laboratory technician). Of these, 71% (27/38) reported running BG tests daily, 10% (4/38) weekly, and 5% (2/38) monthly. The 5 HCPs who reported they never performed BG testing were 4 of the POC coordinators and the laboratory technician. After the HCPs read the operator’s guide, they were observed during simulated use of the meter and then surveyed about its proper use and their perceptions of its usability.
Results
A total of 191 patients with diabetes were enrolled from the U.K. sites for the testing of capillary and venous blood, and a further 34 volunteers were enrolled for the testing of residual arterial blood. The U.S. site enrolled 35 patients for the testing of residual arterial blood only.
Clinical Evaluation and Accuracy
A total of 200 arterial blood measurements (from 67 subjects) and a total of 177 venous and 189 capillary BG measurements (from 190 subjects) were made with the meter. The hematocrit of these samples ranged from 22.9 to 59.8%, with means of 34.0%, 42.2%, and 41.35% for arterial, venous, and capillary blood, respectively.
Overall, 97% of the meter measurements from all blood sample types met proposed accuracy standards (Table 1).17 Figure 2 shows the bias plots for arterial, venous, and capillary blood. The percentage of data meeting these accuracy standards was 95.5%, 98.3%, and 97.4% for arterial, venous, and capillary blood, respectively.
Table 1.
Meter Performance with Arterial, Capillary, and Venous Blood Sample Tests Performed by Health Care Professionals at Four Clinics Compared with YSI Reference Values
| Blood sample type | Glucose levels and accuracy limitsa | ||
| Glucose <100 mg/dlData within ±12 mg/dl n/N (%) |
Glucose ≥100 mg/dlData within ±12.5% n/N (%) |
Data within ±12 mg/dl or 12.5%n/N (%) | |
| Arterial | 27/27 (100) | 164/173 (94.8) | 191/200 (95.5) |
| Venous | 25/26 (96.2) | 149/151 (98.7) | 174/177 (98.3) |
| Capillary | 21/23 (91.3) | 163/166 (98.2) | 184/189 (97.4) |
| All blood sample types | 73/76 (96.1) | 476/490 (97.1) | 549/566 (97.0) |
As proposed by the CLSI.15
Figure 2.
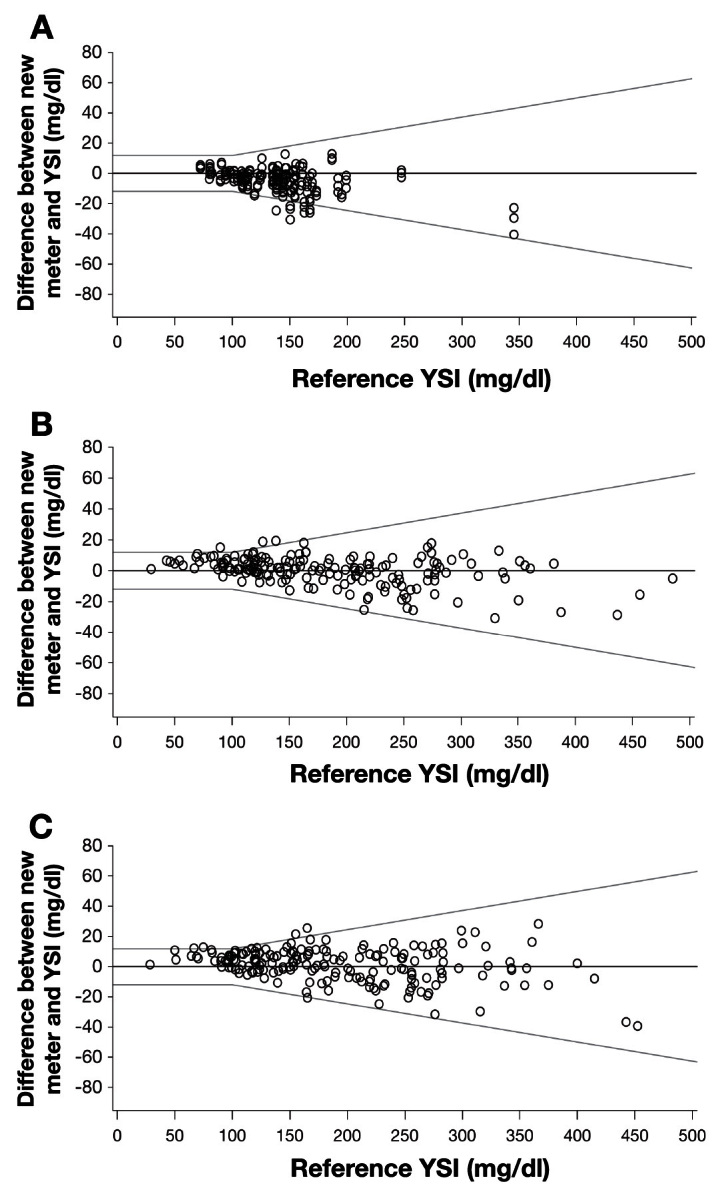
Figure 2. Meter performance compared with YSI reference for (A) arterial, (B) venous, and (C) capillary blood. Accuracy lines refer to the draft standards criteria proposed by the CLSI for hospital BG testing.15
Previously, Bailey and coauthors14 reported that the test strips used with the VerioIQ meter were not significantly affected by hematocrit over a range of 19–61%, with the exception of a small (7.5 mg/dl) positive bias for a hematocrit value of 61% at BG values <65 mg/dl. In a more extensive evaluation, Teodorczyk and coauthors13 showed similar results, with 100% (2700 of 2700) of measurements falling within current International Organization for Standardization error tolerances. In the current evaluation, the meter was not significantly affected by hematocrit within the range of 22.9–59.8% (data not shown).
Effect of Anticoagulants
Venous blood from 22 patients with diabetes with normal-range hematocrit levels (37–45%) was collected. The relative bias for sodium heparin (less than ±0.5 mg/dl for <80 mg/dl and less than ±1.0% for ≥80 mg/dl), sodium citrate (less than ±1.6 mg/dl for <80 mg/dl and less than ±3.1% for ≥80 mg/dl), and potassium EDTA (less than ±1.6 mg/dl for <80 mg/dl and less than ±4.1% for ≥80 mg/dl) when compared with lithium heparin (control) met the acceptance criteria of within ±4 mg/dl (for glucose <80 mg/dl) or ±5% (for glucose ≥80 mg/dl), both for individual lots and for combined data from all lots. The delta bias for sodium citrate (less than ±1.8 mg/dl for <80 mg/dl and less than ±3.3% for ≥80 mg/dl) and potassium EDTA (less than ±1.5 mg/dl for <80 mg/dl and less than ±4.0% for ≥80 mg/dl) compared with sodium heparin also met the acceptance criteria.
Effect of Interferents
Performance of the meter met the accuracy acceptance criteria in the presence of normal-to-high or high therapeutic concentrations of all the endogenous and exogenous interferents evaluated, except for xylose and pralidoxime iodide (PAM; Table 2).17–20 In many cases, the meter met the accuracy acceptance criteria in the presence of abnormally high concentrations of endogenous interferents or supratherapeutic concentrations of exo-genous interferents.
Table 2.
Interference by Endogenous and Exogenous Compounds, Reported as the Maximum Concentration Accepted by the Meter
| Endogenous compound | Normal-to-high endogenous concentration,mg/dl (mmol/liter) | Maximum acceptable concentration,mg/dl (mmol/liter) |
| Bilirubina | 1.20 (0.021) | 18.97 (0.32) |
| Glutathionea | 0.535 (0.017) | 64.85 (2.11) |
| Uric acida | 8.00 (0.48) | 9.55 (0.57) |
| Cholesterol | 200 (5.2) | 750.9 (19.42) |
| Creatinine | 1.3 (0.11) | 4.74 (0.45) |
| Hemoglobin | 200 (0.031) | 200 (0.031) |
| Lactic acid | 19.8 (2.2) | 56.33 (6.20) |
| Triglycerides | 250 (3.7) | 3331 (37.6) |
| Urea | 85.8 (14.3) | 249.1 (41.48) |
| Exogenous compound | High therapeutic exogenous concentration,mg/dl (mmol/liter) | Maximum acceptable concentration,mg/dl (mmol/liter) |
| Acetaminophena | 3.0 (0.20) | 16.88 (1.12) |
| Ascorbic acida | 2.0 (0.114) | 5.71 (0.32) |
| Dopaminea | 0.03 (0.002) | 0.08 (0.005) |
| Levodopaa | 0.326 (0.02)18 | 0.95 (0.048) |
| Theophylline | 2.0 (0.11) | 3.83 (0.18) |
| Ampicillin | 1.8 (0.051) | 5.14 (0.15) |
| Atenolol | 0.2 (0.008) | 0.95 (0.036) |
| Atorvastatin | 2.67 (0.048) | 7.11 (0.128) |
| Cefazolin | 40.0 (0.88) | 227.86 (5.01) |
| Chlorpromamide | 36.0 (1.3) | 70.4 (2.54) |
| Cimetidine | 0.75 (0.030) | 1.94 (0.077) |
| Digoxin | 0.00020 (0.000003) | 0.00057 (0.000007) |
| Dobutamine | 4.67 (0.15) | 11.84 (0.39) |
| Ephedrine | 0.01 (0.00061)19 | 0.19 (0.011) |
| Epinephrine | 0.032 (0.00175) | 0.092 (0.00502 |
| Erythromycin | 2.0 (0.03) | 5.81 (0.079) |
| Ezetimibe | 0.33 (0.0081) | 0.96 (0.023) |
| Fenofibrate | 1.5 (0.04) | 4.31 (0.12) |
| Furosemide | 3.0 (0.09) | 5.69 (0.17) |
| Gentamycin | 1.0 (0.02) | 0.8 (0.017) |
| Gentisic acid | 0.6 (0.04) | 1.71 (0.11) |
| Glimepiride | 0.27 (0.0055) | 0.76 (0.015) |
| Glipizide | 0.10 (0.00224) | 0.19 (0.00426) |
| Glyburide | 0.06 (0.0012) | 0.19 (0.0038) |
| Hydralazine | 10 (0.62) | 23.65 (1.87) |
| Hydrochlorothiazide | 0.2 (0.00672) | 0.58 (0.019) |
| Exogenous compound | High therapeutic exogenous concentration,mg/dl (mmol/liter) | Maximum acceptable concentration, mg/dl (mmol/liter) |
| Hydrocodone | 0.0051 (0.00017) | 0.0200 (0.00067) |
| Ibuprofen | 7.0 (0.34) | 47.42 (2.30) |
| Insulin | 73.3 IU/day | 213 IU/day |
| Lidocaine | 0.60 (0.03) | 1.16 (0.05) |
| Lisinopril | 0.01 (250) | 0.03 (640) |
| Mannitol | 600 (32.9) | 1707 (93.70) |
| Metformin | 0.40 (0.03) | 3.87 (0.30) |
| Methyldopa | 0.75 (0.04) | 1.44 (0.068) |
| Phenytoin | 2.0 (0.08) | 4.79 (0.19) |
| Pioglitazone | 1.5 (0.04) | 3.87 (0.11) |
| PAMa | 80.0 (4.76) | System not compatibleb |
| Procainamide | 1.20 (0.05) | 2.33 (0.099) |
| Quinidine | 0.60 (0.02) | 0.93 (0.029) |
| Salicylate | 30 (2.17) | 57.48 (4.16) |
| Simvastatin | 2.7 (0.066) | 7.6 (0.18) |
| Tetracycline | 0.50 (0.01) | 1.43 (0.032) |
| Tolazamide | 3.31 (0.11)20 | 9.42 (0.30) |
| Tolbutamide | 10.8 (0.4) | 61.94 (2.29) |
| Warfarin | 0.31 (0.01) | 0.95 (0.031) |
| Galactose | 5 (0.28) | 60.39 (3.35) |
| Lactose | 0.5 (0.01) | 3.93 (0.11) |
| Maltose | 120 (3.51) | 363.6 (10.05) |
| Sucrose | 2670 (78.0) | 5142 (150.20) |
| Xylosea | 60.00 (4.000) | 7.55 (0.50) |
Tested in a dose-response study; all other compounds were tested in a paired-difference study.
When testing with PAM, the meter system gave only error messages.
Disinfection
Disinfection for 1 min with 0.55% hypochlorite wipes demonstrated complete inactivation of duck HBV from each of the meter housing materials.
User Acceptance
After reading the meter operator’s guide, most HCPs (32 of 38, 84.2%) successfully ejected the used test strip with the test strip ejector after the simulated BG tests. In response to the user survey, most respondents (34 of 38, 89.5%) indicated they “agreed” or “strongly agreed” with the statement that the meter “gives me an immediate and accurate reading that helps me quickly adjust treatments.” All users reported that they knew how to respond correctly to an onscreen QC prompt, and 34 of 38 (89.5%) agreed with the statement that the meter “reminding me to conduct a quality control test when it is due will help me be compliant with our institution’s regulatory requirements.” For the simulated BG test, the observers noted that, although 29 of 38 (76.3%) HCPs met acceptance criteria for properly keeping the meter pointed downward during the test, some of the HCPs were observed holding the meter upright in order to pull the test strip out. It was reported by the study facilitator that difficulties arose during this task because of the meter’s repeated use with control solution, which led to the strip sticking in the meter. As this error is not expected when blood is tested, no changes were made to the user instructions or the operator’s guide.
Discussion
For all sample types tested, clinical evaluation of the meter indicated that >95% of measurements were within 12 mg/dl or 12.5% of reference YSI values. The accuracy and precision of the system met appropriate performance standards when testing blood with hematocrit values of 22.9−59.8% (data not shown) and when tested with four different anticoagulants. The system was unaffected by 57 of the 59 potential interferents evaluated. Bleach wipes were sufficient to disinfect the meter against HBV in conformance with FDA standards. Users indicated that the system would help them comply with their institutional requirements for POCT and AMBG.
In critically ill patients, the sampling site (i.e., arterial, venous, and capillary blood) may yield different BG values based on the patient’s underlying medical condition.10 Glucose determinations by finger stick are not appropriate in certain clinical conditions, because they can provide misleading data. Finger stick samples are not recommended for patients with arterial insufficiency, which can occur in patients in shock21 and those with Raynaud’s syndrome, severe hypotension, severe peripheral arterial disease,22 vasoconstriction, ischemia, edema,10 or hypothermia.23 All of these conditions can produce a reduction in peripheral perfusion (hypoperfusion) and a decrease in the peripheral BG concentration compared with concomitant venous or arterial values. For hypothermia and other physiologic conditions, sampling of venous or arterial blood samples is recommended for an accurate assessment of a patient’s glycemic status. Therefore, POCT devices capable of measuring BG from venous and arterial, as well as capillary, blood may offer advantages in some clinical settings. This study assessed accuracy of the OneTouch VerioPro+ BG meter using arterial, venous, and capillary blood samples and found that 97% of BG measures from all blood sample types, and 95.5% of arterial blood samples, were within 12 mg/dl (for BG values < 100 mg/dl) or 12.5% (for BG values ≥ 100 mg/dl) of reference YSI values. Although performance of the test strips with venous and capillary blood has been reported previously,13,14 this study demonstrates they also provide accurate results when used with arterial blood.
Several substances, including anticoagulants, acetaminophen, ascorbic acid, dopamine, maltose, and mannitol12,24–26 have been reported to affect the accuracy of some BG monitoring systems. The findings from the present study indicate that the meter accuracy and precision were unaffected by exposure to these compounds. Meter accuracy and precision were affected by xylose (occasionally used during intestinal malabsorption tests) and PAM (used to treat poisoning by organophosphates or acetylcholine esterase inhibitors). Therefore, this meter should not be used if PAM is known or suspected to be in the patient’s blood. In addition, the meter should not be used within 24 h of xylose administration, when xylose levels may be high.27
Outbreaks of HBV in assisted living facilities and other institutions have been traced to lack of adherence to standard precautions and/or recommended infection control procedures during AMBG. Unsafe practices include (1) sharing equipment that should be restricted to single patient use among multiple patients, (2) failure to clean and disinfect meters properly between patient uses, (3) storage of clean and used equipment together, and (4) inadequate glove use.1–5,28–30 It has been proposed that technology designed specifically for AMBG could mitigate some of the issues that lead to HBV transmission and that devices to be used in POCT facilities should meet higher standards from those intended for SMBG.1,5 The current study demonstrated that the meter, developed for AMBG use in POCT facilities, is compatible with bleach disinfectant and meets new meter performance standards as proposed by the CLSI.15 The use of a test strip ejector to reduce the risk of bloodborne pathogen transmission remains unproven. Other design features, such as automatic detection of control solution and onscreen QC prompts, are likely to assist HCPs to meet institutional requirements for POCT and AMBG.
Conclusions
A clinical evaluation of the meter indicated that 97% of BG measures from all blood sample types, and 95.5% of arterial blood samples, met accuracy standards compared with reference YSI measurements. The accuracy and precision of the meter met applicable standards when testing blood with hematocrit ranging from 22.9 to 59.8% and blood supplemented with four different anticoagulants. The system was unaffected by 57 of 59 potential interferents evaluated; interference testing indicated that it should not be used to test the BG of individuals within 24 h of xylose administration or when PAM in the blood sample is known or suspected. Bleach wipes were sufficient to disinfect the meter to FDA standards. The test strip ejector, the ability to disinfect quickly with bleach wipes, and the onscreen alert to the need for QC tests are examples of technology designed to support adherence to infection control procedures and regulatory requirements of POCT and AMBG, as called for by public health authorities.
Acknowledgments
Parts of this study were presented as an abstract and poster at the American Diabetes Association’s 72nd Scientific Sessions, Philadelphia, PA, June 8–12, 2012, and the American Association for Clinical Chemistry Annual Meeting, Los Angeles, CA, July 15–19, 2012. The authors thank Loretta Jones for her contributions to the study and review of early drafts of this manuscript. The authors received editorial and writing assistance from Excerpta Medica.
Glossary
- (AMBG)
assisted monitoring of blood glucose
- (BG)
blood glucose
- (CLSI)
Clinical and Laboratory Standards Institute,
- (EDTA)
ethylenediamine tetra-acetic acid
- (FDA)
Food and Drug Administration
- (HBV)
hepatitis B virus
- (HCP)
health care professional,
- (PAM)
pralidoxime iodide
- (POCT)
point-of-care testing
- (QC)
quality control
- (SMBG)
self-monitoring of blood glucose
Funding
This work was funded by LifeScan, Inc., Milpitas, California.
Disclosures
John Mahoney and Aparna Srinivasan are employees of LifeScan, Inc. and shareholders of Johnson & Johnson. Sandra MacRury has received research funding from LifeScan, Inc.
References
- 1.Klonoff DC, Perz JF. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. J Diabetes Sci Technol. 2010;4(5):1027–31. doi: 10.1177/193229681000400501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffell EF, Milne LM, Seng C, Young Y, Xavier S, King S, Shukla H, Ijaz S, Ramsay M, local incident teams Five hepatitis B outbreaks in care homes in the UK associated with deficiencies in infection control practice in blood glucose monitoring. Epidemiol Infect. 2011;139(3):327–35. doi: 10.1017/S0950268810001007. [DOI] [PubMed] [Google Scholar]
- 3.Patel AS, White-Comstock MB, Woolard CD, Perz JF. Infection control practices in assisted living facilities: a response to hepatitis B virus infection outbreaks. Infect Control Hosp Epidemiol. 2009;30(3):209–14. doi: 10.1086/595693. [DOI] [PubMed] [Google Scholar]
- 4.Thompson ND, Schaefer MK. “Never events”: hepatitis B outbreaks and patient notifications resulting from unsafe practices during assisted monitoring of blood glucose, 2009-2010. J Diabetes Sci Technol. 2011;5(6):1396–402. doi: 10.1177/193229681100500611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson ND, Perz JF. Eliminating the blood: ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J Diabetes Sci Technol. 2009;3(2):283–8. doi: 10.1177/193229680900300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Infection prevention during blood glucose monitoring and insulin administration. http://www.cdc.gov/injectionsafety/blood-glucose-monitoring.html. Accessed August 9, 2012.
- 7.U.S. Department of Health and Human Services; U.S. Food and Drug Administration. Letter to manufacturers of blood glucose monitoring systems listed with the FDA. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm227935.htm. Accessed August 9, 2012.
- 8.Department of Health and Human Services; Centers for Medicare and Medicaid Services; Center for Medicaid, CHIP, and Survey & Certification/Survey & Certification Group. Point of care devices and infection control in nursing homes. August 27, 2010. Ref: S&C: 10-28-NH. http://www.cms.gov/surveycertificationgeninfo/downloads/SCLetter10_28.pdf. Accessed August 9, 2012.
- 9.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr. Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta. 2008;396(1-2):10–3. doi: 10.1016/j.cca.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Ichai C, Preiser JC, Société Française d’Anesthésie-Réanimation; Société de Réanimation de langue Française; Experts group International recommendations for glucose control in adult non diabetic critically ill patients. Crit Care. 2010;14(5):R166. doi: 10.1186/cc9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124(2):257–66. doi: 10.5858/2000-124-0257-POCGT. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann L. Quality of glucose measurement with blood glucose meters at the point-of-care: relevance of interfering factors. Diabetes Technol Ther. 2010;12(11):847–57. doi: 10.1089/dia.2010.0076. [DOI] [PubMed] [Google Scholar]
- 13.Teodorczyk M, Cardosi M, Setford S. Hematocrit compensation in electrochemical blood glucose monitoring systems. J Diabetes Sci Technol. 2012;6(3):648–55. doi: 10.1177/193229681200600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey T, Chang A, Rosenblit PD, Jones L, Teft G, Setford S, Mahoney J. A comprehensive evaluation of the performance of the test strip technology for OneTouch Verio Glucose meter systems. Diabetes Technol Ther. 2012;14(8):701–9. doi: 10.1089/dia.2011.0260. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 3rd ed. Wayne: Clinical and Laboratory Standards Institute; 2012. Point-of-care blood glucose testing in acute and chronic care facilities; draft guideline. CLSI document POCT12 (Draft 2) [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2nd ed. Wayne: Clinical and Laboratory Standards Institute; 2005. Evaluation of matrix effects; approved guideline. CLSI document EP14-A2. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2nd ed. Wayne: Clinical and Laboratory Standards Institute; 2005. Interference testing in clinical chemistry; approved guideline. CLSI document EP07-A2. [Google Scholar]
- 18.Khor SP, Hsu A. The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol. 2007;2(3):234–43. doi: 10.2174/157488407781668802. [DOI] [PubMed] [Google Scholar]
- 19.Wu AH. 4th ed. St. Louis: Saunders Elsevier; 2006. Tietz clinical guide to laboratory tests. [Google Scholar]
- 20.Welling PG, Patel RB, Patel UR, Gillespie WR, Craig WA, Albert KS. Bioavailability of tolazamide from tablets: comparison of in vitro and in vivo results. J Pharm Sci. 1982;71(11):1259–63. doi: 10.1002/jps.2600711119. [DOI] [PubMed] [Google Scholar]
- 21.Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, Harrison JG. Accuracy of fingerstick glucose values in shock patients. Am J Crit Care. 1995;4(1):44–8. [PubMed] [Google Scholar]
- 22.Tanvetyanon T, Walkenstein MD, Marra A. Inaccurate glucose determination by fingerstick in a patient with peripheral arterial disease. Ann Intern Med. 2002;137(9):W1. doi: 10.7326/0003-4819-137-9-200211050-00031-w1. [DOI] [PubMed] [Google Scholar]
- 23.Biem J, Koehncke N, Classen D, Dosman J. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168(3):305–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Kost GJ, Louie RF, Tang Z, Lee JH, Somsanith KJ, Tran NK. Reducing potential medical errors in point-of-care testing: clinical effects of heparin and EDTA on handheld glucose meter results. Point Care. 2002;1(1):2–8. [Google Scholar]
- 25.Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113(1):75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- 26.Lyon ME, Baskin LB, Braakman S, Presti S, Dubois J, Shirey T. Interference studies with two hospital-grade and two home-grade glucose meters. Diabetes Technol Ther. 2009;11(10):641–7. doi: 10.1089/dia.2009.0035. [DOI] [PubMed] [Google Scholar]
- 27.Craig RM, Murphy P, Gibson TP, Quintanilla A, Chao GC, Cochrane C, Patterson A, Atkinson AJ., Jr Kinetic analysis of D-xylose absorption in normal subjects and in patients with chronic renal failure. J Lab Clin Med. 1983;101(3):496–506. [PubMed] [Google Scholar]
- 28.Louie RF, Lau MJ, Lee JH, Tang Z, Kost GJ. Multicenter study of the prevalence of blood contamination on point-of-care glucose meters and recommendations for controlling contamination. Point Care. 2005;4(4):158–63. [Google Scholar]
- 29.Götz HM, Schutten M, Borsboom GJ, Hendriks B, van Doornum G, de Zwart O. A cluster of hepatitis B infections associated with incorrect use of a capillary blood sampling device in a nursing home in the Netherlands, 2007. Euro Surveill. 2008;13(27) [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Notes from the field: deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted-living facility--North Carolina, August-October 2010. MMWR Morb Mortal Wkly Rep. 2011;60(6):182. [PubMed] [Google Scholar]