Abstract
Continuous glucose monitoring (CGM) is an essential tool for modern diabetes therapy. Randomized controlled studies have provided evidence that hemoglobin A1c (HbA1c) results can be improved in patients with type 1 diabetes with elevated baseline HbA1c when using CGM frequently enough and that the frequency and duration of hypoglycemic events can be reduced in patients with satisfactory baseline HbA1c. The CGM group within the Working Group Diabetes Technology (AGDT) of the German Diabetes Association (DDG) has defined evidence-based indications for the practical use of CGM in this consensus statement related to hypoglycemia (frequent, severe, or nocturnal) or hypoglycemia unawareness, insufficient metabolic control despite use of all possible therapeutic options and patient compliance, pregnancy associated with inadequate blood glucose results, and the need for more than 10 blood glucose measurements per day. Contraindications and defined preconditions for the successful use of CGM should be considered.
Keywords: clinical evidence of indications for continuous glucose monitoring, conditions for continuous glucose monitoring use, consensus, continuous glucose monitoring
Introduction
Of the various continuous glucose monitoring (CGM) technologies available that are based on different physical or (bio-) chemical principles, only minimally invasive approaches have been proven to be practicable in everyday situations.1,2 These approaches are all based on electrochemical conversion of glucose using biocatalysts.3,4 Accordingly, the glucose sensor of these CGM systems must have direct access to a compartment with a fluid containing glucose. This means that the sensor, in the form of a membrane-clad, needle-shaped enzyme electrode, must be inserted through the skin into the subcutaneous fatty tissue to have access to interstitial fluid. The first CGM system (CGMS® Gold), released by Medtronic in 1999, registered changes in the interstitial glucose values but did not display them immediately to the patient. The readings were evaluated retrospectively.
Conversely, subsequent CGM systems developed for practical use by patients with diabetes (to which this article will limit itself) display glucose values in real time after an initial calibration: Guardian® REAL-Time, Enlite, Paradigm® VEO (insulin pump with optional attachable glucose sensor; all from Medtronic); FreeStyle® Navigator (from Abbott); and DexCom® Gen 4 and Animas Vibe (from Animas, with a CGM system from DexCom).
A complete CGM system comprises the glucose sensor itself that is inserted through the skin, a small electronic unit that is fixed onto the skin, and a separate display/storage device. The electronic unit contains the current source of the sensor, the amplifier of the sensor signal, and the data transmitter. The data are transferred to the pager-like display device via radio wave; the range supported is up to 3 m. With combined systems, i.e., where the CGM system is combined with an insulin pump (Paradigm VEO and Animas Vibe), it is the screen of the insulin pump that is used to display glucose data, which therefore replaces the separate display device. Table 1 shows the key characteristics of the CGM systems that promptly display actual glucose values.4
Table 1.
Characteristics of Current Continuous Glucose Monitoring Systems. Systems Combined with an Insulin Pump Are not Included4
| Guardian REAL-Time (Enlite) | DexCom G4TM | FreeStyle Navigator II | |
| Thickness of the sensor electrode [gauge (mm)] | 27 (0.36) |
31 (0.23) |
23 (0.6) |
| Length of the sensor electrode (mm) | 8.75 | 13 | 5 |
| Angle of insertion (degrees) | 90 | 45 | 90 |
| Period of use (days) | 6 | 7 | 5 |
| Onset of use after positioning of the sensor (h) | 2 | 2 | 1 |
| Calibration by blood glucose measurements after x h | 2, 8, then every 12 | 2 then every 12 | 1, 2, 10, 24, 72 |
| Display of current measuring values every x min | 5 | 5 | 1 |
| Display of glucose levels over x hours | 3, 6, 12, 24 | 1, 3, 6, 12, 24 | 2, 4, 6, 12, 24 |
| Data download | Possible | Possible | Possible |
As with all electrochemical measurement principles, calibration of the sensor recordings is essential. The objective is to convert the (electrical) signal registered by the CGM system into a glucose value by using a blood glucose concentration value determined enzymatically. This calibration value cannot be recorded—for obvious biological reasons—in a sample taken from the same compartment. The interstitial glucose measurement (CGM) must therefore be calibrated by the result of a measurement from a capillary blood sample [i.e., self-monitoring of blood glucose (SMBG)].
The analytical measuring accuracy of good blood glucose meters stands at approximately 3–5%, but when patients use them in everyday settings, they can produce deviations of up to 20%.5 Therefore, calibration of the CGM systems represents a very significant source of error, and patients must carry out the calibration procedure with appropriate care, i.e., they must be properly trained in this procedure.
Due to certain differences caused by dynamic changes in glucose concentration in blood versus interstitial fluid, glucose values in both compartments are equal only in time periods when there are no rapid changes in glycemia (i.e., “steady state” conditions). During periods of rapidly changing glucose values, e.g., after meals or during and after physical activity, differences between both compartments occur as a result of physiological conditions, which hampers the success of the calibration procedure.6–9
In this context, clearly both the analytical accuracy and the precision of the glucose measurement by CGM systems per se is of importance. Different parameters or models have been developed to assess the accuracy of CGM systems:10–16
Mean absolute relative difference (MARD), i.e., the mean value of the relative differences: MARD = 1/N × ∑ (CGM glucose – reference glucose)N × 100 / reference glucose); N = number of measuring values.
Clarke error grid plot (classification of value pairs from the CGM recording and reference measurement, where only the version “continuous glucose error grid plot” is useful, which takes into account the dynamics of glucose changes13); the assessment measures the percentage of paired values in zone A (optimal) or zone B (still regarded as acceptable from a clinical/diabetological perspective).
- Percentage of values according to International Organization for Standardization criteria for measuring accuracy:
- For reference glucose values ≤75 mg/dl (4.2 mmol/liter), proportion of CGM values within the tolerance of ±15 mg/dl, and
- For reference glucose values ≥75 mg/dl (4.2 mmol/liter), percentage of CGM values within the tolerance of ±20%.
- Reproducibility of registrations of two simultaneously measuring CGM systems in one patient.
To assess the accuracy of CGM systems, a directive has been created by the Clinical Laboratory and Standards Institute (CLSI).17 This CLSI directive (POCT05-A) stipulates requirements for investigating CGM systems in studies. The aim is to achieve comparable results and to introduce investigation and assessment standards. In this case, a distinction is made between the following aspects to be examined:
Accuracy of the measuring point (point accuracy);
Accuracy of the glucose trend (trend accuracy); and
Sensitivity and specificity, measuring stability, cali-bration, and “lag time” of the CGM measurement (physiologically related, chronological differences between the blood glucose and interstitial glucose during a glucose rise and fall).
Clinical Use of Continuous Glucose Monitoring Systems
Publications with the CGM system CGMS Gold, which is to be used exclusively for diagnostic purposes, show MARD values of 11% to 14%; in the continuous glucose error grid plot analysis, 98% of the measured value pairs were in zones A and B.10,11 For CGM systems that display the current glucose values immediately (i.e., in real time), the results of a study evaluating the accuracy of CGM systems—Guardian REAL-Time (with Sof-Sensor), FreeStyle Navigator, DexCom STM™, and the GlucoDay micro-dialysis system—are listed in Table 2.12 These results are comparable with data from other studies.13–16
Table 2.
Accuracy of Three Different Continuous Glucose Monitoring Systems in the Euglycemic and Hypoglycemic Range (These Are Older Generations of These Continuous Glucose Monitoring Systems)
| MARD in the range of 70–180 mg/dl (mean value) |
| Guardian REAL-Time: 15.2% |
| FreeStyle Navigator: 15.3% |
| DexCom STMTM: 21.2% |
| Continuous glucose error grid plot, zone A or zones A and B in the range of 70–140 mg/dl |
| Guardian REAL-Time: 91.3% (only A), 98.9% (A and B) |
| FreeStyle Navigator: 93.7%, 98.6% |
| DexCom STM: 91.1%, 98.3% |
| Continuous glucose error grid plot, zone A or zones A and B in the range of <70 mg/dl |
| Guardian REAL-Time: 81.9%, 84.4% |
| FreeStyle Navigator: 95.5%, 97.0% |
| GlucoDay: 87.3%, 96.2% |
Alongside mean value, median value is often also calculated in the MARD; this value is usually smaller because it is less affected by outliers.18 It is of clinical relevance to assess the accuracy of CGM systems in the hypoglycemic, euglycemic, and hyperglycemic ranges separately and not just the accuracy over the entire measured range from 40–400 mg/dl (2.2–22.2 mmol/liter); the MARD can be quite different in different glycemic ranges (Table 3).18
Table 3.
Comparison of Deviations between Paired Glucose Values with the Continuous Glucose Monitoring Systems DexCom STM and FreeStyle Navigator in Four Different Glycemic Ranges and Measurements with a Blood Glucose Meter (OneTouch® Ultra) and a YSI Laboratory Glucose Analyzer as Mean Absolute Relative Difference12
| Deviation from blood glucose monitor (%) | Deviation from laboratory device (YSI) (%) | |||
| DexCom | FreeStyle Navigator | DexCom | FreeStyle Navigator | |
| MARD (40–400 mg/dl) | 16.2 | 15.9 | 16.8 | 16.1 |
| MARD (median): 40–80 mg/dl | 16.1 | 19.6 | 15.8 | 22.8 |
| 81–180 mg/dl | 13.6 | 11.4 | 15.1 | 12.3 |
| 181–300 mg/dl | 11.7 | 10.1 | 12.6 | 6.9 |
| 301–400 mg/dl | 8.9 | 12.1 | 8.3 | 8.1 |
In the assessment of measuring quality, it is important to note that CGM systems are constantly being improved, both in terms of their hardware (i.e., actual measuring technology) and their software (i.e., algorithms for the analysis and presentation of measured values). Such developments are often implemented without the device name being altered to reflect the change(s). Since such “development cycles” are relatively short, only the very latest literature can be used to assess the measuring quality of a given CGM system; this also applies to the details given in Table 1. The Sof-Sensor was used in the first “blind” measuring system—the CGMS—from Medtronic, which has been available since 1999. It was also used in the CGM systems that display the current glucose values immediately. However, seven generations of this Sof-Sensor were used up until 2011 when the successor model “Enlite” was launched commercially in the European Union. Compared with the last Sof-Sensor, the Enlite offers not only improved comfort when inserting the actual sensor and wearing it, but also a greater accuracy, with a MARD of 14.1% over 6 days versus 15.2% for the Sof-Sensor.19,20 With the DexCom CGM systems, the fourth generation is currently on the market, with similarly improved measuring quality.21
Even if the accuracy of spot glucose measurements in glucose profiles recorded with CGM systems is lower than that of meters used for SMBG, they are regarded as a reliable dynamic monitoring system, and approval for patient use was granted under defined conditions by the regulatory authorities (Food and Drug Administration in the United States and Communauté Européenne mark in Europe). However, patients are supposed to perform conventional SMBG before any therapeutic measure, such as the determination and delivery of an insulin dose. One reason for these regulatory requirements is the differences in glucose levels between the two compartments in which the CGM systems are recording glucose levels and the glucose levels measured by SMBG, especially in the case of rapidly changing glucose levels (discussed earlier).7,8,16 Another reason is errors made by the patients while calibrating CGM systems.
Indications for Use of Continuous Glucose Monitoring Systems
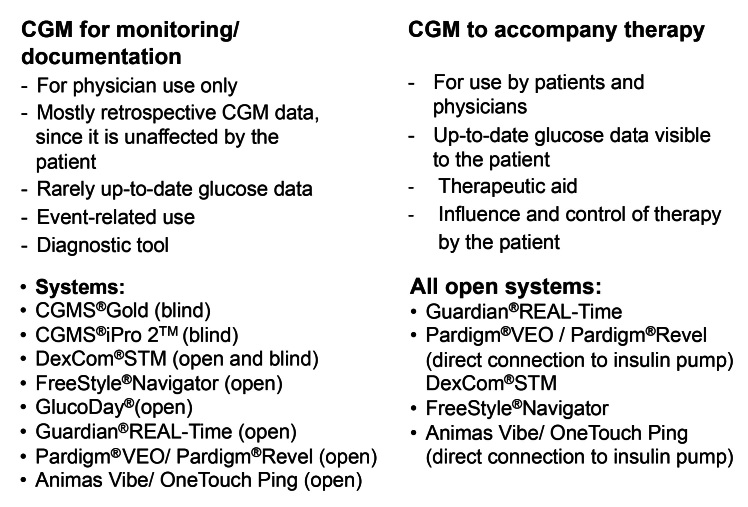
Continuous glucose monitoring systems can be used to monitor the current “disease status,” in this case, glucose levels (Figure 1). Initially, it is irrelevant whether the measured values are displayed immediately or are available only at a later stage; these measuring results help the treating physician to assess a patient’s therapy. However, CGM can also be used as a tool to accompany therapy, provided that the given patient with diabetes is able to determine the delivery of insulin based on the current glucose values and make his own treatment decisions.
Figure 1.
General differences in the use of CGM systems.
Therapy-related use of CGM by patients requires real-time display of glucose values. This does not rule out the need for patients to perform SMBG for safety reasons before making any changes to their insulin therapy. Such a monitoring of glucose levels is comparable with long-term electrocardiogram recordings. Support and evaluation is required from trained medical personnel and it is not considered a direct tool for treatment.
Through personalized and flexible adaptation of their therapy in response to their current glucose levels and from knowing their current glucose status, and—more importantly, the trends in changes in their glucose levels—patients can achieve improved metabolic control and a minimized risk of acute metabolic deteriorations. A number of clinical studies have confirmed such metabolic improvement.22–31 These studies also provide evidence of the increased safety of diabetes treatment and an associated improvement in quality of life.32,33
Monitoring of Glucose Levels
The two CGM systems, CGMS Gold and CGMS iPro 2 from Medtronic, essentially work “blind,” and the DexCom STM system has the option of switching the unit to “blind” mode. “Blind” means that the glucose values are not displayed to the patient and therefore cannot be used on a direct and situational basis for diabetes treatment. The data are stored in the CGM system and are evaluated retrospectively following the completion of the recording period, which provides an insight into glucose metabolism mechanisms during the measuring period that has not been influenced by the patient.
In principle, even an “open, nonblind” CGM system can be used to record glucose levels for (diagnostic) assessment of metabolic adjustments. To assess glucose levels or to optimize treatment, however, it is essential that all activities (meals, physical activity) and therapeutic measures (administration of insulin) that can influence these levels are meticulously documented by the patient.
In Germany, with regard to the reimbursement of costs, this area of application for CGM systems needs to be taken into account in the catalog of the uniform assessment standard, which has not been the case. Only in the physicians’ scale of fees for private medical treatments can the use of CGM systems be represented under “diagnostic” investigations and be billed in the same manner as long-term electrocardiograms.34
Use of Continuous Glucose Monitoring as Guidance for Treatment
Continuous glucose monitoring systems that immediately display the current glucose values provide the patient with continuous information about their current metabolic status and, therefore, additionally with constant indicator information about the trend in glucose changes. In everyday situations, this information facilitates the countless therapeutic decisions that patients with diabetes need to make in relation to food intake, exercise, and insulin delivery (although this therapeutic decision intervention requires additional performance of SMBG). Consequently, this type of monitoring actually satisfies a long-held wish of patients with diabetes and the physicians who treat them.
The everyday suitability of CGM systems specifically includes the relatively simple insertion and removal of sensors and the handling of the CGM system itself. To ensure that the monitored glucose values can be interpreted adequately, the display shows the current glucose value as a number and also displays the glucose levels in the form of a little graph over a time period, which can be set from 1 to 24 h retrospectively, depending on the CGM system (Table 1). An arrow after the numerical value also represents the current glucose trend; its direction clarifies the magnitude of change in glucose levels. Combination of these three pieces of information (current value, magnitude of change in glucose levels, and glucose trend) allows patients to quickly assess their current metabolic control and to check the success (or failure) of recent changes made in their antidiabetic treatment and other measures that influence glycemia (e.g., exercise). The associated (early) warnings against acute glycemic deteriorations play a particularly important role in this context. Patients are immediately able to tell whether
They are at risk of a hypoglycemic event;
There is an inappropriate increase in postprandial glycemia;
The insulin dose selected was appropriate for the current meal; or
Physical activity needs to be compensated for with, e.g., the intake of additional carbohydrates.
The CGM systems can alert patients to glucose values that are too low or too high via audible alarms and/or vibration alarms, which can be turned on and off. This reduces the risk of nighttime hypoglycemic events.25,30 The CGM systems offer different versions of these warning signals, which can be customized for each patient. For example, a pre-alarm can be triggered if rapid changes occur in the patient’s glucose levels, which means that if the glucose values are expected to become too low or too high within a preset period of time, then the system will alarm. This gives patients time to respond appropriately. Of course, false alarms cannot be ruled out in this instance.
Using CGM systems in daily life can be beneficial if patients with diabetes systematically make therapeutic interventions to optimize their diabetes management. This is the case for all patients who respond flexibly to current metabolic requirements by applying an intensive insulin therapy (IIT; multiple daily injections of insulin). This involves not only adapting the prandial insulin dose, but also responding appropriately to rapidly decreasing or rising glucose values. Patients who use an insulin pump [continuous subcutaneous insulin infusion (CSII) therapy] for their treatment can respond with additional flexibility. In this case, recorded glucose profiles and SMBG can be used for adapting insulin therapy adequately to current needs, e.g., by varying the individually adjusted multibasal rate programming, making temporary changes to the basal rate, and using various bolus options to adapt to various meals. These opportunities can be utilized even more effectively with CGM than is the case with SMBG alone (with usually 4–6 measurements per day). Consequently, the next logical step was to combine the insulin pump with a CGM system. This combination led to a new therapeutic option, the so-called sensor-augmented pump (SaP) therapy. With the two device combinations, Animas® Vibe™ (available in Europe) and the model Paradigm REAL-Time (between 2006 and 2009), the measuring results of the CGM system have/had no automatic influence on the pump’s delivery of insulin.
With the Paradigm VEO model (on the market in Europe since 2010), an automatic intervention by the combined system takes place for the first time: in case of low glucose levels, or if there is a risk of hypoglycemia (depending on the set threshold values), the device first triggers an alarm. If the patient does not respond to this alarm within a defined period, for example, because he is in a deep sleep, the insulin pump autonomously suspends the insulin infusion for a maximum of 120 min. Once this time has elapsed, it automatically resumes insulin infusion if the patient has not already done this manually. The risk of developing hypoglycemia can thus be reduced with this system.35–40 This direct coupling of a CGM system to an insulin pump, and the resulting first-ever automated feature, represents the first important step toward a fully automatic insulin application (i.e., artificial pancreas).
Clinical Evidence for the Use of Continuous Glucose Monitoring Systems: Possibilities and Limitations
As with any form of therapeutic intervention, the evidence for CGM system use must be confirmed with randomized, controlled clinical studies [randomized controlled trials (RCTs)] . The search for evidence for the purely “diagnostic” CGM application to record glucose levels makes limited sense, as its use is comparable with the method of magnetic resonance tomography or an X ray (the term “professional CGM” is used for this application of CGM systems). A diagnostic measure per se has no impact on any outcome; it is always the “translation” into a therapeutic intervention that has such an impact. It is the physician who defines—after interpreting the CGM data—a change of treatment; for example, the physician counsels the patient on this therapy and therefore ultimately effects the patient’s adaptation to the new therapy. In this case, it is not actually possible to use the hemoglobin A1c (HbA1c) value, which is a measure to assess metabolic control, nor the number of hypoglycemic events as clinical evidence for CGM systems because, in reality, the “success” of CGM as a diagnostic tool is determined by the physicians’ experience, the skills of the counseling team, and the patient’s training and compliance.
Nevertheless, there are a number of studies on CGM use with “blind” recording of glucose levels, from which clinical evidence was derived; however, rather than strictly adhering to CGM use only, the skills of the physicians and patient were also evaluated with regard to the treatment optimization.41–43 Various RCTs have also been carried out in which the investigation was geared toward treatments and methods with a diagnostic background, and in these instances, CGM supported the assessment of these interventions.44–46 A typical example of this is the detection of nocturnal hypoglycemic episodes.47
The situation is fundamentally different with “open,” real-time use of CGM. In this case, the patient sees his current glucose values and can respond immediately by adjusting therapy accordingly. However, there are again several aspects with this form of CGM use that limit the demonstrative power of RCTs in confirming evidence for this method. Essentially, it requires that patients use this method correctly and draw adequate conclusions from the data for immediate adaptation of their therapy. If they are unable to respond appropriately, CGM is unable to have any effect; again, CGM has no effect per se. This is clearly different from double-blind, placebo-controlled studies with drugs. A measuring procedure that accompanies treatment is largely affected by the training and motivation of patients to interpret the glucose results and draw corresponding therapeutic conclusions; it appears that not enough weight has been given to this fact in all respective studies.
Given that CGM cannot be investigated in a double-blind manner, evidence is also limited in this regard. Therefore, the best possible evidence for the use of any monitoring method, such as CGM, to accompany therapy comes from open, multicenter RCTs with competent diabetes teams and appropriately trained and motivated patients. It has been mentioned before that, due to the rapid development of technology, new generations of CGM systems become available before such RCTs are completed; in effect, the outcomes of such data refer to outdated systems when they are published. Again, this is in contrast to antidiabetic drugs that remained “stable” once they enter later phases of clinical development and in the decades after their approval. However, in view of the improvements made in the technical performance and reliability of CGM systems, this is, at the same time, an inherent part of all technologies that must be taken into account.
Clinical Evidence for the Use of Continuous Glucose Monitoring to Accompany and Guide Therapy
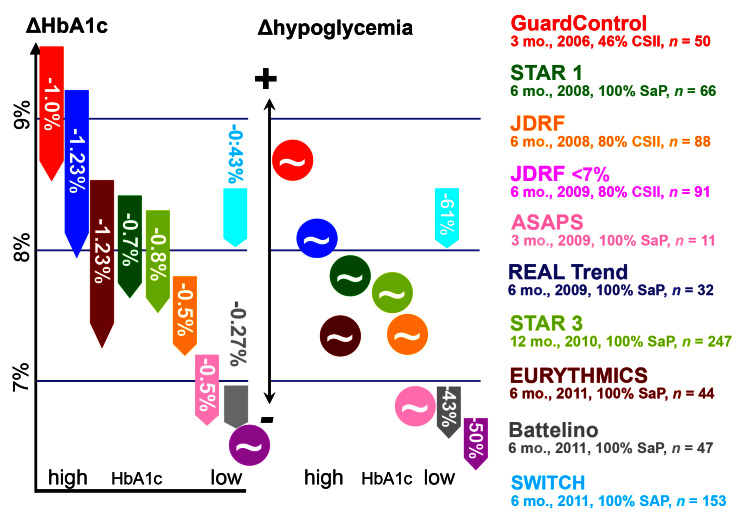
The benefits of CGM have been studied in several RCTs. The main aim of these studies was to confirm if CGM use is associated with an improvement in metabolic control (i.e., HbA1c) and/or a reduction in the frequency of hypoglycemic events or the duration in which the glucose values remain in the low (hypoglycemic) range.22–31 A comprehensive overview about these studies is given in Table 4 and Figure 2. Subsequently, the use of CGM systems for various clinical questions and the evidence for these is discussed.
Table 4.
Overview of Randomized, Controlled Studies on the Use of Continuous Glucose Monitoring Systems to Accompany Therapy
| Study/first author | RCT? (country) | System or systems manufacturer(s) | Inclusion criteria | N | Baseline HbA1c, blood glucose | Duration (weeks) | Result | Comment | Industry sponsored? | |
| Algorithm-derived (special sensor training); data algorithm explained at http://direcnet.jaeb.org and by Buckingham and coauthors48 | JDRF24 | Yes (U.S.) | Dexcom, Abbott, Medtronic | >8 years old; duration of diabetes > 1 year; type 1 diabetes; HbA1c 7–10%; at least three blood glucose self-tests daily; one week of sensor prior knowledge | 322 | >25 years: 7.6 (experimental), 7.6 (control); 15–24 years: 8.0 (experimental), 7.9 (control); <15 years: 8.0 (experimental), 7.9 (control) | 26 | 7.1 (experimental), 7.6 (control), p < .001; 7.8 (experimental), 7.7 (control), p < .52; 7.6 (experimental), 7.7 (control), p < .29 |
Real-time CGM has the ability, in adults who are motivated and who integrate it into their everyday lives, to reduce the HbA1c; this primary end point was achieved in adults but not in children and young people; the cause was the wearing time of less than 70%, which can be assumed from a per-protocol analysis | No |
| JDRF14 | Yes (U.S.) | Dexcom, Abbott, Medtronic | >8 years old; duration of diabetes > 1 year; type 1 diabetes; HbA1c <7%; at least three blood glucose self-tests daily; one week of sensor prior knowledge | 129 | 6.4 (experimental),6.5 (control) | 26 | 6.4 (experimental),6.8 (control); time < 70 mg/dl/day (median): 91 min → 54 min, p = .02 (experimental); 96 min → 91 min, not significant (control)10 versus 11 patients with severe hypoglycemia |
A reduction in HbA1c was not intended and not a primary end point; the primary end point was time of <70 mg/dl; well-controlled, motivated diabetes patients, including children, use the real-time CGM frequently and sustainably; reduction in time |
No | |
| MITRE Study49 | Yes (U.K.);CGM group, control group, and attention control group | CGMS (without RT),Glucowatch (RT) | Adults, HbA1c 2 × >7.5%; type 1 and type 2 diabetes 50/50 | 404 | 9.2 % Glucowatch,9.0 % CGMS,8.9 % attention,9.4% control | 79 | 9.1% Glucowatch,8.55% CGMS,8.3 % attention,8.8 % control | Glucowatch results in harmony with earlier DirecNet study; wearing behavior of Glucowatch markedly restricted due to side effects;JDRF and Mitre not in harmony in adults, which may be due to the JDRF’s real-time CGM | No | |
| DirecNet50 | No (U.S.); no control group without sensor; IIT | Abbott | 3–18 years old; type 1 diabetes; DATA algorithm | 45 | 7.1 (CSII), 7.8 (IIT) |
26 | 7.0 (CSII + CGM), p = .53, n= 24; 7.6 (CSII + CGM), p = .26, n= 21 |
Wearing time fell over time; sensor was worn, on average, for half of the weekly hours; baseline characteristics revealed no predictors for successful wearing | Yes | |
| Algorithm-derived (special sensor training); data algorithm explained at http://direcnet.jaeb.org and by Buckingham and coauthors48 | Bailey51 | No (U.S.) | Dexcom | IIT or CSII; >18 years old; no pregnancy; type 1 and type 2 diabetes | 140 | 7.6 | 12 | 7.2; p < .001 | Unfortunately, there was no control group, type 2 diabetes patients and those with poor baselines without insulin pump also benefited | Yes |
| SaP | STAR123 | Yes (U.S.) | Medtronic | 12–72 years old | 146 | 8.49 (experimental), 8.39 (control) |
26 | 7.77 (experimental), 7.84 (control),experimental versus control; not significant, p = .37; 11 severe hypoglycemia with real-time CGM;3 severe hypoglycemia in control |
The primary end point was not reached; the authors conclude: “The results are strongly suggestive of the fact that the right patient selection should include the willingness and ability to use the technology correctly”; they felt this was an important “screening tool” for recognizing potential candidates | Yes |
| STAR329 | Yes (U.S.) | Medtronic | 7–70 years old; HbA1c 7.4–9.5%; previously IIT | 485 | 8.3 (experimental; SaP), 8.3 (control; IIT) |
XXXX | 7.5 (experimental; SaP; experimental vs. control: p < .001),8.1 (control; IIT), 7.9 (experimental, SaP; children; experimental versus control; p < .001), 8.5 (control, children); severe hypoglycemia in both groups identical (5 per 100 patient years) |
In the IIT group (control group), the sensor gathers data; the current glucose values are not displayed; in this study, it is not possible to determine the influence of the insulin pump per se or the additional influence of the sensor-augmented algorithm separately | Yes | |
| ONSET52 | Yes (Europe); multicenter | Medtronic | 1–16 years old; new manifestation of type 1 diabetes in the past four weeks | 160 | 11.2 (experimental; SaP), 11.5 (control; CSII) |
52 | 7.4 (experimental; SaP), 7.6 (control; CSII), experimental versus control not significant |
The SaP (experimental) is compared with conventional blood glucose self-monitoring (control), even with C-peptide and depression scale in patients following the new manifestation of type 1 diabetes; no significant differences | Yes | |
| SaP | REAL-TREND27 | Yes (France) | Medtronic | HbA1c > 8% below IIT; duration of diabetes > 12 months; in SaP group: agreed to wear CGM for >70% of the time; versus CSII (control) | 132 (51 children, 81 adults) | 9.11 (experimental; SaP), 9.28 (control; CSII) |
26 | 8.3 (experimental; SaP), p < .001; 8.7 (control; CSII), p < .001; experimental versus control, p = .006 (all patients);experimental versus control, p = .004 (patients per protocol); severe hypoglycemia not significant |
The aim was an intention-to-treat analysis; however, a per-protocol population was formed that did not contain 23 patients who had measured less than 70% of the time; in this case, the HbA1c difference achieved significance; 14 from the sensor group (SaP) and 6 from the CSII group left the study | Yes |
| EURYTH-MICS28 | Yes (Europe); multicenter | Medtronic | 18–65 years old; HbA1c > 8.2%in patients on IIT | 87 | 8.46 (experimental), 8.59 (control) |
26 | 7.23 (experimental; SaP), p < .001; 8.46 (control; IIT), not significant;experimental versus control, p < .001; no significant difference in severehypoglycemia |
Control group, IIT; the result is a summation effect of the insulin pump, sensor, and BolusExpert and cannot be ascribed solely to the sensor | Yes | |
| SWITCH31 | Yes (Europe); cross-over; multicenter | Medtronic | 6–70 years old; CSII > 6 months; HbA1c 7.5–9.5% | 124 | Not yet published | 26 | Study participants first had to complete three tests and were required to use the device at least 80% of the time | Yes | ||
| Battelino30 | Yes (Europe) | Abbott | 10–65 years old; IIT or CSII; HbA1c < 7.5% | 120 | 6.92 (experimental), 6.91 (control) |
26 | 6.69 (experimental), p < .05; 6.95 (control), not significant; experimental versus control, p = .008; time in hypoglycemia 0.48 h/day (experimental), 0.97 h/day (control), p = .03; no severe hypoglycemia in either group |
Patients were given instructions on how to use the sensor, but no algorithms; the primary outcome was the time spent below 63 mg/dl | Yes | |
| Therapy left up to the patient (no special training, no computer), “patient-led use” |
ASAP Study26 | Yes (Australia) | Medtronic | Type 1 diabetes; 13–40 years old; HbA1c < 8.5%; motivated, relatively well-adjusted insulin pump patients with prior knowledge of bolus calculation | 62 | 7.3 (experimental), 7.5 (control) |
13 | 7.1 (experimental), 7.8 (control), difference adjusted -0.43, p = .009; difference -0.51 with sensor use > 70%; no severe hypoglycemia in either group |
Motivational tool, “The relatively high rate of patients who left the study and who did not follow the protocol showed that this technology is not universally acceptable and is for some too much of a burden.” | Yes |
| Danne53 | No (Europe); concealed and unconcealed phase |
Abbott | Type 1 diabetes; >18 years old; duration of diabetes > 1 year; primary end point blood glucose outside 3.9–10 mmol/liter | 48, of which 39 were CSII | Hours outside 3.9–10 mmol/liter: 11 ± 4.5 | 60 | 9.5 ± 4.0, p = .002 | An age-independent effect was found to occur on glycemic variability; all apart from one patient wore the device for >85% of the time | Yes | |
| Garg54 | Yes (U.S.) | Dexcom | Type 1 diabetes, type 2 diabetes, with insulin; comparison of concealed versus unconcealed CGM | 91 (75 type 1 diabetes, 16 type 2 diabetes) | Time frame/day <55 mg/dl: 0.94 h, >240 mg/dl: 6.46 h | 3 x 72 h | Secondary end point: time/day (h/day), <55 mg/dl, 0.94 (concealed) → 0.74 (unconcealed) -21%, p < .0001; >240 mg/dl, 6.46 (concealed) → 4.99 (unconcealed) -23%, p < .0001 |
The study was too short for an HbA1c comparison; primary end points were errors and accuracy; the secondary end point showed that the timeframe in the near-normoglycemic range rose; pilot study for Dexcom | Partially | |
| Guard Control22 | Yes (Europe) | Medtronic | Three study arms: permanent real-time CGM, every two weeks for three days, and monitoring only IIT or CSII | 162 (81 children, 81 adults) | 9.5 (experi-mental 1), 9.6 (experi-mental 2), 9.7 (control) |
13 | 8.5 (experimental 1 versus control, p = .003); 8.9 (experimental 2 versus control, not significant); 9.3 (control) |
First study with real-time CGM and measurement of HbA1c values; the high baseline value of the HbA1c should be noted; permanent use (experimental 1) with more effective improvement than intermittent use (experimental 2) | Yes |
Figure 2.
Changes in metabolic control (reduction in HbA1c compared with the baseline value) and in the hypoglycemic frequency in randomized, controlled studies (marked with appropriate colors) with usage of CGM systems, in some cases also with SaP therapy.23,26–31 The data relate to the change in hypoglycemic frequency, not direct values. (Details of studies: duration in months, year of publication, number of patients with preexisting insulin pump therapy, number of patients with sensor-augmented insulin pump therapy [adjustment to this option in the study])
Improvement in Metabolic Control
The GuardControl study demonstrated for the first time that displaying the current glucose values and trends results in an improvement in the metabolic control of inadequately controlled patients with diabetes.22A total of 162 children and adults with type 1 diabetes in suboptimal metabolic control (initial HbA1c 9.6% ± 1.2%) were monitored over a period of 3 months. Subjects were randomized into three groups in relation to the intensity of use of the Guardian RT system: patients in group 1 (n = 54) used this system constantly, patients in group 2 (n = 54) used it every second week for 3 days, and patients in group 3 (n = 54) served as control group. Patients in group 1 had a significant reduction in HbA1c (1.0% ± 1.1%) without an increase in the number of hypoglycemic episodes. In half the patients, HbA1c fell by ≥1%; 26% even achieved a reduction of ≥2%. Occasional use of the CGM system led to a mean improvement in HbA1c of 0.6% in group 2; however, as shown by the patients in the control group, participation in the study per se led to a mean reduction in HbA1c by 0.4%.
Use of CGM also improved metabolic control in patients with a good baseline HbA1c value as shown in the Juvenile Diabetes Research Foundation (JDRF) study, which included 322 patients with type 1 diabetes (inclusion criterion, HbA1c 7–10%).24 The patients were divided into three age groups: children aged 8–14 years, young adults aged 15–24 years, and adults of 25 years and older. This study, which was commissioned by the independent JDRF (funded exclusively by donations), methodically presents the best evidence for CGM use and was published in a highly ranked journal. All three CGM systems that were available on the market in the United States at this point in time were studied; however, the data were not analyzed according to the individual CGM system used. Contrary to a company-driven study, the aim of this study was to study the “class effect” of CGM. Patients in the intervention group used CGM in addition to standard SMBG throughout the study period of 12 months, while patients in the control group initially used SMBG only to monitor their metabolism. After 6 months into the study, the patients in the control group were switched to CGM systems also. In the adult patients, use of the CGM systems led to a significant improvement in metabolic control: the average HbA1c was reduced by 0.53% from 7.60% to 7.07% after 6 months. However, the children and adolescents in the two other age groups showed no significant improvement in their metabolic control compared with those in the control group. Among children, the HbA1c improved by 0.37% when the CGM systems were used; however, the metabolic control in the children in the control group also improved by 0.22%. In this study, the obvious reason for this “negative” outcome was that these patients used the CGM devices for shorter periods of time than the patients in the adult group (discussed later).
In another study, the use of a CGM system versus SMBG was studied with 120 well-controlled patients with type 1 diabetes (baseline HbA1c 6.9%).30Patients in the intervention and the control group were comparable [average age, 26 years; duration of diabetes, 11.5 years; proportion of pediatric patients (age 10–17 years), 26%]. However, the proportion of patients on CSII in the intervention group was higher (76% versus 59%). Patients in both groups used a “blind” CGM system every 2 weeks to record their glucose profiles over 5 days. Over a period of 26 weeks, the study documented not only the time spent in the hypoglycemic range (<63 mg/dl), but also changes in metabolic control and parameters describing glycemic variability. The HbA1c declined in the CGM group to 6.69% (p = .008), while it remained virtually at the baseline level in the control group, at 6.95%. This improvement was seen in both age groups, namely, the adults (reduction of 0.31% from 6.83% to 6.51%) and children (reduction of 0.23% from 7.15% to 6.92%).
Reduction in Hypoglycemic Episodes
The studies carried out to date were not using patients specifically selected according to an indication for CGM, such as those who are prone to frequent, severe hypoglycemic episodes or hypoglycemia unawareness. However, in all RCTs in which HbA1c was the primary end point, there was no increase in hypoglycemia observed [measured as hypoglycemic frequency and/or area under the curve (AUC) in the range ≤70 mg/dl] while the mean HbA1c was lowered.22–24,26–29
In two RCTs with well-controlled patients with type 1 diabetes, the primary end point was time spent in the low glucose range.25,30 In the JDRF <7 study, 129 patients aged between 8 and 69 years were studied (inclusion criterion, HbA1c values <7.0%).25 In the intervention group, in which patients used CGM for 6 months, time spent in the hypoglycemic range of <70 mg/dl was reduced by 41% (from a daily average of 91 to 54 min; p = .002). Among the patients in the control group, which used only SMBG, the time spent in the hypoglycemic range remained virtually unchanged (reduction from 96 to 91 min, as recorded using “blinded” CGM). Time spent in the hypoglycemic range of <60 mg/dl was reduced even further from 40 min daily to 18 min when the CGM system was used. However, there was no significant difference in the number of hypoglycemic episodes between the groups. There were also no changes with regard to metabolic control in patients in the CGM group; the HbA1c remained constant at 6.4%. In the control group, the HbA1c rose slightly from 6.5% to 6.8%. In the other study with 120 well-controlled patients (baseline HbA1c value 6.9%), time spent below a glucose level of <63 mg/dl was lower in the CGM group than the control group (0.48 ± 0.57 versus 0.97 ± 1.66 h/day; p = .03).30 There was no difference between the age groups in the intervention group regarding time spent per day in this range: children (48%) and adults (54%).
Influence of the Period of Use of Continuous Glucose Monitoring Systems on their Effectiveness
As described previously, a key outcome of the GuardControl study was that only long-term and continuous use of CGM systems leads to a significant improvement in metabolic control. This was not the case when the CGM system was used only on an intermittent basis.22 This finding was also noted in other RCTs. A more detailed analyses of the JDRF study showed that the lack of improvement in the HbA1c in the age groups from 8–14 and 15–24 years was not an age-group-specific effect, but rather the result of the shorter period of use of the CGM systems compared with the adult group. Continuous wear of a CGM system for 6 days per week was defined as 100% compliance; however, the analyses of the 8–14-year-olds yielded a usage of just 50%, which fell to a mere 30% in the 15–24-year-old age group.24 In contrast to this, the JDRF <7 study revealed no differences in the frequency of use between the age groups.25 Baseline HbA1c values of <7%, however, strongly indicate high levels of compliance on the part of the patients in this study. In another RCT, only regular CGM use was of benefit with respect to metabolic control.30
Evidence of Continuous Glucose Monitoring Use in the Context of Sensor-Augmented Pump Therapy
In the studies in which this advanced therapeutic option was used, patients with IIT and CSII were included. However, each study had a different proportion of patients who used CSII therapy; in some RCTs, only patients on CSII were included (see Table 4 and Figure 2). In the multicenter REAL trend study, patients with inadequate metabolic control (HbA1c > 8%) using IIT were randomized to “classic” CSII therapy with SMBG or SaP therapy.27 Analyses were conducted separately for patients with good compliance (wearing the CGM system ≥70% of the study time) and poor compliance. In patients with good compliance, usage of SaP therapy led to a reduction in HbA1c of 1.23% over the 6 months of the study. Use of CSII resulted only in an improvement of HbA1c of 0.55% (p = .004). When the data from all patients were analyzed together (i.e., those with good and poor compliance), a significant HbA1c difference was also observed between both groups. However, this difference was smaller than that seen in the group with good compliance; SaP use led to HbA1c reduction of 1.14%, and CSII use led to HbA1c reduction of 0.57% (p = .006).
Similar results for SaP use were observed in the Eurythmics Study and the STAR 3 study.28,29 Compared with the REAL trend study mentioned earlier, these two studies had a similar study design: subjects were randomized to SaP (intervention group) or continued optimization of IIT (control group). There was a significant improvement in metabolic control in the STAR 3 intervention group composed of 170 adults and 70 children and teenagers (aged between 7 and 17 years). In the RCTs in which patients were randomized to SaP,26–29 the use of CGM systems led to an even greater improvement in HbA1c than when patients used IIT plus CGM.22,24
The SWITCH trial, a randomized, controlled, crossover multicenter study over 17 months included a run-in period and two 6-month sequences, separated by a 4-month washout period.31 Seventy-two children and 81 adults with HbA1c between 7.5% and 9.5% using CSII alone were randomized to CGM sensor-on or sensor-off arms for 6 months, then crossed over. The primary outcome was the end-of-period difference in HbA1c between sensor-on and sensor-off arms. The mean difference in HbA1c was -0.43% in favor of sensor-on arm, with a difference of -0.46% (p < .001) in children and -0.41% (p < .001) in adults. Stopping CGM system use resulted in HbA1c reverting to baseline levels.
Initial Automatic Influence on Sensor-Augmented Pump Therapy by Continuous Glucose Monitoring Systems to Avoid Hypoglycemic Episodes
The automated features of the CGM system for the delivery of insulin from pumps promises further improvement of metabolic control and safety of treatment. Evidence for this comes from the use of a combined CGM/pump system with automatic suspension of the insulin delivery (basal rate as well as any remaining prolonged bolus) when glucose levels are low [“low-glucose suspend” (LGS) function in the case of the Paradigm Veo™ insulin pump].36 In a prospective study of children and young adults, using LGS, the number of events (<70 mg/dl, 1.27 ± 0.75 versus 0.95 ± 0.49, p < .01; <40 mg/dl, 0.28 ± 0.18 versus 0.13 ± 0.14, p < .005), their duration (<70 mg/dl, 101 ± 68 versus 58 ± 33 min/day; p < .002) and the area under the glucose profile curve (<70 mg/dl, 0.76 ± 0.61 versus 0.53 ± 0.37 mg/dl/day; p < .05) were reduced. In another RCT, it was shown that LGS reduced time spent in hypoglycemia in a population of 50 subjects with type 1 diabetes (LGS-on 139 ± 77 min versus LGS-off 171 ± 76 min; p = .006).40 In principle, this option appears to be of help to prevent hypoglycemic episodes, particularly if the algorithm used for this is optimized further.
Meta-Analyses of Continuous Glucose Monitoring and Sensor-Augmented Insulin Pumps
In all RCTs, the reduction in HbA1c values observed was greatest when patients had elevated baseline HbA1c values and used the CGM system regularly.22,24,26 This is confirmed by a current meta-analysis of six studies that analyzed individual patient data, i.e., not just the mean results published.55 By way of comparison, in patients with a low baseline HbA1c value, rather than a further improvement in HbA1c, the primary outcome was a reduction in the frequency of hypoglycemic events or a reduction in the time and AUC in the glucose range of <70 mg/dl.25,30 This has been confirmed in other meta-analyses.56,57
A Cochrane report for CGM was published.58 A group of researchers from the Netherlands searched in The Cochrane Library, MEDLINE, EMBASE, and CINAHL for the identification of studies on using CGM systems (reported studies until June 2011). Selection criteria (1366 references found) were RCTs comparing retrospective or real-time CGM with SMBG or with another type of CGM system in patients with type 1 diabetes mellitus. Twenty-two RCTs meeting the inclusion criteria of this review were identified. The results of the meta-analyses performed (across all age groups) indicate benefit of CGM for patients using CGM compared with patients using multiple daily injections of insulin and SMBG. After 6 months, there was a significantly larger decline in HbA1c for real-time CGM users starting insulin pump therapy compared with patients using multiple daily injections of insulin and SMBG (mean difference in change in HbA1c level, -0.7%).
Consensus of the Continuous Glucose Monitoring Working Group of the German Diabetes Association Regarding the Use of Continuous Glucose Monitoring Systems to Accompany and Guide Therapy and the Conditions Necessary for Their Use in Practice
Based on the study evidence presented, there are already consensus statements in a number of countries with regard to the indications of CGM systems.59–68 These differ in certain aspects (see Appendix 1). The CGM working group [Working Group Diabetes Technology (AGDT)] of the German Diabetes Association regards the following indications as relevant for CGM systems in the context of treating patients with type 1 diabetes:
Hypoglycemia, i.e., frequent, severe hypoglycemic episodes (requiring assistance from third parties), severe nocturnal hypoglycemia, and/or proven hypoglycemia unawareness;
Unsatisfactory metabolic control if, despite the use of all available forms of treatment (including also CSII), good compliance and the exclusion of severe psychological/psychiatric problems, the target HbA1c level cannot be achieved;
Before/during pregnancy with inadequate metabolic control using conventional forms of treatment; and
The need to perform more than 10 blood glucose measurements per day to achieve the target HbA1c level.
Conversely, the following factors are considered to be contraindications of CGM systems:
Unwillingness to increase the effort made in relation to treatment, i.e., a lack of motivation and compliance;
Fear of technical systems and/or a lack of trust in them;
Alcohol and/or drug misuse; and
Severe psychological/psychiatric problems that are not the result of failed efforts to achieve improved metabolic control (e.g., bulimia, anorexia, psychoses).
Requirements for the use of CGM systems include
Selection of patients by the professional diabetes team, including
Good compliance,
Exclusion of contraindications,
Exhaustion of all other available measures to optimize the metabolism (including CSII), and
Participation in CGM counseling and training;
Support from a trained diabetologist experienced in CGM with a treatment team; and
Where necessary, a personalized trial phase of CGM over a period of a few weeks with evidence of success.
Summary
A number of clinical studies have shown evidence in favor of CGM systems with respect to an improvement of metabolic control, reduced glucose variability, and a smaller AUC and duration of time in the hypoglycemic and hyperglycemic ranges. The extent of positive metabolic effects depends directly on the relative time for which CGM systems are worn and loses significance if they are worn for less than 50–60% of the time. Patients with high baseline HbA1c values are able to realize greater improvements. In patients with a low HbA1c at the start of treatment, a further reduction in HbA1c may not be expected; however, a reduction in the number and duration of hypoglycemic events is possible.
From the perspective of the AGDT, CGM system use represents a valuable option that can offer patients with diabetes practical support with successful insulin therapy. Structured education is an essential requirement if patients are to benefit from this treatment option. In Germany, the “ConClusio” program provides a structured, albeit not yet validated, patient training program.69
Taking into account the conditions listed here, the personal and material-related efforts required for the use of CGM systems appears to be justified. From our point of view, CGM systems are indispensable in certain patient groups with type 1 diabetes, e.g., patients with hypoglycemia unawareness.
Glossary
- (AGDT)
Working Group Diabetes Technology
- (AUC)
area under the curve
- (CGM)
continuous glucose monitoring
- (CLSI)
Clinical Laboratory and Standards Institute
- (CSII)
continuous subcutaneous insulin infusion
- (HbA1c)
hemoglobin A1c
- (IIT)
intensive insulin therapy
- (JDRF)
Juvenile Diabetes Research Foundation
- (LGS)
low-glucose suspend
- (MARD)
mean absolute relative difference
- (RCT)
randomized controlled trial
- (SaP)
sensor-augmented pump
- (SMBG)
self-monitoring of blood glucose
Appendix 1
Overview of the indications of CGM that are listed in consensus statements from various countries (arranged by indication).59–68
-
Use of CGM indicated for “hypoglycemia”
Hypoglycemia unawareness (United States, United Kingdom, Spain);
Nocturnal hypoglycemia (United Kingdom, Spain, France);
Severe hypoglycemia with hospital admission (Switzerland);
Suspected hypoglycemia with low HbA1c value (United Kingdom);
Fear of hypoglycemia (United Kingdom); and
≥2 severe hypoglycemic episodes per year requiring third-party assistance (Sweden).
-
Use of CGM indicated for “unsatisfactory metabolic control”
HbA1c value too high [United States, United Kingdom (“high” is defined as patients with an insulin pump and an HbA1c of >7.5%, pregnant patients with an HbA1c of >6.1%), France (patients with IIT and HbA1c of >8.1%), Sweden (patients with HbA1c of >10.0%), Switzerland, Netherlands (HbA1c of >8.0%)];
High glycemic variability [United States, United Kingdom, Italy (Lake Como region)]; and
Discrepancy between documented blood glucose values and the mean metabolic control/HbA1c value (Spain).
-
Further indications of CGM systems:
Pregnant patients with type 1 diabetes (Israel) or type 1 and type 2 diabetes (Netherlands, France);
CGM as an instrument for teaching patients how to understand the influence of meals, exercise/sport, and stressful situations on metabolic control (Spain);
Children with type 1 diabetes [Netherlands (all), Slovenia (limited to ages ≤7 years)]; and
Children with more than 10 blood glucose measurements per day to achieve the therapeutic goal (Sweden).
Disclosures
Andreas Liebl is chief physician of the Department of Internal Medicine at the m&i Specialist Hospital in Bad Heilbrunn and receives lecture fees, consultancy fees, and assistance on scientific projects from Roche and Medtronic. Lutz Heinemann is chairman of the Association of Diabetic Technology of the German Diabetes Association and is a consultant to companies such as Roche Diagnostics, Senseonics, and C8 on the development of new diagnostic approaches. Guido Freckmann is the medical director and general manager of the Institute for Diabetes Technology Research and Development GmbH at the University of Ulm (IDT), which performs studies evaluating devices for diabetes therapy on behalf of various companies, and is co-chair of the AGDT. Andreas Thomas is head of Science at Medtronic, Germany, Diabetes Division, a manufacturer and distributor of insulin pumps and glucose sensors.
References
- 1.Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine. 2013;43(1):41–50. doi: 10.1007/s12020-012-9765-1. [DOI] [PubMed] [Google Scholar]
- 2.Siegmund T, Kolassa R, Thomas A. London: Unimed Science Verlag Bremen; 2012. Continuous glucose monitoring (CGM) und sensor-augmented pump therapy (SAP) [Google Scholar]
- 3.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277(3 Pt 1):E561–71. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 4.Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: Current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540–62. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6(5):1060–75. doi: 10.1177/193229681200600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazze RS, Strock E, Stout P, Racchini J, Wesley D, Borgman S. A novel methodology to evaluate continuous glucose monitoring accuracy and clinical representation of glucose exposure and variability. Diabetes. 2007;56(Suppl 1):A107. [Google Scholar]
- 7.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–9. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 8.Jungheim K, Koschinsky T. Glucose monitoring at the arm: risky delays of hypoglycemia and hyperglycemia detection. Diabetes Care. 2002;25(6):956–60. doi: 10.2337/diacare.25.6.956. [DOI] [PubMed] [Google Scholar]
- 9.Kovatchev BP, Shields D, Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11(3):139–43. doi: 10.1089/dia.2008.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Research in Children Network (DIRECNET) Study Group. The accuracy of the CGMS in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5(5):781–9. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tansey MJ, Beck RW, Buckingham BA, Mauras N, Fiallo-Scharer R, Xing D, Killman C, Tamborlane WV, Ruedy KJ, Diabetes Research in Children Network (DirecNet) Study Group Accuracy of the modified continuous glucose monitoring system (CGMS) sensor in an outpatient setting: results from a Diabetes Research in Children Network (DirecNet) study. Diabetes Technol Ther. 2005 Feb;7(1):109–14. doi: 10.1089/dia.2005.7.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovatchev B, Heinemann L, Anderson S, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160–4. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke WL, Anderson S, Farhy L, Breton M, Gonder-Frederick L, Cox D, Kovatchev B. Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis. Diabetes Care. 2005;28(10):2412–7. doi: 10.2337/diacare.28.10.2412. [DOI] [PubMed] [Google Scholar]
- 14.Garg SK, Voelmle MK, Gottlieb P. Feasibility of 10-day use of a continuous glucose-monitoring system in adults with type 1 diabetes. Diabetes Care. 2009;32(3):436–8. doi: 10.2337/dc08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastrototaro J, Oundararajan S, Cooper K, Shah R. Accuracy of real-time continuous glucose monitoring in the MiniMed Paradigm System. Diabetes. 2007;56(Suppl 1):A422. [Google Scholar]
- 16.Mazze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the accuracy, reliability, and clinical applicability of Continuous Glucose Monitoring (CGM): Is CGM ready for real time? Diabetes Technol Ther. 2009;11(1):11–8. doi: 10.1089/dia.2008.0041. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory and Standards Institute. Performance metrics for continuous interstitial glucose monitoring; approved guideline. POCT05-A. http://shopping.netsuite.com/s.nl/c.1253739/it.A/id.413/.f.
- 18.Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmle M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11(2):65–72. doi: 10.1089/dia.2008.0109. [DOI] [PubMed] [Google Scholar]
- 19.Bailey TS, Brazg R, Cooper K, Gautham RV, Janowski R, Kaufman FR, Lee WD, Shah R, Welsh JB, Zisser H. Performance evaluation of the Medtronic MiniMed Enlite subcutaneous glucose sensor. Diabetes. 2011;60(Suppl 1):A238. [Google Scholar]
- 20.Nogueira K, Gautham R, Cooper K, Shah R. The Medtronic ENLITE Sensor: the next step towards fingerstick replacement. Diabetes Technol Ther. 2011;13:257. [Google Scholar]
- 21.Book R, Zhang T. Long term performance of a prototype 4th generation continuous glucose monitoring (CGM) device. Diabetes. 2011;60(Suppl 1):A257. [Google Scholar]
- 22.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–83. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 24.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 25.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–83. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell MA, Donath S, O’Neal DN, Colman PG, Ambler GR, Jones TW, Davis EA, Cameron FJ. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–7. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 27.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, Jeandidier N, Nicolino M. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32(12):2245–50. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermanides J, Nørgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, Diem P, Fermon C, Wentholt IM, Hoekstra JB, DeVries JH. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158–67. doi: 10.1111/j.1464-5491.2011.03256.x. [DOI] [PubMed] [Google Scholar]
- 29.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA, STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 30.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795–800. doi: 10.2337/dc10-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, Schierloh U, Sulli N, Bolinder J, SWITCH Study Group The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–62. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diabetes Research in Children Network (DirecNet) Study Group. Youth and parent satisfaction with clinical use of the GlucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28(8):1929–35. doi: 10.2337/diacare.28.8.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12(9):679–84. doi: 10.1089/dia.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorlas S. Abrechnung der kontinuierlichen Blutzuckermessung. Dtsch Ärzteblatt. 2010;107(27):A–1374. [Google Scholar]
- 35.Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther. 2009;11(2):93–7. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danne T, Kordonouri O, Holder M, Haberland H, Golembowski S, Remus K, Bläsig S, Wadien T, Zierow S, Hartmann R, Thomas A. Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Ther. 2011;13(11):1129–34. doi: 10.1089/dia.2011.0084. [DOI] [PubMed] [Google Scholar]
- 37.Pickup JC. Semi-closed-loop insulin delivery systems: Early experience with low-glucose insulin suspend pumps. Diabetes Technol Ther. 2011;13(7):695–8. doi: 10.1089/dia.2011.0103. [DOI] [PubMed] [Google Scholar]
- 38.Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JA, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34(9):2023–5. doi: 10.2337/dc10-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal P, Welsh JB, Kannard B, Askari S, Yang Q, Kaufman FR. Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol. 2011;5(5):1137–41. doi: 10.1177/193229681100500514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, Shin J, Welsh JB, Kaufman FR. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther. 2012;14(3):205–9. doi: 10.1089/dia.2011.0292. [DOI] [PubMed] [Google Scholar]
- 41.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111(5 Pt 1):933–8. doi: 10.1542/peds.111.5.933. [DOI] [PubMed] [Google Scholar]
- 42.Kestilä KK, Ekblad UU, Rönnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res Clin Pract. 2007;77(2):174–9. doi: 10.1016/j.diabres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes. 2006;7(3):159–64. doi: 10.1111/j.1399-543X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 44.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80(3):371–9. doi: 10.1016/j.diabres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yates K, Hasnat Milton A, Dear K, Ambler G. Continuous glucose monitoring-guided insulin adjustment in children and adolescents on near-physiological insulin regimens: a randomized controlled trial. Diabetes Care. 2006;29(7):1512–7. doi: 10.2337/dc05-2315. [DOI] [PubMed] [Google Scholar]
- 46.Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, Fowler D, Campbell PJ, Temple RC. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmet A, Dagenais S, Barrowman NJ, Collins CJ, Lawson ML. Prevalence of nocturnal hypoglycemia in pediatric Type 1 diabetes: a pilot study using continuous glucose monitoring. J Pediatr. 2011;159(2):297–302. doi: 10.1016/j.jpeds.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 48.Diabetes Research In Children Network (DirecNet) Study Group. Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator) Pediatr Diabetes. 2008;9(2):142–7. doi: 10.1111/j.1399-5448.2007.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooke D, Hurel SJ, Casbard A, Steed L, Walker S, Meredith S, Nunn AJ, Manca A, Sculpher M, Barnard M, Kerr D, Weaver JU, Ahlquist J, Newman SP. Randomized controlled trial to assess the impact of continuous glucose monitoring on HbA(1c) in insulin-treated diabetes (MITRE Study) Diabet Med. 2009;26(5):540–7. doi: 10.1111/j.1464-5491.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 50.Diabetes Research in Children Network Study Group. Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, Beck R, Tamborlane W, Ruedy K. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10(2):91–6. doi: 10.1111/j.1399-5448.2008.00476.x. [DOI] [PubMed] [Google Scholar]
- 51.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203–10. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 52.Kordonouri O, Pankowska E, Rami B, Kapellen T, Coutant R, Hartmann R, Länge K, Knip M, Danne T. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53(12):2487–95. doi: 10.1007/s00125-010-1878-6. [DOI] [PubMed] [Google Scholar]
- 53.Danne T, de Valk HW, Kracht T, Walte K, Geldmacher R, Sölter L, von dem Berge W, Welsh ZK, Bugler JR, Lange K, Kordonouri O. Reducing glycaemic variability in type 1 diabetes self-management with a continuous glucose monitoring system based on wired enzyme technology. Diabetologia. 2009;52(8):1496–503. doi: 10.1007/s00125-009-1408-6. [DOI] [PubMed] [Google Scholar]
- 54.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 55.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Floyd BD, Chandra P, Hall SP, Phillips CO, Alema-Mensah E, Strayhorn G, Ofili E, Umpierrez G. Comparative analysis of the efficacy and safety of continuous glucose monitoring and self-monitoring blood glucose in type 1 diabetes mellitus. Diabetes. 2009;59(Suppl 1):A33. doi: 10.1177/193229681200600513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rys P, Mucha A, Koprowski M, Nowicki M, Malecki MT. Efficacy and safety of continuous glucose monitoring systems vs self-monitoring blood glucose in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2011;54(Suppl 1):S116. [Google Scholar]
- 58.Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus (Review) Cochrane Database Syst Rev. 2012;1:CD008101. doi: 10.1002/14651858.CD008101.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Diabetes Association. Summary of revisions for the 2009 Clinical Practice Recommendations. Diabetes Care. 2009;32(Suppl 1):S3–5. doi: 10.2337/dc09-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F, AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 62.National Institute for Health and Clinical Excellence. Diagnosis and management of type 1 diabetes in children, young people and adults. Clinical guidelines, CG15. http://www.nice.org.uk/Guidance/CG15.
- 63.Hirsch IB, Armstrong D, Bergenstal RM, Buckingham B, Childs BP, Clarke WL, Peters A, Wolpert H. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM) Diabetes Technol Ther. 2008;10(4):232–46. doi: 10.1089/dia.2008.0016. [DOI] [PubMed] [Google Scholar]
- 64.Hammond PJ, Amiel SA, Dayan CM, Kerr D, Pickup JC, Shaw JA, Campbell FM, Greene SA, Hindmarsh PC. ABCD position statement on continuous glucose monitoring: use of glucose sensing in outpatient clinical diabetes care. Pract Diab Int. 2010;27:66–8. [Google Scholar]
- 65.Andersson M, Eliasson B, Gustafsson J, Hanas R. 2009. Guidelines for the clinical use of CGM in Sweden. Letter to Tandvårds-Och Läkemedelsförmånsverket. [Google Scholar]
- 66.Diabetolog Nytt. Riktlinjer för kontinuerlig mätning av vävnadsglukos vid diabetes mellitus. http://diabetolognytt.se/extra/artikel4.html.
- 67.Adana MR, Rigla M. Position statement on continuous glucose monitoring. Av Diabetol. 2009;25(2):96–8. [Google Scholar]
- 68.Benhamou PY, Catargi B, Delenne B, Guerci B, Hanaire H, Jeandidier N, Leroy R, Meyer L, Penfornis A, Radermecker RP, Renard E, Baillot-Rudoni S, Riveline JP, Schaepelynck P, Sola-Gazagnes A, Sulmont V, Tubiana-Rufi N, Durain D, Mantovani I, Sola-Gazagnes A, Riveline JP, Société Francophone du Diabète; Société Française d’Endocrinologie; EVAluation dans le Diabète des Implants ACtifs Group Real-time continuous glucose monitoring (CGM) integrated into the treatment of type 1 diabetes: consensus of experts from SFD, EVADIAC and SFE Diabetes Metab. 2012;38(Suppl 4):S67–83. doi: 10.1016/S1262-3636(12)71538-0. [DOI] [PubMed] [Google Scholar]
- 69.Medtronic. Professionals. http://www.medtronic-diabetes.de/fachkreise/index.html.