Abstract
Introduction:
The options available to CKD 5 patients with donor shortage due to incompatibilities is to either get enlisted in cadaver transplant program or opt for three other alternatives viz; ABO-incompatible transplant (ABO-I), ABO-incompatible transplant with Rituximab (ABO-R) or paired-kidney exchange transplant (PKE). At our institute we have performed ABO-I, ABO-R and PKE transplants and we are presenting the results of these transplants performed at our institution. Here, we report our experiences of living donor kidney transplantation in highly sensitized patients.
Objective:
To review the options available to CKD 5 patients with incompatible donor.
Materials and Methods:
Between January 2008 and June 2011, 7 PKE, 26 ABO-I and 7 ABO-R transplants were carried out at our institute. Evaluation of both the recipients and donors involved biochemical, serological and radiological investigations. In case of PKE, recipients were operated simultaneously in different operation theaters. In ABO-I splenectomy was done while in ABO-R was given. Post-transplant the recipient management protocol remained the same. Expenditure following each transplant was calculated.
Results:
The graft and patient survival of ABO-I, ABO-R and PKE transplants 12-18 months after transplant were 78.9%:80%, 85.7%:85.7% and 100%:100%, respectively.
Conclusions:
The inclusion of Rituximab in the transplant protocol appears promising. The existing donor shortage could be addressed by encouraging other options like PKE. The limiting factor for ABO-R and PKE transplants is time and cost, respectively. The decision depends on the informed consent between the patient and the nephrologists.
Keywords: ABO incompatibility, CKD 5, living donor, paired-kidney exchange transplants, Rituximab, splenectomy
INTRODUCTION
Renal transplantation is the treatment of choice for patients with end-stage renal disease,[1] since it offers improved survival and quality-of-life benefits as compared with dialysis. Also it is considerably less costly. The recipient receives renal graft from either cadaver or a living donor. Cadaver transplant entails a long waiting period which is around 6 months to more than a year at our institute. Those who continue to remain on chronic hemodialysis during the interim period frequently experience complications and some succumb to them. Living donor entails availability of an ABO compatible, fit and willing donor. Unfortunately such donors may not be available for all recipients. This leads to an increasing waiting list and also with the annual incidence rate of CKD 5 patients expected to rise at 5-8%[2] the need for alternative viable options has increased.
We face a similar scenario at our institute and the patients are offered three options 1) ABO-incompatible transplant with conventional splenectomy being done in the recipient (ABO-I), 2) ABO-incompatible transplant using Rituximab (ABO-R) and 3) Paired kidney exchange transplant also known as Swap transplant [The amendments made in the Transplantation of Human Organs Act have legalized swapping of vital organs.[3,4,5]]
AIMS AND OBJECTIVES
To review the options available to CKD 5 patients with incompatible donor.
MATERIALS AND METHODS
Between January 2008 and June 2011, 7 paired-kidney exchange transplants (PKE) [Figure 1, Table 1], 14 recipients and 14 donors (Both conventional and unconventional) and 26 ABO-incompatible transplants [Table 2] were carried out at our center out of which seven patients received Rituximab regimen (anti-CD20 monoclonal antibody) which avoided splenectomy in them.
Figure 1.
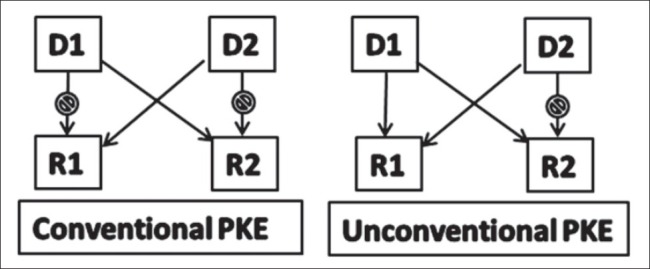
Pattern of paired-kidney exchange transplantation
Table 1.
Data for PKE transplants
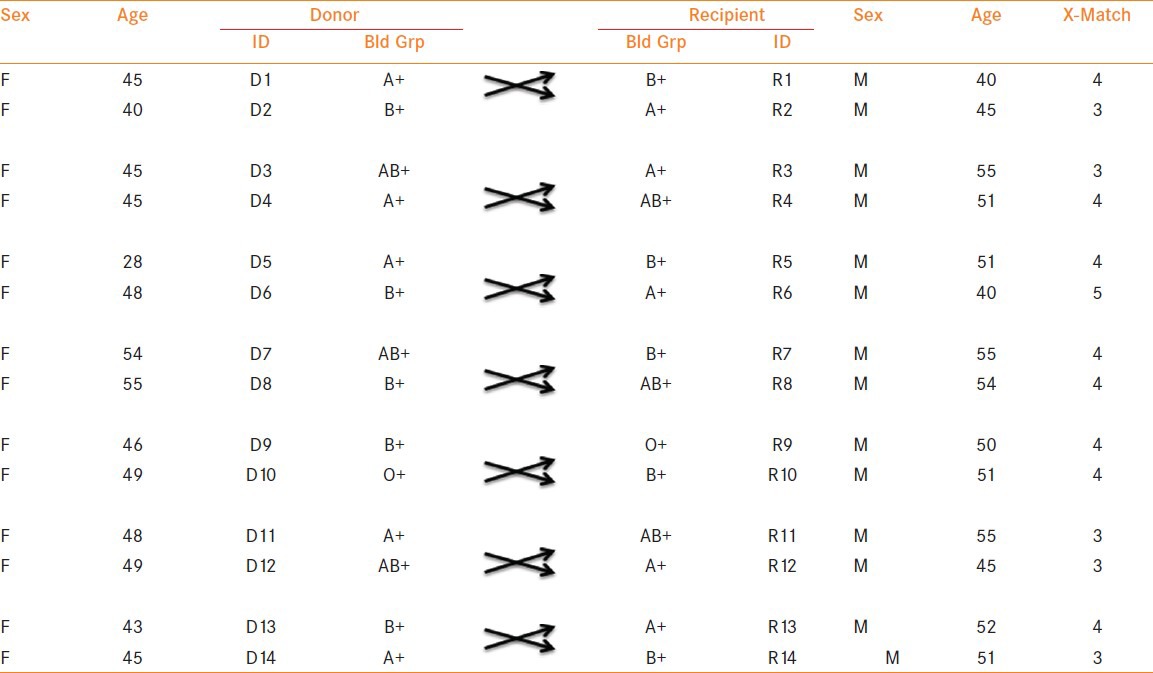
Table 2.
Patients and their blood groups involved in ABO-I transplants

Evaluation of both the recipients and donors involved standard biochemical, serological and radiological investigations. The donor underwent 64 slice CT angiography with volume rendering for anatomical evaluation. Prior to every transplant the donor and the recipient were counseled by the respective surgeons, nephrologists and transplant social workers for potential complications. No objection certificates were received from the authorization committees and only then the transplants were scheduled. The transplants were carried out in a single institute.
In case of PKE transplants both the recipients were operated simultaneously in different operation theatres and the recipients in ABO-I transplants underwent open splenectomy after receiving pneumococcal vaccines prior to the transplant. All donors underwent open donor nephrectomies. Post-transplant the recipient management protocol remained the same with minor changes related to immunosuppressive therapy.
In ABO-R transplant the protocol called for a 10 days pretransplant conditioning period starting with one dosage of Rituximab and followed by full dose. Tacrolimus (0.125 × body weight in kg), Mycophenolate Mofetil (1500-3000 mg/ day), prednisolone [(30 mg) 1 day prior to transplant. On the day of transplant 500 mg Solumedrol is given which is tapered to 125 mg over 3 days. On Day 4 post-transplant prednisolone was started at 1 mg/kg which was tapered @ 5 mg/day till dose of 15-30 mg/day depending on the serum creatinine value. The dose was then gradually tapered over a week and discontinued.] Antigen-specific immunoadsorption was performed starting 6 days prior to transplant. The cut-off for anti-A and anti-B titres after Plasmapheresis done prior to transplant was between 1:1 to 0. After the last session, 0.5 g/kg of intravenous immunoglobulin is administered. Postoperatively three more apheresis sessions were given every third day. The duration of hospitalization were same for all on average: Recipients (10-14 days) and Donors (5-7 days).
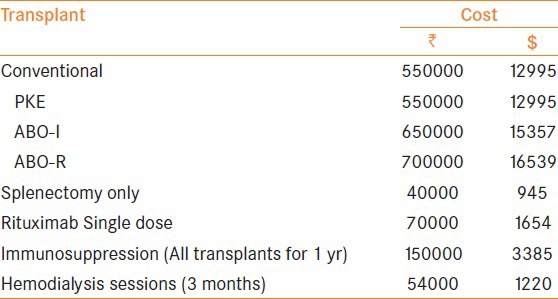
The cost incurred after every transplant was calculated. This included the operative and the perioperative expenditure incurred (including hospitalization, immunosuppression, plasmapheresis, routine investigations, etc) for a period of 1 year. The cost borne by the recipient for maintenance immunosuppression and follow-up investigations after 1 year of transplant has not been included. [1$= 44.325 Rs]
RESULTS
ABO-Incompatible Transplants with Conventional Splenectomy
Out of 19 recipients 4 expired: 2 due to septicemia secondary to community-acquired pneumonia 4-6 months post-transplant, 1 expired due to flaring up of underlying incidentally detected lymphoproliferative disorder –B cell Lymphoma within a week post-transplant. One recipient had a cardiac event a year after transplant to which she succumbed. One recipient suffered from subsequent graft rejection 6 months later and went back to dialysis. One patient had renal artery thrombosis, the graft was not salvageable. He underwent graft nephrectomy within a week of the transplant and subsequently went back to dialysis. One recipient had severe post-transplant coagulopathy immediately the next day after transplant and was managed with intravenous infusion of Factor VII. He recovered well. Figure 2 depicts graft and patient survival data over a follow-up period of 12-18 months. Five out of the 19 recipients had subclinical pancreatitis which was suspected when the recipients developed either prolonged paralytic ileus or mild pain in abdomen. These symptoms were supplemented by a rise in serum amylase and lipase values.
Figure 2.
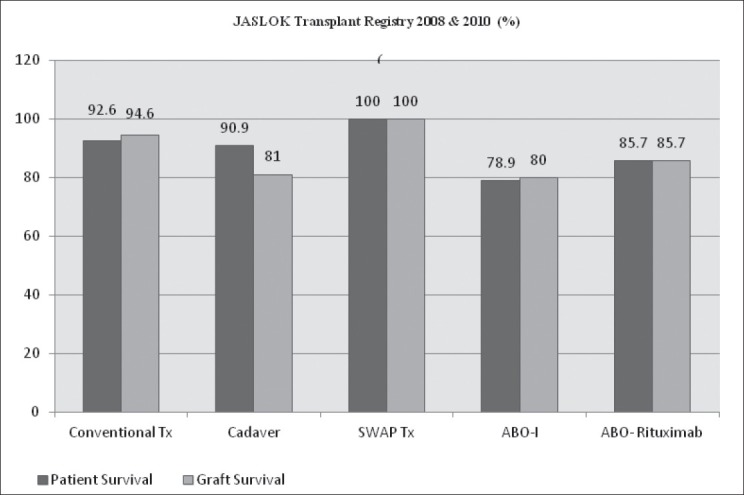
Jaslok Transplant Registry 2008 and 2011 (%)
ABO-Rituximab Transplants
All six recipients recovered well with an average drop of creatinine of 7.7 (8.9 pre-Tx to 1 Post-Tx) except one who expired due to sepsis secondary to community acquired pneumonia 1 month after transplant. Figure 2 depicts graft and patient survival data over a follow-up period of 12-18 months. Morbidities seen in ABO-I transplants were not seen.
PKE Transplants
Following transplant the recipient responded well. The creatinine value dropped from 11.6 to 1.1 on average. Two recipients had prolonged hospital stay secondary to prolonged lymphatic drainage. One recipient had renal vein thrombosis postoperatively. He was immediately explored and later recovered, though slowly. Two recipients had acute cellular rejection which was immediately treated with anti-thymoglobulin. No antibody-mediated rejection was seen. Figure 2 depicts graft and patient survival data over a follow-up period of 12-18 months. Comparatively less/no complications were seen in PKE (SWAP) transplants.
Patient and Graft Survival
The graft survival and patient survival of ABO-I, ABO-R and PKE transplants 12-18 months after transplant were 78.9%:80%, 85.7%:85.7% and 100%:100%, respectively.
Cost Factor
We believe that cost is one of the important factors needed to be considered in a developing country like ours. The approximate expenditure incurred has been summarized [Table 3]. Expenditure incurred due to transplant per se, immunosuppression given for the subsequent year post transplant, Rituximab & dialysis sessions which the patients underwent prior to transplant were included also. This information added a new dimension for looking at and analyzing the options available for end-stage renal disease patients
Table 3.
Approximate transplant expenditure
DISCUSSION
CKD 5 patient who wants kidney transplant has to find a suitable living donor or wait for cadaver kidney. In parts of India, where cadaveric transplant programme has not taken off fully, when a patient with end-stage renal disease (CKD 5) decides to opt for undergoing transplant he looks for a compatible donor mostly in his own family. The search comes to a standstill when he is unable to find one due to ABO incompatibility in the donor. Cadaver transplant entails a long waiting period which is around 6 months to more than a year at our institute. Those who continue to remain on chronic hemodialysis during the interim period frequently experience complications and some succumb to them. Living donor entails availability of an ABO compatible, fit and willing donor. Unfortunately such donors may not be available for all recipients. This leads to an increasing waiting list. With limited options available the choice is difficult to make.
In our study we found that the graft and patient survival in an ABO-I transplant with conventional splenectomy is relatively poor as compared to others. ABO-I transplants were associated with morbidity secondary to splenectomy, which the recipient had to undergo prior to the transplant, let alone the repeated plasmapheresis and the risks involved. Also those recipients who developed pancreatitis probably did so following minor trauma to the pancreas during splenectomy. These patients settled with conservative management. To avoid the morbidity following splenectomy we started including Rituximab as a part of protocol for the ABO-I Tx and the results are encouraging. Moreover it avoids splenectomy in the recipient. Tayden et al.[6] and Fuchinoue S et al.[7] in their studies have upheld Rituximab as a viable and safe option for those who undergo ABO-I transplants.
PKE
Showed good results at par with the results found in world literature[8,9] [Figure 3]. PKE transplants are safe except for the delay in availability of ‘eligible’ pairs. Also once the eligible pairs are available they have to be counseled individually. PKE also includes going through the process of obtaining permission from the authorization committees of respective state legislatives which may take time which can amount to weeks, sometimes months in developing countries like India. Many countries after a lot of hesitation resorted to PKE transplants to meet the donor deficit, including South Korea, Switzerland and USA.[1,10]
Figure 3.
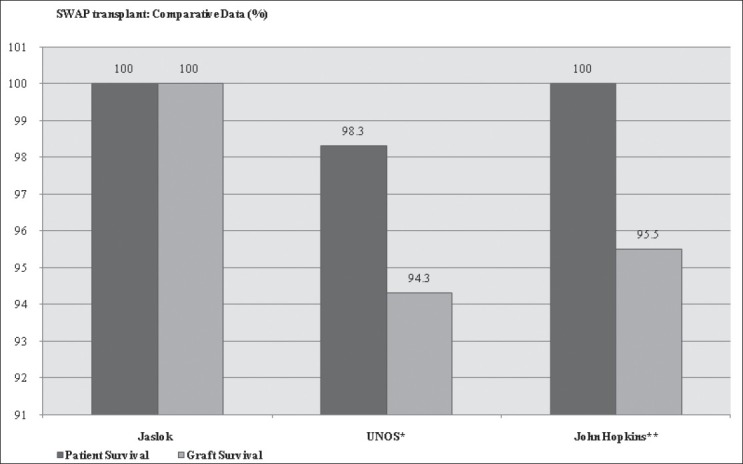
PKE ransplant: Comparative Data
Interestingly the patient and graft survival of ABO-R and PKE transplants have been parallel to the conventional transplants which is encouraging. PKE requires a large pool of patients on dialysis and donors. It also requires more than one team for simultaneous surgery in two donors and two recipients. In a smaller unit ABO-R appears a viable alternative.
In a developing country like ours, cost factor always place a vital role in decision making even if it involves important health issues. The PKE transplants cost the same as conventional transplants. However, the ABO transplants with or without the use of Rituximab raises the cost a bit more due to splenectomy, plasmapheresis and Rituximab itself but, We guess it is insignificant if we are looking at improving the graft outcome and the QoL of the patient. On an average dialysis costs around 55,000 rupees for 3 months. Instead if the recipient can be counseled and is willing he can be encouraged to undergo transplant using Rituximab. It would save him from the morbidity associated with dialysis at a very small price.
On comparing each option and looking at the pros and cons of each. We found that.
PKE are just like any other conventional transplant but it entails 1) eligible pair availability, 2) state legislative permission which would take a long time, 3) a large pool of recipients and donors to choose from and most importantly 4) more than one transplant teams.
ABO-I using conventional splenectomy is a less costly venture needing a ‘smaller unit’ but the associated risks/ complications is something to reckon with. On the other hand ABO-R transplant using Rituximab is a viable option in a ‘smaller unit’. Using Rituximab is no doubt a costly venture but it spares the recipient of the associated morbidities of undergoing second surgery i.e., splenectomy and also of its indolent risks. Also the results are encouraging.
CONCLUSIONS
The inclusion of Rituximab in the transplant protocol holds good promise for the future of ABO incompatible transplants. The existing donor shortage (especially due to incompatibility between recipient and donor) could be addressed by encouraging other options which includes PKE transplants also. The limiting factor for ABO-R and PKE transplants in a country like India is time and cost, respectively. The decision depends on the informed consent between the patient and the nephrologists.
ACKNOWLEDGMENTS
Transplant Surgeons and Nephrologists: Dr. P. J. Chibber, Dr. A. Raval, Dr. S. Raina, Dr. P.F Soonawala, Dr. J. Lalmalani, Dr. Vinit Shah, Dr. Ketan Desai, Dr. Aseem Thamba and Dr. Vimal Patel for sharing their experiences.
The National Institute of Immuno-Hematology, Parel, Mumbai, INDIA for carrying out the Anti-A and Anti-B titers for the recipients undergoing ABO-I or ABO-R transplants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Eng J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Veerappan I, Neelakantan N, Tamilarasi V, John GT. Medical and non-medical factors that affects voluntary living-related kidney donation: A single-centre study. Indian J Nephrol. 2011;21:14–20. doi: 10.4103/0971-4065.75223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Government of India. Transplantation of Human Organs Act, 1994. Central Act 42 of 1994. Available from: http://mohfw.nic.in/WriteReadData/l892s/S12011122007MS-18018705.pdf .
- 4.The Report of Transplant of Human Organs Act Review Committee. Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. 2005. Available from: http://www.mohanfoundation.org/tho/thoa-review-committee.asp .
- 5.Gazette - Transplantation of Human Organs Rules. 1995. (GSR NO. 51(E), dr 4-2-1995) [As amended vide GSR 571(E), dt31-7-2008] [cited 2008 Jul]. Available from: http://wwwmedindianet/indian_health_act/The-Transplantation-of-Human-Organ-Rules-1995-Definitions.htm .
- 6.Tydén G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145–8. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Fuchinoue S, Ishii Y, Sawada T, Murakami T, Iwadoh K, Sannomiya A, et al. The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011;91:853–7. doi: 10.1097/TP.0b013e31820f08e8. [DOI] [PubMed] [Google Scholar]
- 8.United Network for Organ Sharing Web site. Organ procurement and transplantation network data. [Accessed March 10, 2005]. Available at: http://www.unos.org .
- 9.Montgomery RA, Zachary AA, Ratner LE, Segev DL, Hiller JM, Houp J, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294:1655–63. doi: 10.1001/jama.294.13.1655. [DOI] [PubMed] [Google Scholar]
- 10.Modi P, Rizvi SJ, Pal B, Baradwaj R, Gupta S, Shah V, et al. Living donor paired-kidney exchange transplantation: A single institution experience. Indian J Urol. 2010;26:511–4. doi: 10.4103/0970-1591.74446. [DOI] [PMC free article] [PubMed] [Google Scholar]


