Abstract
Bone health is affected in patients with prostate cancer, both by the disease and its treatment. Metastases to bone leads to pain, fractures, and spinal cord compression; bone loss due to androgen deprivation therapy (ADT) leads to osteoporosis and its complications. Both these scenarios are a major cause of morbidity and adversely affect the quality of life of these patients. Maintaining an optimum bone health throughout the natural course of prostate cancer is an important aspect in the management of this disease. An understanding of the complex interplay between osteoclasts, osteoblasts, receptor activator of nuclear factor κB (RANK), and various other tyrosine kinases involved in the pathophysiology of bone metastases is essential. Zoledronic acid (ZA), an intravenously administered bisphosphonate, and Denosumab, a subcutaneously administered inhibitor of nuclear factor B ligand (RANKL), have already been approved by Food and Drug Administration (FDA) for their use in treatment of bone metastases. This article discusses the pathophysiology of bone metastases and bone loss due to ADT in prostate cancer, role of biomarkers, newer modalities of imaging, World Health Organization (WHO)/FRAX nomogram in evaluation of these patients, utility of currently available drugs and evidence supporting their use, and newer therapeutic agents like alpha-emitting Radium-223, endothelin-A receptor antagonists (Atrasentan and Zibotentan) and the proto-oncogene tyrosine-protein kinase (SRC) inhibitor, Dasatinib.
Keywords: Bisphosphonates, bone metastasis, osteoclasts, prostate cancer, skeletal related events, zoledronic acid
INTRODUCTION
Prostate cancer (PCa) as a urological malignancy has the highest incidence of bone metastases. Half of PCa patients with bone metastases will succumb within 30 months of detection. Bone metastasis causes some of the most distressing symptoms of advanced stage cancer, with 22% patients requiring treatment for single or multiple pathological skeletal fractures, 7% for spinal cord compression, and 3-4% for hemiparesis or paresis.[1] Bone metastases due to disease and bone loss due to treatment are the major contributors to patient morbidity in PCa. Unfortunately, this aspect of PCa often remains neglected and ignorance about optimum management of bone health in PCa patients is common even today. In a recent survey amongst 108 randomly selected, qualified Indian urologists, it was found that a majority of them have not been following the recommended guidelines related to bone health management.[2]
PROSTATE CANCER AND BONE COMPLICATIONS
Normal bone physiology
A healthy bone maintains its structural integrity by a continuous process of bone remodeling that involves osteoblasts for bone formation and osteoclasts for its resorption. The formation of bone by osteoblasts involves the production of typeI procollagen, followed by its processing, modification, and secretion. Secreted typeI procollagen is cross-linked to form aligned helical arrangements of multiple collagen fibrils and fibers. This typeI collagen-rich matrix which also contains other matrix proteins undergoes mineralization to form bone. Monocyte/macrophage progenitors give rise to osteoclasts, which after maturation bind to bone to create a sealed vacuole. Acidification of this vacuole by lytic enzymes leads to bone resorption.
Receptor activator of nuclear factor κB (RANK) is a member of the Tumor Necrosis Factor (TNF) receptor superfamily and is expressed by osteoclasts. Receptor activator of nuclear factor κB ligand (RANKL) activates RANK, which in turn activates osteoclasts. RANKL is expressed by osteoblasts and bone marrow stromal cells. Osteoprotogerin (OPG), on the other hand, is a decoy receptor for RANKL, expressed by osteoblasts and protects bone from resorption.[3]
Pathophysiology of bone metastasis
Bone metastasis in PCa is a state of high bone turnover involving both osteoblasts and osteoclasts. Osteoclasts erode trabecular bone and osteoblasts form sclerotic woven bone. The woven bone thus generated, though dense on radiography, is structurally weak and associated with increased risk of fracture.[4]
New bone formation causes hypocalcemia, leading to secondary hyperparathyroidism. Parathryroid hormone induces RANKL expression in osteoblasts and bone marrow stromal cells, which further stimulates osteoclast formation [Figure 1].
Figure 1.
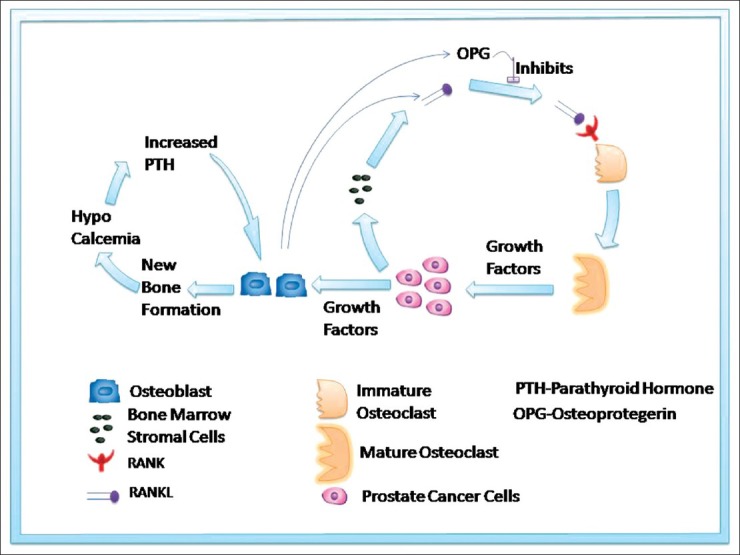
Schematic illustration of bone metabolism in prostate cancer
Pathophysiology of age and ADT-related osteoporosis
Age has its own impact on bone strength. The incidence of osteoporosis in men older than 50 years is roughly 13% in the western world. Both testosterone and estrogen contribute to bone mineral density (BMD) and are correlated with increased fracture risk among older men in general population. Men older than 60 years have a 25% chance of experiencing fracture due to osteoporosis. Furthermore, the mortality of hip fracture in men is almost quadruple that in women.[5] Factors that increase the risk for osteoporosis in men include age (>70 years), low body weight (body mass index <20-25 kg/m2), weight loss [>10% (compared with the usual young or adult weight or weight loss in recent years)], physical inactivity, corticosteroid use, and previous fragility fracture.[6]
Androgen deprivation either by surgical castration or by gonadotropin-releasing hormone (GnRH) analogs or antagonists is a pivotal treatment modality in PCa. Surgical or medical castration generally lowers serum testosterone to <20 ng/ml. Estrogen, being produced by peripheral aromatization of testosterone, is also low during androgen deprivation therapy (ADT). Both testosterone and estrogen contribute to BMD and are correlated with increased fracture risk among older men in general population. BMD decline of approximately 3% at lumbar spine and 2% at hip has been shown during the first year of ADT therapy.[7] Retrospective studies have estimated fracture rates in the range of 6-9% in men on ADT and 13-40% in those treated with bilateral orchiectomy.[8] Intermittent ADT has been found to be no better than continuous ADT with regards to bone loss.[9]
Clinical manifestation of bone metastases and bone loss
80-90% of men with metastatic castration-resistant PCa (mCRPC) have radiologically detectable bone metastases.[10] Pain is the most common symptom. Vertebral metastases can cause compression fracture, spinal cord compression, and/or nerve root compression. Axial skeleton (vertebral bodies, pelvis, ribs, skull) is involved much more commonly compared to long bones. Ineffective erythropoiesis leads to anemia. Hypocalcemia is generally not symptomatic.[10]
ADT causes an annual bone loss of 4.6%. The most significant loss of BMD occurs within the first year of ADT. Men receiving GnRH analogs with or without antiandrogens have similar BMD losses.[11]
Evaluation of bone metastases
Metastasis to bone is a poor prognostic factor in PCa. Early detection of bone metastases is important in deciding patient management and improving their quality of life, and it has become all the more crucial with the availability of bisphosphonates and other new drugs. The ideal time to investigate for bone metastases and the choice of right imaging modality are important in maximizing clinical benefit.
In addition to history and physical examination, serum calcium, alkaline phosphatase (ALP), and X-ray, newer bone markers, bone scintigraphy, positron emission tomography (PET), computed tomography (CT) scan, and magnetic resonance imaging (MRI) have an important role to play in detecting bone metastases.
Biomarkers of bone metabolism have been used to assess bone formation and resorption, especially in drug trials. Markers of bone formation include ALP, osteocalcin, and procollagen. The specificity of ALP has been improved by developing monoclonal antibodies recognizing bone isoenzymes of ALP (bALP). This is a marker of osteoblast cell activation, and so more representative of bone metastases.[12]
The enzyme Tartrate-Resistant Acid Phosphatase (TRAP), products of bone breakdown like calcium and products of bone matrix degradation like hydroxyproline, pyridinium cross-links, and telopeptides are the markers of bone resorption. However, none of them are being used in clinical practice.[13]
Plain X-ray requires 30-75% of normal bone mineral content to be lost before radiolucency is apparent. This limits its sensitivity to detect bone metastases to 44-50%.[14]
Conventionally, bone scintigraphy with 99mTc-methylene diphosphonate (99mTc-MDP) has been the imaging modality of choice in detecting bone metastases.
The recommended indications for performing bone scan in the initial workup of PCa have been summarized in Table 1. Briganti et al. have developed a risk stratification tool to select patients requiring initial imaging from a series of 853 patients.[15] Their classification and regression tree (CART) stratifies patients into low risk [biopsy Gleason ≤7, cT1-3, and prostate-specific antigen (PSA) <10 ng/ml], intermediate risk (biopsy Gleason ≤7, cT2-3, and PSA >10 ng/ml), and high risk (biopsy Gleason >7), conferring a risk of bone metastases of 1.8%, 8.5%, and 16.4%, respectively. Briganti et al.'s regression tree shows higher sensitivity (87.5%) compared to the European Association of Urology (EAU), American Urological Association (AUA), and National Comprehensive Cancer Network (NCCN) guidelines.
Table 1.
EAU and NCCN recommendations
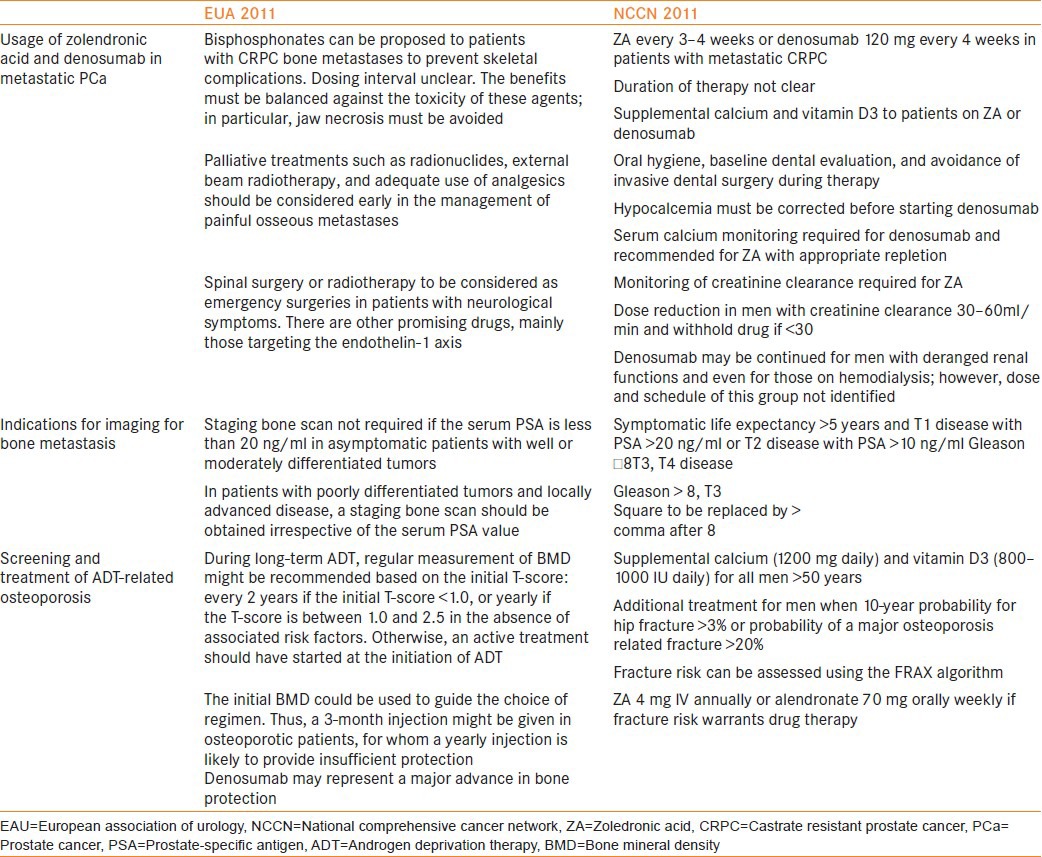
99mTc-MDP is a nonspecific marker of osteoblastic activity. Therefore, Bone Scan (BS) detects bone metastases at an advanced stage of tumor infiltration, when osteoblastic reaction to metastatic cell deposit has occurred.[16] Sensitivities reported in the literature range between 62 and 89%.[16]
99mTc-MDP accumulates in response not only to tumor but also to degenerative joint disease, benign fractures, and inflammation, thus decreasing its specificity.[17] Images in bone scans are limited to anterior and posterior planes. Standard planar bone scan can be improved by single-photon emission computerized tomography (SPECT) on selected areas.[18,19] Further, with advent of new radiopharmaceutical agents like 18F-fluorocholine and 18F-fluoride, PET/CT has been found to be more accurate than 99mTc-MDP bone scan. Even-Sapir et al. have compared bone metastases detection by 99mTc-MDP scan, SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT in 44 patients with high-risk PCa.[20] The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of planar 99mTc-MDP bone scan were 70%, 57%, 64%, and 55%, respectively, of SPECT were 92%, 82%, 86%, and 90%, respectively, of 18F-fluoride PET were 100%, 62%, 74%, and 100%, respectively, and of 18F-fluoride PET/CT were 100% for all parameters.[20]
CT scan has not been used as a screening test, but rather as a second-line imaging technique to evaluate abnormal bone scan uptakes remaining unexplained after standard X-ray or to image suspicion of neurological disorders.
The superiority of MRI over bone scan in detecting bone metastases has been repeatedly demonstrated.[21,22] MRI can not only detect bone metastases, but also quantify it, and so can measure tumor response to therapy.[23] However, the use of MRI in first line is not feasible owing to its limited availability and cost. MRI identifies bone metastases at an early stage, before host reaction of the osteoblasts becomes visible on bone scan and X-ray.[16] Recently, the diagnostic performance of one-step MRI of the axial skeleton (MRIas) has been compared to the routinely used imaging modalities. Sensitivities were 46% for Tc-99m MDP bone scan alone, 63% for BS/X-ray, 83% for BS/X-rays/MRI, and 100% for MRIas. Corresponding specificities were 32%, 64%, 100%, and 88%.[24]
Evaluation of bone loss due to ADT
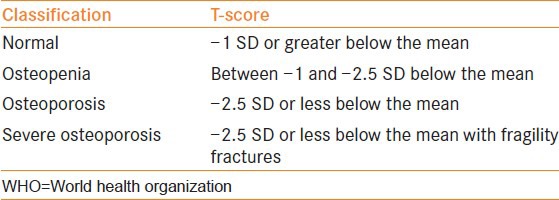
Early identification of bone loss in men on ADT guides selection of candidates for lifestyle modifications, dietary changes, and medical therapy. BMD measured in g/cm2 accounts for about 70% of bone strength. Conventionally, dual-energy X-ray absorptiometry (DXA) scan has been used to quantify BMD. This method measures BMD changes in spine, hip, proximal femur, and total body. A DXA scan can be rapidly performed in the office and uses radiation doses lower than those of conventional X-ray.[25] This technology uses two X-ray beams, which are attenuated differently by bone and soft tissues, specifically to image bone with a high degree of accuracy. The X-ray images are internally analyzed to determine bone mineral content in grams and then divided by the image area to determine BMD. Results of BMD measurement are reported as T-score, which is the number of standard deviations with which patient's measured bone loss deviates from the mean of young normal population of the same sex at a given site.[26] WHO has classified osteoporosis on the basis of T-score [Table 2]. This classification was originally developed for white postmenopausal women, but is also applied to men now. Deterioration in T-score is directly related to fracture risk. A 10-20% bone loss represented by a T-score of −1 increases the relative risk of fracture by 1.5- to 2-fold.
Table 2.
WHO osteoporosis classification
Until recent past, BMD has been used as a surrogate for fracture risk due to ADT-mediated osteoporosis. Though BMD is one of the important risk factors predicting fracture risk, it has now been found that most fractures occur in men whose BMD is not in osteoporotic range.[27]
Several other factors like prior fragility fracture, family history of hip fracture, current tobacco smoking, chronic use of glucocorticoids, daily use of at least 3 units of alcohol, rheumatoid arthritis, and other causes of secondary osteoporosis have been implicated in causing fragility fractures.[28,29,30,31,32,33,34,35] Based on these risk factors with or without measured BMD, WHO has developed an algorithm that quantitatively assesses fracture risk. NCCN guidelines for the management of PCa recommend this online algorithm (http://www.shef.ac.uk/FRAX/tool.jsp) for fracture risk assessment and for guiding pharmacological therapy to decrease the risk of fragility fractures in patients on ADT.[36]
DRUGS USED IN THE MANAGEMENT OF BONE COMPLICATIONS IN PROSTATE CANCER
Bisphosphonates
These are the most commonly used drugs in the management of bone complications in PCa. Their structural similarity to inorganic phosphate helps them to incorporate into bone and bind to hydroxyapatite. Once inside the bone, they act in the following ways to decrease bone resorption:
Decrease the availability of hydroxyapatite crystals and osteoclast-mediated resorption.
Inhibit recruitment, differentiation, attachment, and survival of osteoclasts.
Act on osteoblasts to indirectly inhibit osteoclast differentiation and activation.
Inhibit RANKL expression in PCa cells, further diminishing osteoclast activity.[37]
Potency of bisphosphonates is governed by the presence or absence of an amino side chain at R2 location. Etidronate, Clodronate, Pamidronate, alendronate, ibandronate, risedronate, and zoledronic acid (ZA) are the currently available bisphosphonates, listed from lowest to highest potency. ZA is 100 times more potent than Pamidronate and 1000 times more potent than Etidronate.[38]
Based on the results of three randomized controlled trials involving more than 3000 patients, intravenous ZA received Food and Drug Administration (FDA) approval in 2002 for treatment of bone metastases from any solid tumor including PCa.[39,40,41]
The Zometa 039 was a popular trial in which 643 men with CRPC and asymptomatic or minimally symptomatic bone metastases were assigned randomly to intravenous ZA (4 or 8 mg every 3 weeks) or placebo.[41] All men continued ADT and received additional cancer therapy at the discretion of the treating physicians. The primary study end point was the proportion of men who experienced one or more skeletal related event (SRE; pathologic fracture, spinal cord compression, surgery or radiation therapy to bone, or change in antineoplastic treatment to treat bone pain) by 15 months. Adverse renal events led to two amendments. First, ZA was infused over a longer time period (from 5 to 15 min) and in greater infusate volume (from 50 to 100 ml). Second, the ZA 8 mg treatment dose was reduced to 4 mg, with serum creatinine monitoring before each dose, and the primary efficacy assessment became the comparison of the 4 mg group versus placebo. After these amendments, adverse renal events between the groups were similar.
ZA was associated with fewer SREs than the placebo group at 15 months (33.2% vs. 44.2%; P = 0.021). ZA increased the median time to first SRE (488 days vs. 321 days; P = 0.009). Median survival was longer in the ZA group than in the placebo group (546 days vs. 464 days; P = 0.091) and the observed difference in overall survival (OS) was not statistically significant.
The Medical Research Council (MRC) PR05 is an important study evaluating the role of oral clodronate in metastatic castration-sensitive PCa.[42] Long-term results of this study published recently have shown a significant benefit in OS in the clodronate group compared with placebo (8-year OS 22% vs. 14%, P = 0.032). CALGB/CTSU 90202 is an ongoing study evaluating the role of ZA in castration-sensitive metastatic PCa.[43] Six hundred and eighty men with PCa who have started ADT within 3 months have been assigned to ZA (4 mg intravenously every 4 weeks) or placebo. The primary end point is SRE or PCa death. Given the encouraging results in MRC PR05 with the comparatively weak bisphosphonate Clodronate, data from CALGB/CTSU 90202 are eagerly anticipated.
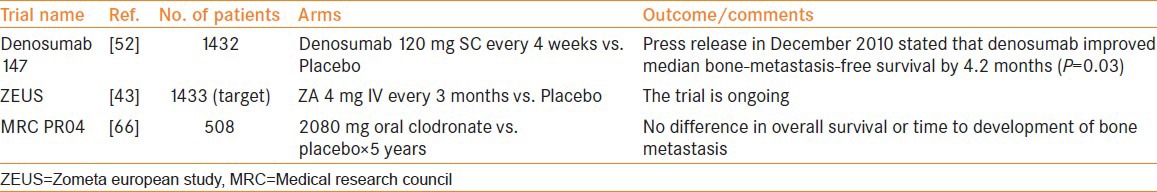
The role of ZA in preventing bone metastases in non-metastatic PCa is being evaluated in an ongoing randomized, controlled trial called The Zometa European Study (ZEUS).[43] The study enrolls men without bone metastases who have at least one of the following factors that put them at high risk: PSA >20 ng/ml, lymph node metastases, or Gleason score ≥8 primary tumor. It will randomly assign a total of 1433 men to ZA (4 mg IV every 3 months for 48 months) or to standard care without ZA. The primary end point is the proportion of men with at least one bone metastasis after 48 months of therapy.
Alendronate is another bisphosphonate that has gained popularity, especially with it's once a week oral dosage. It has been shown that once a week 70 mg oral alendronate significantly increases the BMD in osteopenic or osteoporotic men with PCa treated with ADT, compared to those not receiving bisphosphonate therapy. It has also shown to significantly decrease the risk of femoral neck fracture after 1 year of follow-up in these patients.[44]
In men with metastatic CRPC, NCCN recommends ZA 4 mg intravenously every 3-4 weeks, but the total duration of therapy is not clear. For prevention of ADT-related fragility fractures, NCCN recommends risk assessment using FRAX nomogram, and if this risk warrants therapy, ZA 4 mg IV annually or Alendronate 70 mg orally weekly should be administered.
Toxicity is an important concern with Bisphophonates. Hypocalcemia is a common side effect, and it is prudent to start Vitamin D before the initiation of therapy and to monitor calcium levels during therapy. Some patients can have a self-limiting acute phase reaction within 24 h of infusion. Renal insufficiency requires dose modification of ZA and the drug is not recommended if GFR is <30 ml/min/1.7 m2. Nephrotoxicity of ZA can be reduced by increasing its infusion time from 5 to 15 min and by using 4 mg dose instead of 8 mg. If a normal baseline creatinine increases ≥0.5 mg% or abnormal baseline creatinine increases ≥1.0 mg%, further doses should be withheld till the creatinine returns to 10% of baseline.[37]
Another dreaded side effect of ZA is osteonecrosis of jaw (ONJ). Duration of therapy, dosage, and dental extraction during therapy are the risk factors associated with ONJ. Oral examination before the initiation of therapy, extraction of non-restorable teeth before therapy, and a 2-3 weeks gap between extraction and initiation of therapy have been proposed to reduce the risk of osteonecrosis.[45] Antimicrobial rinses with chlorhexidine, antibiotics, surgical debridement, and laser therapy are recommended by the American Association of Oral and Maxillofacial Surgeons for the management of ONJ.[46]
Denosumab
As RANKL is an important regulator of osteoclast activity, RANKL inhibition is a rational strategy in the management of osteoclast-mediated bone complications. Denosumab is a fully human monoclonal IgG2 antibody with a high affinity for human RANKL. It has a longer circulatory half-life (46 days) compared to bisphosphonates, and suppresses bone turnover markers for 84 days.[47]
Ease of subcutaneous administration (120 mg subcutaneously every 4 weeks), lack of nephrotoxicity, and absence of acute phase reaction are the other advantages of Denosumab over Bisphoshonates. However, ONJ does occur with denosumab also.
Denosumab received FDA approval for the treatment of patients with bone metastases derived from solid tumors in November 2010 following the positive results of a randomized trial where its efficacy was compared with zoledronate in breast cancer patients with bone metastases.[48]
Denosumab Trial 103 compared denosumab (120 mg SC every 4 weeks) with ZA (4 mg IV every 4 weeks) in 1901 men. The primary objective of this trial was to demonstrate non-inferiority of denosumab compared with ZA, whereas the secondary objective was to evaluate superiority of Denosumab and comparative safety and tolerability of the two drugs. The primary end point was time to first SRE. Median time to first SRE was significantly better in the denosumab arm (20.7 months vs. 17.1 months; P = 0.001 for non-inferiority, P = 0.008 for superiority). OS and overall disease progression were equivalent. Men treated with denosumab experienced higher incidence of hypocalcemia (12.8% vs. 5.8%) and a nonsignificant trend toward higher ONJ (2.3% vs. 1.3%; P = 0.09).[49] An improved efficacy of Denosumab compared with Bisphosphonates was also reported in a phase II trial of patients with multiple tumor types – PCa (45%), breast cancer (40%), and other tumors (15%).[50] Further analysis of a subset of patients with PCa (n = 50) from this trial revealed the superiority of Denosumab compared with Bisphosphonates for normalization of bone turnover based on urinary biomarkers.[51] This was achieved in 69% of patients in the denosumab group and in only 19% of patients in the bisphosphonate group. The first on-study SRE rate observed in this subset of patients was 3% and 19% in the Denosumab and Bisphosphonate groups, respectively.[51]
More recently, the result of Denosumab 147 trial, evaluating the role of this drug in preventing metastases in non-metastatic CRPC, was published.[52] In this phase 3, double-blind, randomized, placebo-controlled study, 1432 men with non-metastatic castration-resistant PCa at high risk of bone metastases (PSA ≥8.0 ng/dl or PSA doubling time ≤10.0 months or both) were enrolled at 319 centers from 30 countries. Patients were randomly assigned (1:1) via an interactive voice response system to receive subcutaneous denosumab 120 mg or subcutaneous placebo every 4 weeks. The primary end point was bone-metastases-free survival, a composite end point determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause.
Denosumab significantly increased bone-metastases-free survival by a median of 4·2 months, compared with placebo (median 29·5 months vs. 25·2 months; P = 0.028). Denosumab also significantly delayed time to first bone metastasis (33·2 months vs. 29·5 months, P = 0.032). OS did not differ between groups (denosumab, 43.9 months vs. placebo, 44.8 months, P = 0.91). Rates of adverse events and serious adverse events were similar in both groups, except for ONJ and hypocalcemia. Thirty-three (5%) patients on denosumab developed ONJ versus none on placebo. Hypocalcemia occurred in 12 (2%) patients on denosumab and 2 (<1%) on placebo.[52]
A request seeking FDA approval for using denosumab to prevent metastases in patients with non-metastatic CRPC had been filed. However, a recent news article reported that a panel of cancer experts from FDA voted 12 to 1 against expanding the current indications of the drug since the benefits of this drug did not outweigh its risks, which included bone disease (ONJ) in about 6% of patients. Panelists termed this delay in bone metastases a “statistical benefit,” but not one that resulted in increased survival or higher quality of life for patients.[53] A final decision in this regard is expected in April 2012 after FDA reviews the application.
ROLE OF PALLIATIVE RADIOTHERAPY TO BONE IN METASTATIC PROSTATE CANCER
Palliative radiotherapy is being used commonly to relieve local pain from uncomplicated bone metastases at single or multiple sites, in metastatic spinal cord compression, and impending pathological fractures. 70-80% of patients who receive local radiotherapy to relieve bone pain respond to it and up to one-third achieve a complete response. A single dose of 8 Gy is adequate and optimal for palliation for the patient with localized metastatic bone pain. There is generally no role for multifraction treatment in uncomplicated bone metastasis.[54] A large proportion of patients receiving single-dose radiotherapy for metastatic bone pain are likely to require retreatment. Overall, around 25% of patients are retreated after a single dose of radiation. Retreatment is both feasible and effective after single doses of 8 Gy, even over the spinal cord, and a further single dose of 8 Gy or a fractionated schedule of 20 Gy in five fractions is perfectly safe.[55] Retreatment should be considered in all patients who have persistent or recurrent bone pain. Generally, response reaches a plateau at 4-6 weeks after treatment, and so patients should be encouraged to wait at least 4 weeks after treatment before considering retreatment. Often patients have pain at multiple sites, flitting from one site to another. In such patients, local radiotherapy is unsatisfactory and may require multiple treatment visits. Wide-field or hemi-body radiotherapy should be considered in such patients. In patients with metastatic spinal cord compression, surgical decompression is the treatment. However, patients with a poor performance status, poor survival prognosis, and involvement of multiple spinal segments are candidates for radiotherapy. Metastatic lesions with a high risk of fracturing require elective surgical stabilisation. Painful low-risk lesions, however, can be treated conservatively using external beam radiotherapy. Radiotherapy is also given after surgical stabilization to induce remineralization of the fractured bone and to stabilize the osteosynthetic prosthesis.
SYSTEMIC RADIONUCLIDE THERAPY
Radiation therapy is known to reduce tumor size and decrease osteolysis and skeletal tumor burden. Some radiopharmaceuticals when systemically administered are preferentially taken up at areas of exposed hydroxyapatite resulting from metastasis. An effective agent will specifically target tumor cells in metastatic bone lesions and spare the adjacent bone marrow.
Most commonly used agents are beta-emitters, Strontium 89 and Samarium 153, and alpha-emitter, Radium 223. These agents have been shown to palliate pain due to bone metastasis.[56] As these agents also irradiate the bone marrow, myelosuppresion is the most prominent toxicity of these agents.
Beta-emitting agents, Strontium 89 (89Sr) and Samarium 153 (153Sm), have been approved for palliation of pain due to bone metastases. 89Sr has shown an increased OS in patients with CRPC. 153Sm has also shown an increased disease-free survival with a trend toward increased OS in patients with CRPC and bone metastases.[57,58] Further, radionuclide therapy in combination with other therapy has shown favorable results. 153Sm in combination with docetaxel showed marked improvement in bone pain and a trend toward increased OS.[59]
Radium 223 or alpharadin (223Ra) is different from 89Sr and 153Sm in being an alpha-emitting agent. Alpha emission has a limited range compared to beta emission, and so the marrow-related toxicity is lesser.
Alpharadin in symptomatic PCa (ALSYMPCA) is a notable recent phase III study in which 900 patients were randomly assigned 2:1 to 223Ra (six IV administrations separated by 4-week intervals) or to placebo. The primary end point was OS. In June 2011, the study was stopped early on the basis of the recommendation of an independent data monitoring committee after a preplanned interim efficacy analysis. Compared with placebo, 223Ra chloride was associated with improved OS (median, 14.0 months vs. 11.2 months; P = 0.0022).[60] It not only significantly prolonged time to first SRE, but also significantly prolonged three out of four SRE components, i.e. time to pathological fracture, time to spinal cord compression, and time to external beam radiation. Hematologic toxicity consisted of neutropenia in 4% of patients and thrombocytopenia in 8%. The most common non-hematologic toxicities were bone pain (43% with radium-223 vs. 58% in the placebo group), diarrhea (22% vs. 13%), nausea (34% vs. 32%), vomiting (17% vs. 13%), and constipation (18% in both groups). The significant improvement in OS in the 223Ra arm was an unexpected but encouraging result. This discreet advantage over the already FDA-approved drugs, denosumab and ZA, has brought 223Ra to the forefront and the results of this interim analysis are expected to expedite its FDA approval for treatment of metastatic PCa.
Selective estrogen receptor modulator
Raloxifene and Toremifeneare orally administered selective estrogen receptor modulators (SERMs) that exert estrogenic effects on bone, and have been shown to improve BMD in men on ADT.[61,62] In a randomized, placebo-controlled trial on 1389 men receiving ADT, toremifene significantly reduced the number of new vertebral fractures, when compared with placebo (2.5% vs. 4.9%; P < 0.05). Toremifene also significantly increased BMD at the lumbar spine by 2% and at the hip by 1.6%. It decreased breast pain, decreased hot flashes, and caused favorable changes in lipid profile.[63] No SERM has yet been approved by FDA for reduction of osteoporotic fracture risk in men receiving ADT for PCa.
ROLE OF DIETARY SUPPLEMENTS
Because calcium is a large constituent of bone mass and Vitamin D improves intestinal calcium absorption, adequate intake of calcium and Vitamin D is necessary to maintain healthy bone mass. Therefore, Ca and VitD supplements are considered an essential component of an ADT-induced bone loss prevention or treatment strategy. NCCN recommends 1200 mg of supplemental calcium daily and 800-1000 IU of vitamin D3 daily in all men >50 years of age on ADT.[36] Further, all men on Denosumab should also be given supplemental calcium and vitamin D3, as this drug is known to cause hypocalcemia.
THERAPEUTIC AGENTS TO TREAT BONE COMPLICATIONS IN PROSTATE CANCER: WHAT DOES THE EVIDENCE SAY?
While evaluating the clinical utility of various bone-targeted therapies, four clinical implications need to be considered:
Can the available drugs reduce the incidence of SREs in metastatic CRPC?
Can these drugs reduce the incidence of SREs in metastatic androgen-sensitive PCa?
Can these drugs prevent metastasis in patients with non-metastatic CRPC?
Can these drugs prevent fragility fractures in men receiving ADT?
Presence of pathological fracture, radiation or surgery to bone, and spinal cord compression have been considered SREs in most studies.
Tables 3-6 summarize the salient studies addressing these four clinical questions.
Table 3.
Prevention of SREs in metastatic CRPC
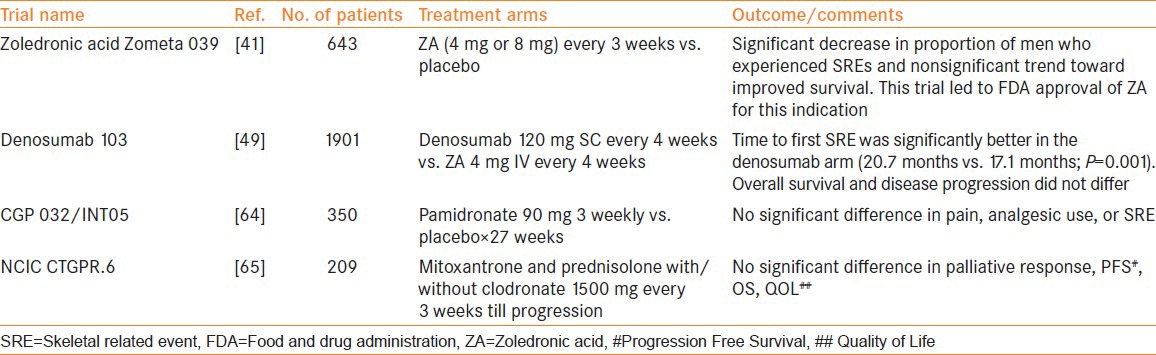
Table 6.
Prevention of fragility fractures in men on ADT

Table 4.
Prevention of SREs in androgen-sensitive prostate cancer

Table 5.
Prevention of metastasis in non-metastatic CRPC
NEWER THERAPEUTIC APPROACHES FOR BONE COMPLICATIONS IN PROSTATE CANCER
Endothelin a receptor-targeted therapies
Endothelin 1 is secreted by the epithelial cells of normal prostate, and its signaling modulates vasomotor tone, nociception, hormone production, and cellular proliferation. It has a role in activation of osteoblasts and formation of bone metastases via its activation of endothelin 1 (ETA) receptor.[68] ETA receptor antagonists Atrasentan and Zibotentan are thus being investigated for their likely beneficial role in the management of bone metastases. Results of phase III trials of both these drugs have been disappointing.[69]
SRC-targeted therapies
SRC (pronounced “sarc” as it is short for sarcoma) is a tyrosine kinase inhibitor that promotes several mechanisms involved in metastasis, like cell proliferation and survival, cell adhesion, migration, invasion, and dissemination to distant organs.[70] Dasatinib is one of the several inhibitors of SRC that are in clinical development for the treatment of prostate and breast cancer.[71] It has been shown to suppress markers of bone turnover and is currently under study in a phase III trial for men with CRPC that compares docetaxel monotherapy and docetaxel with dasatinib.[72] The results of this trial are expected in December 2012.
Saracatinib and bosutinib are the other SRC inhibitors being evaluated as potential therapeutic agents in bone metastasis.[73,74]
WHAT DO THE GUIDELINES SAY?
Changes in the recent EAU and NCCN guidelines speak volumes about the growing concern for management of bone health in PCa patients. Table 1 summarizes EAU and NCCN 2011 recommendations.
CONCLUSION
Maintaining an optimum bone health in patients with metastatic PCa and in those on ADT should be an important consideration for the treating physician. Appropriate use of imaging modalities like PET/CT and MRI can help detect bone metastasis with greater accuracy. WHO/FRAX nomogram should be utilized to identify candidates at risk of fragility fractures so that appropriate treatment can be initiated. Current evidence supports the use of FDA-approved agents ZA and Denosumab in preventing SREs and fragility fractures. However, their approval for use in men with non-metastatic CRPC for preventing bone metastases is awaiting further evaluation. Amongst the newer therapeutic agents likely to have an impact on management of bone metastases, Radium 223, with its added advantage in improving OS, seems to be the most promising.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Smith MR, Brown GA, Saad F. New opportunities in the management of prostate cancer-related bone complications. Urologic Oncology: Seminars and Original Investigations. 2009;27:S1–20. [Google Scholar]
- 2.Pradhan M, Mandhani A, Chipde S, Kumar J, Ansari MS, Srivastava A, et al. Bone mineral densitometry at the time of instituting Androgen Deprivation Therapy in metastatic prostate cancer: Does practice pattern match the guidelines? Indian J Urol. 2012:S72. doi: 10.4103/0970-1591.105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 4.Clarke NW, McClure J, George NJ. Osteoblast function and osteomalacia in metastatic prostate cancer. Eur Urol. 1993;24:286–90. doi: 10.1159/000474311. [DOI] [PubMed] [Google Scholar]
- 5.Bae DC, Stein BS. The diagnosis and treatment of osteoporosis in men on androgen deprivation therapy for advanced carcinoma of the prostate. J Urol. 2004;172:2137–44. doi: 10.1097/01.ju.0000141515.67372.e5. [DOI] [PubMed] [Google Scholar]
- 6.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–4. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 8.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: Recommendations for diagnosis and therapies. Cancer. 2004;100:892–9. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 9.Kiralti BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–32. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 10.Berruti A, Dogliotti L, Bitossi R, Fasolis G, Gorzegno G, Bellina M, et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: Predictive role of bone resorption and formation markers evaluated at baseline. J Urol. 2000;164:1248–53. [PubMed] [Google Scholar]
- 11.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 12.Fontana A, Delmas PD. Markers of bone turnover in bone metastases. Cancer. 2000;88:2952–60. doi: 10.1002/1097-0142(20000615)88:12+<2952::aid-cncr11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Watts NB. Clinical utility of biochemical markers of bone remodeling. Clin Chem. 1999;45:1359–68. [PubMed] [Google Scholar]
- 14.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 15.Briganti N, Passoni M, Ferrari, Capitanio U, Suardi N, Gallina A, et al. When to perform bone scan in patients with newly diagnosed prostate cancer: External validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur Urol. 2010;57:551–8. doi: 10.1016/j.eururo.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jurgens H, et al. Whole body MR imaging for detection of bone metastases in children and young adults: Comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177:229–36. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 17.Thurairaja R, McFarlane J, Traill Z, Persad R. State-of-the-art approaches to detecting early bone metastasis in prostate cancer. BJU Int. 2004;94:268–71. doi: 10.1111/j.1464-410X.2003.04960.x. [DOI] [PubMed] [Google Scholar]
- 18.Venkitaraman R, Sohaib AG, Cook G. MRI or bone scan or both for staging of prostate cancer? J Clin Oncol. 2007;25:5837–8. doi: 10.1200/JCO.2007.14.3875. [DOI] [PubMed] [Google Scholar]
- 19.Schirrmeister H, Glatting G, Hetzel J, Nüssle K, Arslandemir C, Buck AK, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42:1800–4. [PubMed] [Google Scholar]
- 20.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-Fluoride PET/CT. J Nucl Med. 2006;47:287–97. [PubMed] [Google Scholar]
- 21.Frank JA, Ling A, Patronas NJ, Carrasquillo JA, Horvath K, Hickey AM, et al. Detection of malignant bone tumors: MR imaging vs scintigraphy. AJR Am J Roentgenol. 1990;155:1043–8. doi: 10.2214/ajr.155.5.2120933. [DOI] [PubMed] [Google Scholar]
- 22.Kattapuram SV, Khurana JS, Scott JA, El-Khoury GY. Negative scintigraphy with positive magnetic resonance imaging in bone metastases. Skeletal Radiol. 1990;19:113–6. doi: 10.1007/BF00197616. [DOI] [PubMed] [Google Scholar]
- 23.Tombal B, Rezazadeh A, Therasse P, Van Cangh PJ, Vandeberg B, Lecouvet FE. Magnetic resonance imaging of the axial skeleton enables objective measurement of tumor response on prostate cancer bone metastases. Prostate. 2005;65:178–87. doi: 10.1002/pros.20280. [DOI] [PubMed] [Google Scholar]
- 24.Lecouvet FE, Geukens D, Stainier A, Jamar F, Jamart J, d’Othée BJ, et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: Diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007;25:3281–7. doi: 10.1200/JCO.2006.09.2940. [DOI] [PubMed] [Google Scholar]
- 25.Washington, D.C: National Osteoporosis Foundation; 2003. Physician's Guide to Prevention and Treatment of Osteoporosis. [Google Scholar]
- 26.Higano CS. Management of bone loss in men with prostate cancer. J Urol. 2003;170:S59–63. doi: 10.1097/01.ju.0000097351.48848.1f. [DOI] [PubMed] [Google Scholar]
- 27.Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men—where are we? Osteoporos Int. 2006;17:1577–83. doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 28.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 29.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 31.Kanis JA, Johansson H, Oden A, De Laet C, Johnell O, Eisman JA, et al. A meta-analysis of milk intake and fracture risk: Low utility for case finding. Osteoporos Int. 2005;16:799–804. doi: 10.1007/s00198-004-1755-6. [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, et al. A family history of fracture and fracture risk: A meta-analysis. Bone. 2004;35:1029–37. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton IL, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: A meta-analysis. Osteoporos Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Prostate Cancer. 2010. [Last accessed 2012 Aug 23]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf .
- 37.Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80:1652–60. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann NY Acad Sci. 2007;1117:209–57. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 39.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer. 2003;98:1735–44. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 40.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 41.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 42.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: Long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–6. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saylor PJ, Lee RJ, Smith MR. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J Clin Oncol. 2011;29:3705–14. doi: 10.1200/JCO.2010.34.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Planas J, Trilla E, Raventós C, Cecchini L, Orsola A, Salvador C, et al. Alendronate decreases the fracture risk in patients with prostate cancer on androgen-deprivation therapy and with severe osteopenia or osteoporosis. BJU Int. 2009;104:1637–40. doi: 10.1111/j.1464-410X.2009.08622.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advisory Task Force on Bisphosphonate- Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–76. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappa B ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–8. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 48.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 49.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 51.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: Results of a randomized phase II trial. J Urol. 2009;182:509–15. doi: 10.1016/j.juro.2009.04.023. discussion 515-6. [DOI] [PubMed] [Google Scholar]
- 52.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet. 2011;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FDA panel votes against Amgen's Xgeva for prostate cancer. Los Angeles Times on the web. Feb 8 2012. Aug 22 FDA Panel votes against Amgen's Xgeva for prostate cancer. LosAngeles Timesontheweb. [Last accesed on 2012 Aug 22]. Available from: http://articles.latimes.com/2012/feb/08/business/la-fi-amgen-20120208 .
- 54.Haddad P, Wong RK, Pond GR, Soban F, Williams D, McLean M, et al. Factors Influencing the use of single vs multiple fractions of palliative radiotheraphy for bone metastases: A 5-year review. Clin Oncol (R Coll Radiol) 2005;17:430–4. doi: 10.1016/j.clon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Agarawal JP, Swangsilpa T, vander Linden Y, Rades D, Jeremic B, Hoskin PJ. The role of external beam radiotherapy in the management of bone metastases. Clin Oncol (R Coll Radiol) 2006;18:747–60. doi: 10.1016/j.clon.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010;40:89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: A randomised phase II trial. Lancet. 2001;357:336–41. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 58.Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, et al. Samarium153EDTMP in bone metastases of hormone refractory prostate carcinoma: A phase I/II trial. J Nucl Med. 1993;34:1839–44. [PubMed] [Google Scholar]
- 59.Tu SM, Mathew P, Wong FC, Jones D, Johnson MM, Logothetis CJ. Phase I study of concurrent weekly docetaxel and repeated samarium153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:3319–24. doi: 10.1200/JCO.2008.20.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doreen S. Bayer's investigational compound radium-223 chloride met its primary endpoint of significantly improving overall survival in a phase III trial in patients with castration-resistant prostate cancer that has spread to the bone. Bayer Health Care. [Last accessed on 2012 Aug 23]. Available from: http://press.healthcare.bayer.com/en/press/news-details-page.php/14214/2011-0301 .
- 61.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: A randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–6. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 62.Smith MR, Malkowicz SB, Chu F, Forrest J, Price D, Sieber P, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–5. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–21. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–84. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 65.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–42. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 66.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–76. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 67.Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–9. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 69.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: A double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–23. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Xia W, Unger P, Miller L, Nelson J, Gelman IH. The Src-suppressed C kinase substrate, SSeCKS, is a potential metastasis inhibitor in prostate cancer. Cancer Res. 2001;61:5644–51. [PubMed] [Google Scholar]
- 71.Saad F, Lipton A. SRC kinase inhibition: Targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36:177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–8. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang JC, Bai L, Yap S, Gao AC, Kung HJ, Evans CP. Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC3 bone model. Mol Cancer Ther. 2010;9:1629–37. doi: 10.1158/1535-7163.MCT-09-1058. [DOI] [PubMed] [Google Scholar]
- 74.Rabbani SA, Valentino ML, Arakelian A, Ali S, Boschelli F. SKI606 (Bosutiblocks prostate cancer invasion, growth, and metastasis in vitro and in vivo through regulation of genes involved in cancer growth and skeletal metastasis. Mol Cancer Ther. 2010;9:1147–57. doi: 10.1158/1535-7163.MCT-09-0962. [DOI] [PubMed] [Google Scholar]


