Abstract
Background:
Obstructive sleep apnea (OSA) is often not diagnosed in patients presenting for surgical procedures thereby increasing the incidence of adverse perioperative course. Early diagnosis of this disease is important in modifying anesthetic management as well as utilizing specific means which may decrease the complications and improve the patient outcome.
Methods:
Patients greater than eighteen years of age, ASA I-III scheduled for elective surgical procedures under anesthesia were randomly selected. Their demographic data, diagnosis and nature of surgery were noted in a semi-structured performa. They were then screened for the presence of OSA with the help of a STOP BANG questionnaire.
Results:
This study included two hundred four patients randomly selected. Slight female predominance was seen in this sample (55.4%). Mean age of the subjects was 42.7 years (SD=15.08). 24.5% subjects were at high risk for OSA (STOP-BANG>3) with a male predominance (72% versus 37% in low risk group; X2=18.62; P<0.001). High risk OSA subjects had higher prevalence of cardiovascular risk factors (57% vs. 11.7% in low risk group; X2=33.35; P<0.001). Similarly, this group had a higher prevalence of asthma and chronic obstructive pulmonary disease (COPD) (14% versus 3.8% in low risk group; X2=6.54; P=0.03). Prevalence of diabetes mellitus (22%) and hypothyroidism (6%) was also higher in this group (5.2% and 1.9% in low risk group respectively; X2=15.42; P<0.001).
Conclusion:
High degree of suspicion and knowledge of association of OSA and medical diseases may help in detection of such cases and decrease the rate of perioperative complications thus improving patients safety.
Keywords: Anaesthesia, complication, obstructive sleep apnea, STOP BANG
INTRODUCTION
Obstructive sleep apnea (OSA) is defined as occurrence of at least 5 episodes of apnea or hypoapnea per hour in association of symptoms attributed to sleep disordered breathing.[1] OSA is often not diagnosed in patients presenting for surgical procedures thereby increasing the incidence of adverse perioperative course.[2,3,4] Early diagnosis of this disease is important in modifying anesthetic management as well as utilizing specific means which may decrease the complications and improve the patient outcome.
OSA affects nearly 13-19% in India.[5,6] However, it is estimated that 82% of men and 92% of women with moderate-to-severe sleep apnoea have not been diagnosed.[7,8] Previous reports suggested that a sizable number of patients undergoing surgery suffer from OSA. Reported prevalence varied between 3.2% to as high as 24% depending upon the population studied.[9,10] These patients had longer duration of hospital stay, lengthened Post-anesthesia care unit (PACU) stay, un-explained Intensive-care unit (ICU) admissions, higher mortality rates in addition to cardiac, respiratory and neurological complications.[11,12] However, reports are conflicting and a few studies provide contradictory evidences.[13]
There is a paucity of Indian data on the prevalence of patients with OSA presenting for surgical procedures. With this background this study was planned to evaluate the prevalence of OSA in patients presenting to our institute for surgical procedures. We also studied the association between demographic profile, comorbid medical conditions, anthropometric measurements and the risk of OSA.
METHODS
This study was conducted in the department of anesthesia of a tertiary care teaching institute after seeking approval from Institutional Ethics Committee. The patients greater than eighteen years of age, ASA I-III scheduled for elective surgical procedures under anesthesia were randomly selected during the study period of three months (March 2012-June 2012). The patients were explained the purpose of the study and informed consent was obtained. Exclusion criteria were- children below age of eighteen years, pregnant females, history of substance dependence (except tobacco), cranial neurosurgical procedure or emergency surgery. Those not willing to provide consent were also excluded from the study.
Their demographic data, diagnosis and nature of surgery were noted in a semi-structured performa. They were then screened for the presence of OSA with the help of a questionnaire (vide-infra) in the presence of their bed partners or a reliable informant. History of COPD, hypertension, coronary artery disease, hypothyroidism and diabetes mellitus was specifically enquired and relevant medical records were examined. Relevant clinical examination was done as mentioned below.
Questionnaire
STOP-BANG questionnaire was used for the screening of OSA [Table 1]. Approval from the author of the questionnaire used in this study was also sought. (Chung et al. 2008). This questionnaire consists of four items that uncover the history regarding snoring, daytime tiredness, observed pauses in breath during sleep and history of high blood pressure (STOP). Other four variables were assessed on physical examination – Body mass index (BMI), age, neck circumference, and gender (BANG).
Table 1.
STOP-BANG questionnaire

Each variable was given a score of 1 or 0 depending on presence or absence of the characteristic assessed. A total score of greater than 3 was considered as high risk for OSA.
Physical examination
Patients were weighed using a calibrated spring scale that is commonly used in hospitals. Height was measured using a stadiometer after removing footwears. Both the variables were used to calculate BMI. Neck circumference was measured at the level of cricothyroid using a non-elastic tape. Abdominal girth was measured at the level of umbilicus.
Statistical analysis
Analysis was done with the help of SPSS version 17.0 (Illinois, USA). Descriptive statistics was calculated. For the continuous variables mean and standard deviations were calculated. Based upon the STOP-BANG score, the sample was divided into two categories-low risk and high risk for OSA. Chi-square was used to compare proportions and independent sample t test was used to compare means between both these groups.
RESULTS
Sample description
This study two hundred four patients randomly selected from the preoperative list. Slight female predominance was seen in this sample (55.4%). Mean age of the subjects was 42.7 years (SD=15.08). Majority of patients were belonging to the specialties of urological surgery (26%), general surgery (29.4%) and gynecology (18.1%). Rests of the patients were from other specialties like neurosurgery, oncosurgery, otorhinolaryngology, plastic surgery and orthopedics.
Cardiovascular problems i.e., systemic hypertension and coronary artery disease was found in 21.1%, COPD in 3.4%. Comorbid endocrinal disorders were diabetes mellitus (9.3%) and hypothyroidism (2.9%). 13.2% patients complained of frequent headaches. 17.2% subjects were regular smokers and 11.3% were alcohol abusers but not dependent.
Comparison of high risk and low risk group
In the study 24.5% subjects were at high risk for OSA (STOP-BANG >3) with a male predominance (72% vs. 37% in low risk group; X2=18.62; P<0.001) in this group. High risk OSA subjects had higher prevalence of cardiovascular risk factors (57% vs. 11.7% in low risk group; X2=33.35; P<0.001). Similarly, this group had a higher prevalence of asthma and COPD (14% vs. 3.8% in low risk group; X2=6.54; P=0.03). Prevalence of diabetes mellitus (22%) and hypothyroidism (6%) was also higher in this group (5.2% and 1.9% in low risk group respectively; X2=15.42; P<0.001). Surprisingly, alcohol abuse was more frequent in high risk group (24% vs. 7.1% in low risk group; X2=10.72; P=0.001). Table 2 depicts the comparison of other variables. It is interesting to note that neck circumference and abdominal girth had a linear relationship with BMI in both the groups [Figure 1].
Table 2.
Comparison of low risk and high risk groups
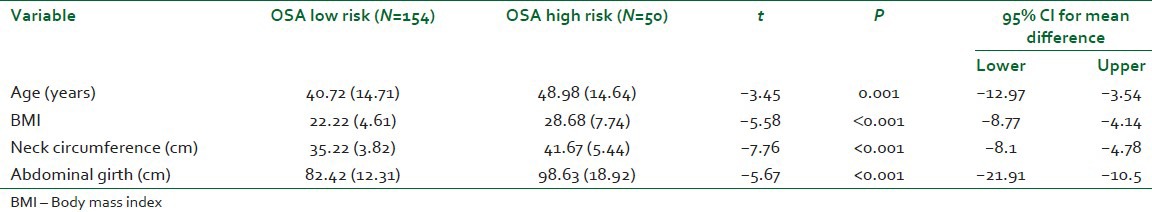
Figure 1.
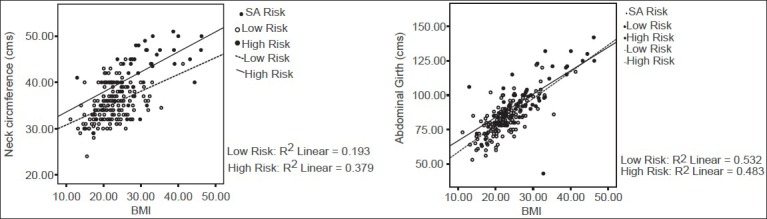
Relationship of BMI with neck-circumference and abdominal girth (N= 204)
Characteristics of high risk group
Since male gender is an established risk factor for the OSA, we compared the study variables between genders in high risk group. We did not find any difference with respects to age, BMI, neck-circumference and abdominal girth between genders. Prevalence of cardiovascular risk factors was higher in females (85.7% vs. 36.1% in males; X2=14.29; P=0.001). Endocrine disorders viz., diabetes and hypothyroidism were also more frequent among female subjects (35.7% and 21.4%, respectively as compared to 16.7% and 0% in males, respectively; X2=16.67; P=0.003).
DISCUSSION
This study demonstrated the prevalence of OSA in patients presenting for surgery in Indian setup. High risk patients had a higher prevalence of cardio-respiratory and endocrinal disorders. In addition, these subjects had higher BMI, larger neck circumference and abdominal girth. Male predominance was also seen in high risk group (72%). Earlier studies have reported varied prevalence of OSA in surgical patients, depending upon the methodologies adopted. In an academic center, nearly 23% surgical patients were screened positive for OSA.[10] On the other hand, 67% males and 28% female patients were found positive on screening for OSA in a dental clinic.[14] The prevalence was astronomically high (78%) in patients undergoing bariatric surgery because obesity is a known risk factor for OSA.[15] Contrary to common belief, this disorder is not limited to adults. Muntz et al.[16] reported that 22% children undergoing cleft surgeries were suffering from OSA. This data suggested that almost one in every four patient undergoing surgery was suffering from OSA. Further, the prevalence depends upon the population included in the study. In present study, sample was drawn randomly and ignoring type of surgical procedure. Our results confirmed the results of earlier study conducted in a teaching institution by Finkel et al.[10] In addition, our results suggested that at least surgical population in India is not different from the Western population with regards to risk of OSA. This is important to recognize considering the potential intra-operative and post-operative complications of OSA.[17] We found male predominance in high risk group. These subjects were fatter than the other group. Male gender and high BMI are known risk factors for OSA and this is why these characteristics have been included in the STOP-BANG questionnaire.[15,16,17,18] These patients had higher rates of cardio-vascular disorders and diabetes. It should be noted that untreated OSA is an independent risk factor for cardio-vascular illness through a myriad of mechanisms.[19,20] However, the condition is reversible and treatment of OSA was found to decrease the incidence of cardio-vascular disorders.[21] OSA is also known to impair glycemic control that was found to improve with the treatment.[22,23] Similarly, hypothyroidism is also known to precipitate OSA by inducing weight gain.[24] Hence, we suggest that subjects with OSA should be screened for cardio-vascular and diabetes. Similarly, subjects with diabetes, hypertension, coronary artery disease (CAD) and hypothyroidism should also be screened for OSA in clinics and during pre-anesthetic check-up.
Interestingly, anthropometric measurements were not different between genders in this study. Smaller sample size could be responsible for this finding and further research is desired in this area. We found that rates of cardiovascular disorders and endocrinal disorders were higher in women in high risk group. Whether female gender increases the cardio-vascular morbidity and diabetes in OSA patients is not known and, is clearly an area for future research. Although in our study, a number of other factors e.g., smaller sample size and perimenopausal age could have contributed to these finding.
Considering all these factors, we propose that a high degree of suspicion should be kept in patients presenting in preanaesthetic clinics. A number of physical factors like short thick neck, nasal obstruction, tonsillar hypertrophy, narrow oropharynx, retrognathia and obesity can be a clue. History of snoring also provide a clue to diagnosis.[25] These patients are also candidates for difficult intubation oxygen desaturation in the post operative period is a common occurrence,[26] as well as occurrence of cardiac arrhythmias,[27] haemodynamic instability, myocardial ischemia or infacction and increased incidence of intubation and mechanical ventilation.[28]
A drawback of the study was it was a single institutional data and cannot be generalized. Secondly, a bias may have been present in selection of patients as ours is a tertiary care teaching institute. We only calculated the incidence of OSA by STOP BANG but did not follow the patients in post operative course. A larger study is needed recruiting patients of higher BMI and their follow up in the perioperative period will give an idea of presentation of OSA in our Indian scenario.
In conclusion we wish to state that a high degree of suspicion and knowledge of association of OSA and medical diseases may help in detection of such cases and decrease the rate of perioperative complications thus improving patient's safety.
ACKNOWLEDGEMENT
We are thankful to Dr. Francis Chung for allowing us to use STOP-BANG in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Loube D, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indication for positive pressure treatment of adults obstructive sleep apnea patients: A consensus statement. Chest. 1999;115:863–6. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 2.Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in surgical patients. Implications for perioperative settings. Chest. 2006;129:198–205. doi: 10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]
- 3.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: A retrospective matched cohort study. Can J Anaesth. 2009;56:819–28. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 4.Memtsoudis S, Liu SS, Ma Y, Chiu YL, Walz JM, Gaber-Baylis LK, et al. Perioperative pulmonary outcome in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–21. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of modified Berlin questionnaire to identify patients at risk for obstructive sleep apnea syndrome. Indian J Med Res. 2006;124:281–90. [PubMed] [Google Scholar]
- 6.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep disordered breathing in community dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Evans L, Flinn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 9.Fidan H, Fidan F, Unlu M, Ela Y, Ibis A, Tetik L. Prevalence of sleep apnoea in patients undergoing operation. Sleep Breath. 2006;10:161–5. doi: 10.1007/s11325-006-0067-9. [DOI] [PubMed] [Google Scholar]
- 10.Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer-Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–8. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–63. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 12.Liu SS, Chisholm MF, John RS, Ngeow J, Ma Y, Memtsoudis SG. Risk of postoperative hypoxemia in ambulatory orthopedic surgery patients with diagnosis of obstructive sleep apnea: A retrospective observational study. Patient Saf Surg. 2010;4:9. doi: 10.1186/1754-9493-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96:1328–35. doi: 10.1213/01.ANE.0000061585.09157.66. [DOI] [PubMed] [Google Scholar]
- 14.Levendowski DJ, Morgan T, Montague J, Melzer V, Berka C, Westbrook PR. Prevalence of probable obstructive sleep apnea risk and severity in a population of dental patients. Sleep Breath. 2008;12:303–9. doi: 10.1007/s11325-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 15.Lopez PP, Stefan B, Schulman C, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: More evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834–8. [PubMed] [Google Scholar]
- 16.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008;118:348–53. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 17.Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: Why is it important? Curr Opin Anaesthesiol. 2009;22:405–11. doi: 10.1097/ACO.0b013e32832a96e2. [DOI] [PubMed] [Google Scholar]
- 18.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 19.Altintas N, Aslan E, Helvaci A, Malhotra A. Relationship between obstructive sleep apnea severity index and left ventricular function and volume. Ann Saudi Med. 2012;32:384–90. doi: 10.5144/0256-4947.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Med Rev. 2012 doi: 10.1016/j.smrv.2012.03.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priou P, Le Vaillant M, Meslier N, Chollet S, Masson P, Humeau MP, et al. The IRSR Sleep Cohort Group. Independent association between obstructive sleep apnea severity and glycated hemoglobin in adults without diabetes. Diabetes Care. 2012;35:1902–6. doi: 10.2337/dc11-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surani S, Subramanian S. Effect of continuous positive airway pressure therapy on glucose control. World J Diabetes. 2012;3:65–70. doi: 10.4239/wjd.v3.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resta O, Pannacciulli Gioia G, Stefàno A, Barbaro MP, De Pergola G. High prevalence of previously unknown subclinical hypothyroidism in obese patients referred to a sleep clinic for sleep disordered breathing. Nutr Metab Cardiovasc Dis. 2004;14:248–53. doi: 10.1016/s0939-4753(04)80051-6. [DOI] [PubMed] [Google Scholar]
- 25.Esclamado RM, Glenn MG, McCulloch TM, Cummings CW. Perioperative complications and risk factors in the surgical treatment of obstructive sleep apnea syndrome. Laryngoscope. 1989;99:1125–9. doi: 10.1288/00005537-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Chemiack NS. Respiratory dysrhythmia during sleep. N Engl J Med. 1981;305:325–30. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 27.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]


