Abstract
Objective:
A comparative study to evaluate the efficacy of dexmedetomidine as a hypotensive agent in comparison to esmolol in Functional Endoscopic Sinus Surgery (FESS).
Methods:
Forty patients ASA I or II scheduled for FESS were equally randomly assigned to receive either dexmedetomidine 1 μg/Kg over 10 min before induction of anesthesia followed by 0.4-0.8 μg/Kg/h infusion during maintenance (DEX group), or esmolol, loading dose 1mg/kg was infused over one min followed by 0.4-0.8 mg/kg/h infusion during maintenance (E group) to maintain mean arterial blood pressure (MAP) between (55-65 mmHg). General anesthesia was maintained with sevoflurane 2%-4%. The surgical field was assessed using Average Category Scale and average blood loss was calculated. Hemodynamic variables (MAP and HR); arterial blood gas analysis; plasma cortisol level; intraoperative fentanyl consumption; Emergence time and total recovery from anesthesia (Aldrete score ≥9) were recorded. Sedation score was determined at 15, 30, 60 min after tracheal extubation and time to first analgesic request was recorded.
Result:
Both DEX group and E group reached the desired MAP (55-65 mmHg) with no intergroup differences in MAP or HR. The for the quality of the surgical filed in the range of MAP (55-65 mmHg) were <=2 with no significant differences between group scores during hypotensive period. Mean intraoperative fentanyl consumption was significantly lower in DEX group than E group. Cortisol level showed no significant changes between or within groups. No significant changes were observed in arterial blood gases. Emergence time and time to achieve Aldrete score ≥9 were significantly lower in E group compared with DEX group. The sedation score were significantly lower in E group compared with DEX group at 15 and 30 minutes postoperatively. Time to first analgesic request was significantly longer in DEX group.
Conclusion:
Both dexmedetomidine or esmolol with sevoflurane are safe agents for controlled hypotension and are effective in providing ideal surgical field during FESS. Compared with esmolol, dexmedetomidine offers the advantage of inherent analgesic, sedative and anesthetic sparing effect.
Keywords: Controlled hypotension, dexmedetomidine, esmolol, functional endoscopic sinus surgery
INTRODUCTION
Functional endoscopic sinus surgery (FESS) is becoming a widely performed operation.[1] Its introduction associated with enhanced illumination and visualization has dramatically improved surgical dissection. However major complications have been reported for FESS under general anesthesia resulting from impaired visibility due to excessive bleeding.[2] Controlled hypotension is a technique used to limit intraoperative blood loss to provide the best possible field for surgery.[3,4] Benefits for controlled hypotension for FESS include reduction in blood loss with improved quality of surgical field. Various agents e.g., magnesium sulfate.[5] Vasodilators (sodium nitroprusside),[4] nitroglycerine,[6] high doses of potent inhaled anesthetics,[7] and beta adrenergic antagonist[8] have been used to achieve controlled hypotension. Some disadvantages have been reported of these techniques including delayed recovery from inhaled anesthetics, resistance to vasodilators, tachyphylaxis, and cyanide toxicity for nitroprusside. Esmolol is an ultrashort acting selective B1 adrenergic antagonist that reduces heart rate and blood pressure. It has rapid onset of action of bolus i.v. injection and infusion. Upon termination of infusion gradual recovery of arterial blood pressure to the pre infusion level occurred without development of rebound hypertension.[9,10]
Dexmedetomidine (DEX) is a potent highly selective α2 adrenergic receptor agonist. It has sedative, analgesic and anesthetic sparing effect, and sympatholytic properties.[11] The central and peripheral sympatholytic action of (DEX) is mediated by α2 adrenergic receptor and is manifested by dose-dependent decrease in arterial blood pressure, heart rate, cardiac output and norepinephrine release.[12,13]
The present work was designed to compare the efficacy and safety of dexmedetomidine or Esmolol as a hypotensive agent in FESS with attention on the amount of blood loss, quality of the surgical field, recovery profile, and tolerability in adult patients.
METHODS
This is a prospective, randomized, single blinded study done in Mansoura University Hospital, Egypt, during the period from December 2010 to November 2011. After approval of the local ethical committee, written informed consent was obtained from patients during the pre-anesthetic evaluation. Forty ASA physical status I or II patients aging 20-50 years scheduled for elective FESS. Patients with recurrent sinus surgery, hypertension, coronary artery diseases and renal, hepatic or cerebral insufficiency and patients with coagulopathies or receiving drugs influencing blood coagulation were excluded from the study. All patients had bilateral nasal polyposis with opacity of all paranasal sinuses. The patients were assessed clinically in addition to ECG, chest X ray and basal laboratory tests. Patients included in this study were randomly assigned according to computer generated randomization to receive either dexmedetomidine (DEX group n=20) or esmolol (E group n=20).
In the operating room, two cannulae were inserted, one for infusion of dexmedetomidine or esmolol and the other for administration of fluids and other drugs. A 22G radial artery catheter was inserted for continuous measurement of arterial blood pressure and blood sampling for arterial blood gas analysis. In DEX group, patients received loading dose of 1 μg/kg dexmedetomidine diluted in 10 ml 0.9% saline infused over 10 min before induction of anesthesia, followed by continous infusion of (0.4- 0.8 μg/kg/h). In E group, patients received esmolol as a loading dose 1 mg/kg was infused over 1 min followed by continuous infusion of (0.4-0.8 mg/kg/h). In both groups infusion rate was titrated to maintain MAP within 55-65 mmHg. All patients were premedicated with IV midazolam 0.05 mg/kg and fentanyl 2 μg/Kg. Patients received standard anesthetic technique with propofol 1-2 mg/kg supplemented if necessary by 0.2 mg/kg aliquots until loss of verbal response. The required induction doses of propofol were recorded. Endotracheal intubation was facilitated with atracurium 0.5 mg/kg with suitable sized cuffed tube. Anesthesia was maintained with sevoflurane 2-4%. All patients were mechanically ventilated with 60% air/O2 mixture. In both groups, signs of inadequate anesthesia as increase in the arterial pressure greater than the targeted MAP or somatic responses as Movement, tearing, or sweating) were treated with additional dose of fentanyl. Nitroglycerine was infused if these target limits could not be achieved with upper most doses. The drug infusion rate was then decreased when targeted MAP was achieved. Respiratory rate (RR) and tidal volume (TV) were adjusted according to body weight to maintain normocapnia. Patients received lactated Ringer's at 3 ml/kg and were placed in a 15° reverse Trendlenburg position to improve venous drainage. In both groups cottonoids soaked with epinephrine in a concentration of 1:80.000 was inserted into the nasal cavity and in between the polyps to minimize blood loss. Oropharyngeal pack was used. Endoscopic sinus surgeries in all patients were in the form of bilateral polypectomy, middle meatal antrostomy, complete ethmoidectomy and sphenoidotomy. The same surgeon performed all operations to ensure consistency in the estimation of the surgical field. He was blinded to the hypotensive agent used. When MAP reached the desired range (55-65 mmHg) and was maintained for at least 10 minutes, the surgeon estimated the quality of the surgical field using a predefined category scale adopted from that of Fromme et al.[14]
Average category scale for assessment of intraoperative surgical field:
0 - No bleeding
1 - Slight bleeding – no suctioning of blood required
2 - Slight bleeding – occasional suctioning required. Surgical field not threatened
3 - Slight-bleeding – frequent suctioning required. Bleeding threatens surgical field a few seconds after suction is removed
4 - Moderate bleeding – frequent suctioning required. Bleeding threatens surgical field directly after suction is removed
5 - Sever bleeding – constant suctioning required Bleeding appears faster than can be removed by suction. Surgical field severely threatened and surgery not possible.
The ideal category scale values for surgical conditions were predetermined to be two and three. The total blood loss was measured from the suction apparatus. Infusion of the study drugs was stopped five minutes before the anticipated end of surgery, and sevoflurane was stopped at the end of the surgery and the residual neuromuscular blockade was antagonized with neostigmine (0.05 mg/kg) and atropine (0.01 mg/kg).
Monitoring included invasive blood pressure measurement, heart rate, arterial blood gases. (PaO2, PaCo2, Ph and HCO3), plasma cortisol level and surgical field score (Avarage Category scale). Plasma cortisol level was measured in three samples taken preoperatively, during hypotensive period and postoperatively. Hemodynamics and (ABGs) were recorded preoperatively (baseline), postinduction (after administration of hypotensive and anesthetic agent), intraoperatively (15, 30, 45 and 60 min), 5 and 10 minutes after stoppage of hypotensive agents and lastly after recovery. Intraoperative fentanyl consumption and requirements for additional hypotensive agent (nitroglycerine) were recorded. Emergency time, defined as the interval between the discontinuation of anesthetics to response of eye opening to verbal command,[15] was recorded. After extubation and full recovery, patients were transferred to the postanesthesia care unit (PACU) to be observed where time to first analgesic rescue was recorded. Postoperative recovery was evaluated using a modified Alderet Score (0-10),[16] and time needed to achieve ≥9 was recorded. Sedation score[17] was measured using the following scale at 15, 30 and 60 minutes after tracheal extubation: 1=anxious, agitated, or restless; 2=cooperative, oriented, and tranquil; 3=responsive to commands; 4=a sleep, but with brisk response to light, glabellar tap, or loud auditory stimulus; 5=a sleep, sluggish response to glabellar tap, or auditory stimulus; and 6=a sleep, no response. Patients also were asked about recalling introperative events or any sign of awareness.
RESULTS
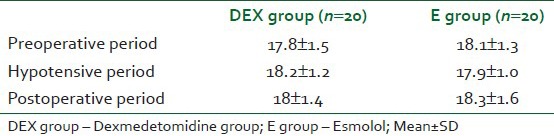
Fifty patients were assigned for study eligibility. Four patients refused to sign consent and six patients failed to meet the inclusion criteria. The remaining 40 patients who fulfilled the entry criteria were enrolled in this study. Patients were able to complete the entire study and their data were included in the final analysis. Patients of the study groups were comparable with respect to demographic data and operative data [Table 1]. The induction propofol dose was significantly lower in DEX group than E group (1.36±0.38 mg/kg) versus (2.32±0.42 mg/kg), respectively (P<0.001).
Table 1.
Demographic and operative data
Baseline values of MAP and HR were comparable in both groups. In DEX and E groups, there was a significant reduction of MAP in both groups compared to baseline value intraoperatively. Both groups reached the desired MAP (55-65 mmHg) with no intergroup significant differences after induction or during hypotensive period. In both groups, there was no need to use nitroglycerine as an additional hypotensive agent intraoperatively. At 5 and 10 minutes after stoppage of hypotensive agents, at end of surgery and after recovery, MAP was significantly lower in DEX group than E group [Figure 1]. Heart rate decreased significantly relative to baseline after administration of loading dose in both groups.
Figure 1.
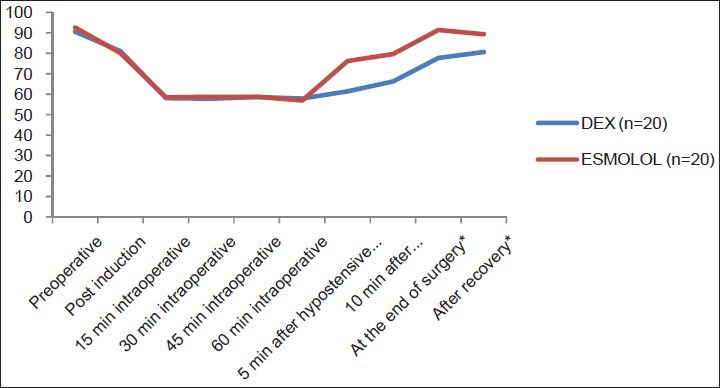
Mean arterial blood pressure (mmHg) of the studied groups
There were no intergroup significant differences in HR after induction or during the hypotensive period. HR showed significant increase in E group 5, 10 min after stoppage of hypotensive agent, at end of surgery and after recovery compared to DEX group [Figure 2].
Figure 2.
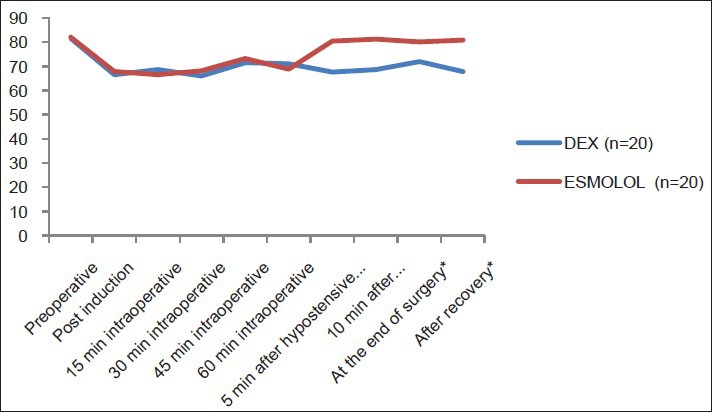
Heart rate (bpm) of the studied groups
Mean intraoperative fentanyl consumption in DEX group was significantly less than E group (25.0±2 μg vs. 60.0±3.5).
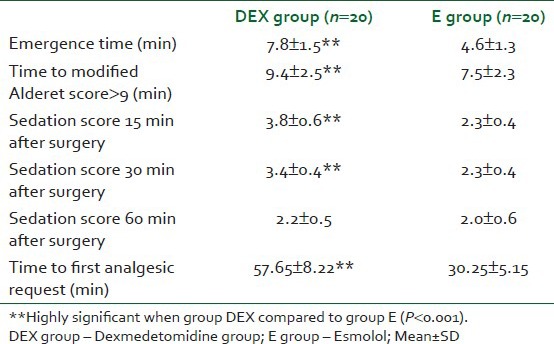
The average category scale (ACS) for quality of surgical field was comparable in both groups in the range of MAP (55-65 mmHg). Scores for a bloodless surgical field were low in both groups; there was no significant difference in between group scores. The median range of scores was 2 (1-3) in both groups. The scores were <=2 through the hypotensive period [Table 2]. There was no significant difference in the amount of blood loss intraoperatively in both groups [Table 2]. No patients presented with excessive blood loss. No significant changes were observed in ABGs (pH, PaCo2, PaO2 and HCO3) for patients in both groups. The plasma cortisol level showed no significant change between the two groups or in the same group during different phases of the study [Table 3]. Emergence time and time needed to achieve ≥9 of modified Aldrete score were significantly shorter in E group than DEX group. (4.6 (1.30) min and 7.5 (2.3) min. versus (7,8 (1.50) min. and 9.4 (2.5) min., respectively) (P<0.01).
Table 2.
Average category scale (0-5) during hypotesive anesthesia periods [Median (Range)]
Table 3.
Serum cortisol (ug/dl)
The mean postoperative sedation scores were significantly lower in E group than in DEX group at 15 min (2.3 (0.4) vs. 3.8 (0.6) min. and at 30 min. 2.3 (0.4) vs, 3.4 (0.4) min (P<0.01). No significant difference was observed in sedation score at 60 min in both groups in both groups no patient complains of any sign of awareness. Time recorded to first analgesic request was significantly shorter in E group than DEX group ((30.25±5.15 min) versus (57.6 ± 58.22 min)) respectively (P<0.01) [Table 4]. No postoperative nausea or vomiting observed in both groups.
Table 4.
Recovery characteristics, sedation scores and time to first analgesic request
DISCUSSION
A lot of efforts have been done to optimize the surgical conditions for FESS. Induced hypotension has been widely advocated to control bleeding during FESS to improve the quality of surgical field.[18,19] In our prospective randomized study of dexmedetomidine or esmolol in combination with sevoflurane we planned to provide this optimal surgical field. Both drugs were effective in achieving MAP of 55 to 65 mmHg, and lowering the heart rate ensured good surgical condition and providing dry surgical field during FESS.
Patients who were treated with dexmedetomidine 10 min before induction of anesthesia had significant decrease in MAP and HR after administration of loading dose. This dexmedetomidine induced hemodynamic profile can be attributed to the known sympatholytic effect of α2 agonists. The α2-receptors are involved in regulating the autonomic and cardiovascular systems. Alpha 2 receptors are located on blood vessels, where they mediate vasoconstriction, and on sympathetic terminal, where they inhibit, norepinephrine release.[20] At lower doses, the dominant action of α2 agonist is sympatholysis.[21] Basar et al.,[22] investigated the effect of single dose of dexmedetomidine 0.5 μg/kg administration 10 min before induction of anesthesia and reported significant reduction in MAP and HR. The efficacy of dexmedetomidine in providing better surgical and less blood loss during controlled hypotension was previously reported during tympanoplasty, septoplasty and maxilofascial surgery.[23,24,25] In the current study, the induction dose of propofol was significantly lower in DEX group than in E group. This effect coinciding with the result of Peden et al.,[26] who reported that dexmedetomidine caused a reduction in the overall dose of propofol required to produce loss of consciousness. Guven et al.[27] and Goksu et al.,[28] reported better hemodynamic stability, visual analog scale for pain and clear surgical field with less side effects in DEX group than placebo group when FESS done under either conscious sedation or local anesthesia respectively. In our study we achieved ASC of <=2 during hypotensive period for quality of surgical field with little bleeding that did not hamper the visual clarity during the surgery. Esmolol administration in the present study was associated with significant decrease in MAP and HR compared to baseline values.
Esmolol lowers arterial blood pressure through a decrease in cardiac output secondary to negative chronotropic and ionotropic effects of β adrenergic antagonism. It provided a stable course of controlled hypotension and produces beneficial effects in the surgical field and in blood conservation.[4,29] The optimal anesthetic technique seems to be relative bradycardia with associated hypotension.[30] In the present study intraoperative fentanyl consumption was significantly less in DEX group compared with E group. Several studies have found that perioperative use of dexmedetomidine was associated with a significant decrease in the consumption of inhalational agent, fentanyl, and analgesic in dose dependent manner.[31,32]
No significant changes were detected in the plasma cortisol level between the two groups during the intra and postoperative periods, this was attributed to the sympathoadrenal blocking action of both dexmedetomidine and esmolol inhibiting the release of catecholamine and other stress hormones.[11,12,29]
This study demonstrated prolonged postoperative analgesia in (DEX) group. This is in accordance with Gurbet et al.,[33] who stated that intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Also the analgesic effects of dexmedetomidine had been appreciated in various setting and various population.[34,35,36,37]
Dexmedetomidine was associated with significant longer emergence time and time to total recovery from anesthesia compared to esmolol.
Richa et al.,[13] reported that extubation time was significantly slower in patients receiving dexmedetomidine compared with those receiving remifentanyl for controlled hypotension. In the present study patients of DEX group had significant higher postoperative sedation scores than those in E group. Dexmedetomidine has sedative and analgesic sparing effects via central actions in the locus ceruleus and in the dorsal horn of the spinal cord.[38]
CONCLUSION
This study demonstrated that dexmedetomidine or esmolol with sevoflurane are safe agents for controlled hypotension and both are effective in providing ideal surgical field during FESS. Compared with esmolol dexmedetomidine offers the advantage of inherent analgesic, sedative and anesthetic sparing effect.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Stammberger H, editor. Functional Endoscopic sinus surgery. Philadelphia: BC Decker; 1991. pp. 321–33. [Google Scholar]
- 2.Stankiewicz JA. Complication of Endoscopic Intranasal Ethmoidectomy. Laryngoscope. 1987;97:1270–3. doi: 10.1288/00005537-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Tobias JD. Controlled hypotension in children: A critical review of available agents. Paediatric Drugs. 2002;4:439–53. doi: 10.2165/00128072-200204070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Degoute CS, Ray MJ, Manchon M, Dubreuil C, Banssillon V. Remifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplasty. Can J Anaesth. 2001;48:20–7. doi: 10.1007/BF03019809. [DOI] [PubMed] [Google Scholar]
- 5.Elsharnouby NM, El Sharnouby MM. Magnesium sulphate as a technique of hypotensive anesthesia. Br J Anaesth. 2006;96:727–31. doi: 10.1093/bja/ael085. [DOI] [PubMed] [Google Scholar]
- 6.Degoute CS, Dubreuil C, Ray MJ, Guitton J, Manchon M, Banssillon V. Effect of posture, hypotension and locally applied vasoconstriction on the middle ear microcirculation in anaesthetized humans. Eur J Appl Physiol Occup Physiol. 1994;69:414–20. doi: 10.1007/BF00865405. [DOI] [PubMed] [Google Scholar]
- 7.Pavlin JD, Colley PS, Weymuller EA, Jr, Van Norman G, Gunn HC. Propofol versus isoflurane for endoscopic sinus surgery. Am J Otolaryngol. 1999;20:96–101. doi: 10.1016/s0196-0709(99)90018-2. [DOI] [PubMed] [Google Scholar]
- 8.Degoute CS. Controlled hypotension: Guide to drug choice. Drugs. 2007;67:1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ornstein E, Young WL, Ostapkovich N, Matteo RS, Diaz J. Deliberate hypotension in patients with intracranial arteriovenus malformations: Esmolol compared with isoflurane and sodium nitroprusside. Anesth Analg. 1991;72:639–44. doi: 10.1213/00000539-199105000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Blowey DL. Anihypertensive agents: Mechanism of action, safety profiles, and current uses in children. Curr Ther Res Clin Exp. 2001;62:298–313. [Google Scholar]
- 11.Bloor BC, Ward DS, Belleville JP, Maze M. Effect of intravenous dexmedetomidine in humans. 11. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Schmelling WT, Kampine JP, Roerig DL, Warltier DC. The effect of the stereoisomers of the α2-adrenergic agonist dexmedetomidine on systemic and coronary haemodynamics in conscious dogs. Anesthesiology. 1991;75:499–511. doi: 10.1097/00000542-199109000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Richa F, Yazigi A, Sleilaty G, Yazbeck P. Comparison between dexmedetomidine and remifentanil for controlled hypotension during tympanoplasty. Eur J Anaesthesiol. 2008;25:369–74. doi: 10.1017/S0265021508003761. [DOI] [PubMed] [Google Scholar]
- 14.Fromme GA, MacKenzie RA, Gould AB, Jr, Lund BA, Offord KP. Controlled hypotension for orthognatic surgery. Anesth Analg. 1986;65:683–6. [PubMed] [Google Scholar]
- 15.Chung F. Are discharge criteria changing? J Clin Anesth. 1993;5:645. doi: 10.1016/0952-8180(93)90011-3. [DOI] [PubMed] [Google Scholar]
- 16.Alderete JA. The post-anesthesia recovery score revisted. J Clin Aesth. 1995;7:89. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay M, Savege T, Simpson BR, Good R. Controlled sedation with alphaxolone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhart LH, Folz BJ, Wulf H, Geldner G. Intravenous anesthesia provides optimal surgical conditions during microscopic and endoscopic sinus surgery. Laryngoscope. 2003;113:1369–73. doi: 10.1097/00005537-200308000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Cincikas D, Ivaskevicius S. Application of controlled arterial hypotension in endoscopic rhino-surgery. Medicina (Kaunas) 2003;39:852–9. [PubMed] [Google Scholar]
- 20.Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980;32:337–62. [PubMed] [Google Scholar]
- 21.McCallum JB, Boban N, Hogan Q, Schmeling WT, Kampine JP, Bosnjak ZJ. The mechanism of alpha-2-adrenergic inhibition of sympathetic ganglionic transmission. Anesth Analg. 1998;87:503–10. doi: 10.1097/00000539-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O. The effect of preanaesthetic single dose dexmedetomidine on induction, hemodynamic and cardiovascular parameters. J Clin Anesth. 2008;20:431–6. doi: 10.1016/j.jclinane.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septoplasty. Eur J Anaesthesiol. 2007;24:447–53. doi: 10.1017/S0265021506002122. [DOI] [PubMed] [Google Scholar]
- 24.Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y. Effectivness of dexmedetomidine in reducing bleeding during spetoplasty and tympanoplasty operations. J Clin Anesth. 2008;20:437–41. doi: 10.1016/j.jclinane.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Richa F, Yazigi A, Hage C. Dexmedetomidine an agent for controlled hypotension in maxilla fascial surgery. Eur J Anaesthesiol. 2004;21:A–242. [Google Scholar]
- 26.Peden CJ, Cloote AH, Stratford N, Prys-Roberts C. The effect of intravenous dexmedetomidine premedication on the dose of requirements of propofol to induce loss of consciousness in patients receiving alfentanil. Anaesthesia. 2001;56:408–13. doi: 10.1046/j.1365-2044.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- 27.Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2011;120:586–92. doi: 10.1177/000348941112000906. [DOI] [PubMed] [Google Scholar]
- 28.Goksu S, Arik H, Demiryurek S, Mumbuc S, Oner U, Demiryurek AT. Effects of dexmedetomidine infusion in patients undergoing functional endoscopic sinus surgery under local anaesthesia. Eur J Anaesthiol. 2008;25:22–8. doi: 10.1017/S0265021507001317. [DOI] [PubMed] [Google Scholar]
- 29.Boezaart AP, Merwe JV, Coetzee A. Comparison of sodium nitropruside and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can J Anaesth. 1995;42:373–6. doi: 10.1007/BF03015479. [DOI] [PubMed] [Google Scholar]
- 30.Timperley D, Sacks R, Parkinson RJ, Harvey RJ. Perioperative and intraoperative maneuvers to optimize surgical outcomes in skull base surgery. Otolaryngol Clin North Am. 2010;43:699–730. doi: 10.1016/j.otc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Aantaa R, Jaakola ML, Kallio A, Kanto J. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997;86:1055–60. doi: 10.1097/00000542-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Scheinin H, Jaakola ML, Sjövall S, Ali-Melkkilä T, Kaukinen S, Turunen J. Intramuscular dexmedetomidine as premediation for general anaesthesia. A comparative multicenter study. Anesthesiology. 1993;78:1065–75. doi: 10.1097/00000542-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 34.Huncke TK, Adelman M, Jacobowitz G, Maldonado T, Bekker A. A prospective randomized, placebo-controlled study evaluating the efficacy of dexmedetomidine for sedation during vascular procedures. Vasc Endovasc Surg. 2010;44:257–61. doi: 10.1177/1538574410363621. [DOI] [PubMed] [Google Scholar]
- 35.Taghinia AH, Shapiro FE, Slavin SA. dexmedetomidine in anaesthetic facial surgery: Improving anaesthetic safety and efficacy. Plast Reconstre Surg. 2008;121:269–76. doi: 10.1097/01.prs.0000293867.05857.90. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Vázquez ME, Hernández-Salazar E, Hernández-Jiménez A, Pérez-Sánchez A, Zepeda-López VA, Salazar-Páramo M. Clinical analgesic efficacy and side effects of dexmedetomidine in the early postoperative period after arthroscopic knee surgery. J Clin Anesth. 2007;19:576–82. doi: 10.1016/j.jclinane.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anaesthesia care combined with tramadol via patient – controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus coeruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]


