Abstract
Posterior reversible encephalopathy syndrome presents with a variety of neurologic features, which, although devastating at some point, are potentially reversible on prompt recognition and institution of appropriated treatment. We report the management of three cases occurring in the last 4 years in our tertiary university hospital.
Keywords: Anesthesia and hypertensive disorders, posterior leukoencephalopathy syndrome, posterior reversible encephalopathy syndrome
INTRODUCTION
The development of focal neurologic deficits in a postpartum patient is a rare event. The differential diagnosis of this condition includes partial seizure, ischemic and hemorrhagic stroke, cerebral venous thrombosis, migraine, eclampsia, cerebral artery dissection, encephalitis and posterior leukoencephalopathy syndrome. Prompt diagnosis and treatment for most of these diseases is crucial, but clinicians often fail to suspect the last one.[1,2,3] We report three cases of peripartum leucoencephalopathy syndrome in our tertiary university hospital during the last 4 years.
CASE REPORTS
Case 1
A 29-year-old woman, G2 P1, at the 35th week of gestation with a history of pregnancy-induced hypertension, was admitted to the emergency department due to two generalized seizures episodes at home without prodromal symptoms. According to the patient's husband, the patient has presented malaise and headache during the past week.
The patient's blood pressure was 180/100 mmHg at admission. Obstetric ultrasound was normal. The laboratory test concerning renal and liver function was normal, except for the presence of 2 + proteinuria. The electrocardiogram showed sinus tachycardia. Emergency cesarean delivery was indicated due to eclampsia. Rapid sequence induction with 200 mg of propofol and 100 mg of succinylcholine was administered and anesthesia was maintained with oxygen and nitrous oxide at a concentration of 50%. 1.5% of sevofluorane and 150 mcg of fentanyl were administered after the umbilical cord was clamped. After induction and during surgery, systolic blood pressure was between 140 mmHg and 130 mmHg. A 2000 g male infant with Apgar scores of 7 at 10 min was delivered. The scalp pH was 7.17 and appropriate resuscitation was performed. The patient was admitted extubated at the Post Anesthesia Care Unit (PACU) with blood pressure over 170/110 mmHg, conscious, hemodynamically stable and disoriented, with a coherent conversation but with blurred vision. No focal neurological symptoms were seen at this time. Her blood pressure decreased from 170/110 mmHg to 140/100 mmHg after an intravenous administration of magnesium sulfate first bolus of 4 gr followed by an infusion of 1 g/24 h associated with intravenous infusion of labetalol and nitroglycerin according to our protocol. The laboratory test in the Intensive Care Unit (ICU) was normal. After surgery, a cranial computerized tomography (CT) and magnetic resonance imaging (MRI) scans were performed because the patient was still Hypertensive and had headache and confusion. The CT scan showed findings consistent with posterior reversible encephalopathy in the context of eclampsia, and brain MRI confirmed these findings [Figure 1]. Ophthalmological examination was requested showing moderate hypertensive retinopathy. An echocardiogram was also performed showing slight pericardial effusion, normal left ventricular size and normal systolic function, moderate functional mitral regurgitation and pulmonary artery pressure slightly elevated. Three days after the seizures, her neurologic examination became normal. Her blood pressure gradually normalized but laboratory values still showed slight anemia and mild proteinuria. She was treated orally with amlodipino 10 mg/day and labetalol 200 mg bid from the second day of stay at the ICU. One week later, the MRI was repeated and results were normal. Nine days later, the patient was discharged home without any neurological sequel.
Figure 1.
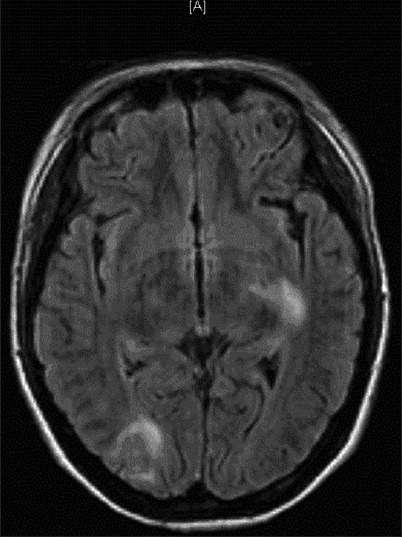
Brain magnetic resonance. The figure shows a normal cerebellar parenchyma with small hyperintense images in the pontine tegmentum and hypersignals in the occipital lobes, with extension to the posterior temporal region on the right side. Left posterior capsular regions, corona radiate and the capsular caudate nucleus area were affected. The injuries were mainly due to vasogenic edema in relation to reversible encephalopathy
Case 2
A 45-year-old woman, G1 P0, at the 39th week of gestation, was admitted to the emergency department after a generalized seizure at home without prodromal symptoms. After seizure, she presented drowsiness. According to the patient's husband, the patient was presenting progressive and widespread edema in the prior weeks but she did not suffer any hypertensive episode or neurologic symptom. Cesarean section was indicated due to eclampsia 1 h from the admission. General anesthesia was performed with rapid intubation sequence using 100 mg of succinylcholine and 200 mg of propofol and 150 ug of fentanyl after the umbilical cord was clamped, without any complications. She maintained systolic blood pressure between 110 mmHg and 80 mmHg during anesthesia. An intravenous administration of magnesium sulfate first bolus of 4 gr followed by a infusion of 1 g/24 h was started. A live male infant with significant clinical deterioration was delivered. The newborn umbilical cord pH was 6.75 and he was transferred to the pediatric ICU for appropriate resuscitation. Emerge from anesthesia in the operating room was delayed for no apparent reason, and once the patient was awake, she was stirred and confused without apparent focal neurological deficit. The patient was admitted extubated to the PACU, conscious, with agitation, disorientation, incoherent speech, exaltation of the patellar reflexes and blurred vision. No neurological focal deficit at that time of clinical assessment was found. The patient presented obesity and foveal edema. The administration of magnesium sulfate was continued and an intravenous infusion of labetalol was added. Despite this treatment, her blood pressure remained high. Dexamethasone 4 mg was added to the treatment. The laboratory test at admission was altered with creatinine 2.12 mg/dL, potassium 5.1 mEq/L, magnesium 5.88 mg/dL and sodium 130 mEq/L, pH 7.26 and HCO3 13 mEq/L, elevated LDH and 3 + proteinuria.
Six hours after surgery, a cranial CT, brain MRI, electroencephalogram and angioresonance were performed. The cranial CT scan was normal. The brain MRI showed subcortical hyperintense lesions, on T2-weighted sequences, with different characteristics [Figure 2]. The electroencephalogram showed signs of brain involvement lateralized in the right hemisphere and the angioresonance was normal. In the PACU, her blood pressure remained high. Creatinine values increased to 3.2 mg/dL; however, these values normalized within 2 days and the creatinine clearance at discharge was 155 mL/min, with persistence of proteinuria 305 mg/mL and albumin 1.4 g/dL. Intravenous infusion of antihypertensive drugs was removed and she was treated orally with amlodipine 10 mg/day, labetalol 200 mg tid, irbesartan 300 mg/day and enalapril 10 mg bid. Because of persistent high blood pressure and renal impairment, a renal Doppler ultrasound was also performed, which showed good bilateral arterial and venous supply. The resistance indices and acceleration, as well as the morphology of the wave, were normal. Due to the patient's improvement, she was transferred to the gynecology ward. The patient was discharged without any neurological deficits.
Figure 2.
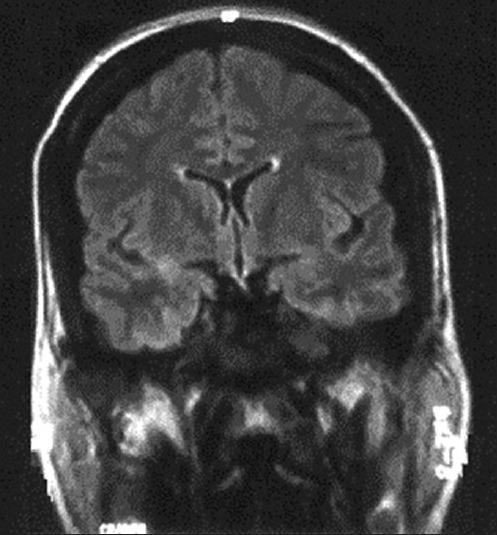
Brain magnetic resonance. The figure shows subcortical hyperintense lesions on T2-weighted sequences, with different characteristics. One draws the subcortical white matter with preservation of U fibers, at the left parietal-occipital, and the other has rounded morphology and was located in right temporal white matter. None of them suggests hemorrhagic transformation
Case 3
A 35-year-old woman, G2 P1, at 39 weeks’ gestation, was admitted to the emergency department with labor work. Her medical record showed no significant disease and her prenatal history was unremarkable. An epidural catheter was placed and epidural analgesia was administered. The blood test was normal. During labor, her systolic blood pressure remained high, with values of 170/90 mmHg being recorded. This blood pressure persisted and intravenous 5 mg hydralazine was administered. A 2700 g newborn was delivered alive with Apgar scores of 7 at 10 min. The umbilical cord pH was 7.26. He was transferred to a pediatric clinic where appropriate resuscitation was performed.
The patient was transferred to the obstetric ward 2 h after delivery, with persisting high blood pressure and headache. Alphamethyldopa 500 mg orally was administered to control arterial pressure, but, 20 min later, the patient had a seizure and intravenous 10 mg of diazepam was administered to control the same. The patient recovered, presenting confusion, and was then admitted to the PACU with stupor, without focal neurological and mild lower limb edema. A cranial CT scan was performed, which showed findings consistent with posterior reversible encephalopathy [Figure 3]. In the laboratory blood test, the hemoglobin, platelet count and prothrombin activity were 11.7 mg/dL, 67,000 × 109/L and 60%, respectively. The patient also presented elevated liver enzymes and proteinuria. Elevated blood pressure persisted, requiring intravenous infusion of labetalol (25 mg bolus dose over 2-5 min). An intravenous infusion of magnesium sulfate was also initiated for 24 h. At 48 h, the intravenous infusions were suspended and oral treatment was initiated with amlodipine 10 mg/day and irbesartan 300 mg/day.
Figure 3.
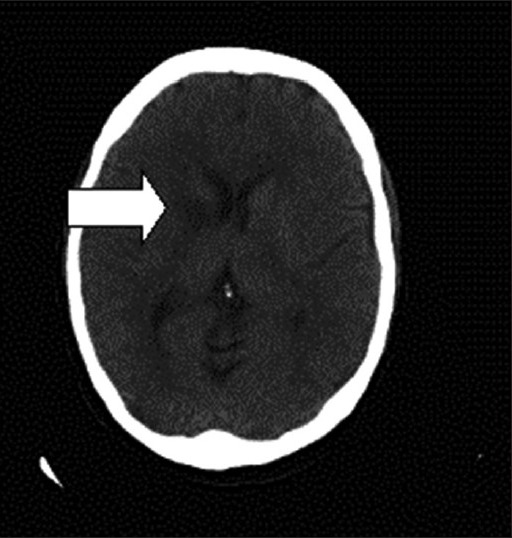
Cranial computerized tomography scan. The figure shows areas that are hypodense in the white matter surface, predominantly bilateral posterior and right frontal, as well as in the right internal white capsule, suggestive of acutesubacute ischemic lesions in the context of reversible posterior leukoencephalopathy
Because of the improvement of patient, she was transferred to the gynecology ward on the third day. Five days later, an MRI was performed. Results were normal. The patient was discharged without any neurological deficits.
DISCUSSION
Hinchey et al. first reported a reversible posterior leukoencephalopathy syndrome in 1996.[4] This name was changed to posterior reversible encephalopathy syndrome (PRES) in 2000, which is now the most widely accepted terminology. The reported incidence of PRES is around 0.01%.[1] At our teaching hospital, we have about 7000 deliveries per year. In the last 4 years, we have had only three cases of PRES. Most cases of PRES are associated with hypertensive disorders, particularly those occurring during pregnancy. In most obstetrics cases found in the literature, there is a history of preeclampsia, and PRES usually develops only after delivery,[5] as has been reported in our cases. Before PRES was first defined in 1996, there were several case reports describing the MRI finding of posterior leukoencephalopathy as an interesting anomaly in the eclamptic patient.[6] In the literature, this clinical feature has also been described in normotensive patients in association with drug-induced[4] or human immunodeficiency virus-associated immunosuppression, with thrombotic thrombocytopenic purpura/hemolytic uremic syndrome[1] and acute intermittent porphyria. The most common clinical symptoms and signs are headache, altered alertness and behavior ranging from drowsiness to stupor, seizures, vomiting, mental abnormalities including confusion and diminished spontaneity and speech, and abnormalities of visual perception. The onset is usually subacute but may be heralded by a seizure. Seizures are common at the onset of neurologic symptoms but can also develop later. Lethargy and somnolence are often the first signs noted, but stupor and frank coma may also develop. Hemianopia, visual neglect and frank cortical blindness may occur. The tendon reflexes are often brisk and some patients have weakness and incoordination of the limbs.[4] Although sometimes preceded by headache, vomiting and confusion, the presenting cases of PRES developed suddenly and included blindness, generalized or focal seizures, impairment of consciousness and, occasionally, motor signs. The main point is that early accurate diagnosis and treatment of PRES may prevent brain damage, hemorrhage and infarction.[7] Early neuroimaging is therefore indicated in cases of profound depression of consciousness in pregnancy or if there are atypical features or visual disturbance in eclamptic presentations.[8] When patients present with a focal neurologic deficit, mainly if visual impairment appears, a CT scan must be performed to rule out a hemorrhagic or ischemic stroke. However, the CT scan is rarely abnormal and MRI is the imaging modality of choice.[9] Distinguishing PRES from acute stroke is especially important to guide treatment. Mild-to-moderate hypertension should not be treated in ischemic stroke but must be managed aggressively in PRES.[7] PRES is an uncommon but devastating condition in which an excellent outcome may be obtained if the correct diagnosis is made. In cases associated with pregnancy-related hypertensive disorders, magnesium sulfate should be used as prophylaxis or treatment of seizures. Seizures should be managed actively in all cases because status epilepticus could occur in more than half of the patients.[5] The clinical differential diagnoses that should be considered are: Vascular diseases as infarct, especially “top of the basilar syndrome” with bilateral ischemia, hemorrhage (congophilic parieto occipital lobar intracerebral hemorrhage) or venous thrombosis. Other differential diagnosis are infections (encephalitis, meningitis), inflammatory or autoimmune diseases like postinfectious encephalomyelitis, vasculitis, etc.[10] An epinephrine-induced case has been reported[11] in the literature recently. Preeclampsia is a progressive disorder that will inevitably worsen if pregnancy continues. Delivery is the definitive management and is followed by resolution, generally over a few days but sometimes taking much longer. At mature gestational age, delivery should not be delayed. Even so, it is important to control severe hypertension and other maternal derangements before subjecting the woman to the stress of delivery.[12] Most of the times, prolongation of pregnancy in the presence of preeclampsia carries no benefit for the mother but is desirable to improve the fetal prognosis because, in general, fetal outcome is proportional to gestational age at delivery.[13] In cases of preeclampsia before 34 weeks, delivery should be delayed for at least 24-48 h if maternal and fetal status permit, to allow fetal benefit from antenatal corticosteroids administered for lung maturation.[14] Recent studies show decrease in the incidence of cerebral palsy when magnesium sulfate was used as a tocolytic agent.[15] In many cases, the timing of delivery will be based upon several factors, such as maternal and/or fetal, rather than a single absolute indication for delivery. Aggressive antihypertensive treatment should be commenced in all women with a systolic blood pressure ≥170 mmHg or a diastolic blood pressure ≥110 mmHg because of the risk of intracerebral hemorrhage and eclampsia.[14] While there is no controlled trial to determine how long severe hypertension may be left untreated, it is recommended that treatment be administered promptly, aiming for a gradual and sustained lowering of blood pressure. Drugs for the treatment of very high blood pressure in pregnancy have been the subject of a Cochrane review, which concluded that no good evidence exists to prove that any short-acting antihypertensive is better than another.[16] The agent of choice for the acute treatment of hypertension is oral nifedipine. This is administered as a 10 mg oral dose initially, with a repeat dose of 10 mg if there is inadequate response after 30 min. Headache is a frequent side-effect. Intravenous labetolol is the agent of choice for intravenous administration. This is administered as a 20-50 mg bolus dose over 2 min. The third agent of choice is hidralacine. This is administered as an intravenous dose of 5-10 mg every 20-30 min to control hypertension of >170 systolic and/or 110 diastolic. Magnesium sulfate therapy is recommended for use antepartum and intrapartum and within the first 24 h postpartum for severe preeclampsia when the following factors are present: (1) persistently elevated blood pressure despite adequate hypotensive therapy and appropriate fluid management and (2) evidence of central nervous system dysfunction, thrombocytopenia or liver disease.[17] In our institution, we use the following therapeutic protocol. As intravenous Treatment, we use labetalol 20 mg loading dose followed by a continuous perfusion between 40 mg/h and 100 mg/h and magnesium sulfate 4 g loading dose followed by a continuous perfusion 1 g/h for 24 h. We also hydralazine 5-10 mg every 20-30 min for the control of hypertensive peaks. And, finally, when adequate control of blood pressure is not achieved, we also use nitroglycerin 0.25-0.5 mcg/kg/min, increasing the dose to 0.5-1 mcg/kg/min every 3-5 min, to a maximum dose of 5 mcg/kg/min. When the blood pressure is controlled, an oral treatment is initiated - labetalol 100 mg q.d.s. to a maximum of 200 mg q.d.s., amlodipine 10 mg q.d. to a maximum of 10 mg bid and irbesartan 150-300 mg od. Home treatment is with labetalol at the same dose with alfa metil dopa 250 mg od. In our case, the first patient was managed with labetalol orally and in the second and third cases with the same drug in intravenous perfusion. All cases reported by us presented good outcome. As a conclusion, PRES in an uncommon but devastating condition in which an excellent outcome may be obtained with a prompt diagnosis and an appropriated management of the hypertension disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. Am J Neuroradiol. 2000;21:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 2.Lipstein H, Lee CC, Crupi RS. A current concept of eclampsia. Am J Emerg Med. 2003;21:223–6. doi: 10.1016/s0735-6757(02)42241-3. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: Evaluation with diffusion-tensor MR imaging. Radiology. 2000;219:756–65. doi: 10.1148/radiology.219.3.r01jn48756. [DOI] [PubMed] [Google Scholar]
- 4.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 5.Striano P, Striano S, Tortora F, De Robertis E, Palumbo D, Elefante A, et al. Clinical spectrum and critical care management of Posterior Reversible Encephalopathy Syndrome (PRES) Med Sci Monitor. 2005;11:CRR 549–53. [PubMed] [Google Scholar]
- 6.Hara N, Fugii T, Tsutsumi O. Postpartum eclampsia associated with cortical blindness. Int J Obstet Gynecol. 1994;47:287–8. doi: 10.1016/0020-7292(94)90576-2. [DOI] [PubMed] [Google Scholar]
- 7.Stott VL, Hurrel MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: A misnomer reviewed. Int Med J. 2005;35:83–90. doi: 10.1111/j.1445-5994.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 8.Karnal DR, Guntupalli KK. Neurologic disorders in pregnancy. Crit Care Med. 2005;33:S362–71. doi: 10.1097/01.ccm.0000182790.35728.f7. [DOI] [PubMed] [Google Scholar]
- 9.Chambers KA, Cain TW. Postpartum blindness: Two cases. Ann Emerg Med. 2004;43:243–6. doi: 10.1016/s0196-0644(03)00633-4. [DOI] [PubMed] [Google Scholar]
- 10.Prout RE, Tuckey JP, Giffen NJ. Reversible posterior leucoencephalopathy syndrome in a peripartum patient. Int J Obstet Anesth. 2007;16:74–6. doi: 10.1016/j.ijoa.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Gharabawy R, Pothula VR, Rubinshteyn V, Silverberg M, Gave AA. Epinephrine-induced posterior reversible encephalopathy syndrome: A case report. J Clin Anesth. 2011;23:505–7. doi: 10.1016/j.jclinane.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Lowe SA, Brown MA, Dekker G, Gatt S, McLintock CK, McMahon L, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–6. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 13.Duley L, Meher S, Abalos E. Management of pre-eclampsia. BMJ. 2006;332:463–8. doi: 10.1136/bmj.332.7539.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD001449.pub2. CD001449. [DOI] [PubMed] [Google Scholar]
- 15.Thomas J, Garite C. Andrew Combs Obstetric Interventions Beneficial to Prematurely Delivering Newborn Babies: Antenatal Corticostetroids, Progesterone, Magnesium Sulfate. Clin Perinatol. 2012;39:33–45. doi: 10.1016/j.clp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with preeclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet. 2002;359:1877–79. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]


