Abstract
Multiciliate cells (MCCs) are highly specialized epithelial cells that employ hundreds of motile cilia to produce a vigorous directed flow in a variety of organ systems. The production of this flow requires the establishment of planar cell polarity (PCP) whereby MCCs align hundreds of beating cilia along a common planar axis. The planar axis of cilia in MCCs is known to be established via the PCP pathway and hydrodynamic cues, but the downstream steps required for cilia orientation remain poorly defined. Here, we describe a new component of cilia orientation, based on the phenotypic analysis of an uncharacterized coiled-coil protein, called bbof1. We show that the expression of bbof1 is induced during the early phases of MCC differentiation by the master regulator foxj1. MCC differentiation and ciliogenesis occurs normally in embryos where bbof1 activity is reduced, but cilia orientation is severely disrupted. We show that cilia in bbof1 mutants can still respond to patterning and hydrodynamic cues, but lack the ability to maintain their precise orientation. Misexpression of bbof1 promotes cilia alignment, even in the absence of flow or in embryos where microtubules and actin filaments are disrupted. Bbof1 appears to mediate cilia alignment by localizing to a polar structure adjacent to the basal body. Together, these results suggest that bbof1 is a basal body component required in MCCs to align and maintain cilia orientation in response to flow.
Keywords: Cilia, Xenopus, Planar cell polarity
INTRODUCTION
Multiciliate cells (MCCs) extend hundreds of motile cilia in order to produce robust fluid flow along luminal surfaces in several organ systems, including the lung airways, the ependymal lining of the brain and the female reproductive tract. The flow produced in these tissues is invariably directed along a specific organ axis, requiring mechanisms that orient beating cilia at the apical surface along points orthogonal to the planar axis (Marshall and Kintner, 2008). Failure to properly orient beating cilia leads to chaotic or misguided flow that may contribute to human diseases such as primary ciliary dyskinesia.
The planar orientation of ciliated cells and cilia requires components of the conserved planar cell polarity (PCP) pathway. Proteins in the PCP pathway localize differentially in epithelial cells at sites of cell-cell contact, adopting a head-to-tail arrangement along the planar axis (Bayly and Axelrod, 2011; Zallen, 2007). A similar planar localization of PCP components occurs in epithelial cells with motile cilia (Guirao et al., 2010; Momose et al., 2012; Vladar et al., 2012), and this pattern likely arises prior to cilogenesis (Vladar et al., 2012). Altering PCP signaling causes cilia misorientation, as seen by a misalignment of cells along a tissue axis, rather than by a misalignment of cilia within a given cell (Guirao et al., 2010; Mitchell et al., 2009; Momose et al., 2012; Vladar et al., 2012). Moreover, as perturbing PCP components can influence the planar orientation of ciliated cells non-cell-autonomously, it is likely that PCP acts at sites of cell-cell contact to cue planar polarity (Mitchell et al., 2009). However, some PCP components also localize to the base of the cilium, raising the possibility that they function at each cilium, or even by trafficking in and out of the cilium (Guirao et al., 2010; Wallingford and Mitchell, 2011). In line with this possibility, cilia orientation within a given cell can be randomized when some PCP components are disrupted cell autonomously, consistent with a defect in the orientation of individual cilia rather than a loss of tissue orientation (Park et al., 2008; Wallingford and Mitchell, 2011). How PCP components might operate at the cilium is still ill-defined, and may differ significantly from the instructive role that the PCP pathway plays at sites of cell-cell contact.
When MCCs first differentiate, cilia orientation is initially imprecise, although biased in one direction, presumably in response to the PCP pathway described above (Mitchell et al., 2007; Vladar et al., 2012). If cilia function is impaired, thus blocking flow, cilia orientation fails to improve, remaining biased along a tissue axis but unrefined within a given cell (Guirao et al., 2010; Mitchell et al., 2007). Conversely, the orientation of cilia in MCCs is extremely responsive to an externally imposed flow, as long as the cilia are mobile (Guirao et al., 2010; Mitchell et al., 2007). The response to flow allows cilia in MCCs to acquire a precise orientation: in this model, flow acts during a plastic refinement period in which cilia produce and respond to flow in a positive-feedback loop. The orienting effect of flow on cilia in MCCs is quite robust, but the underlying mechanism is completely unknown.
The cues that orient cilia ultimately impinge on the basal body: the cylindrical structure that docks at the apical plasma membrane, templates outgrowth of the ciliary axoneme and determines the orientation of cilia beating (Marshall, 2008). Cilia orientation is dictated by appendages that attach to the basal body at specific polar points, and that mediate interaction with polarized networks of actin- and microtubule-based filaments (Marshall and Kintner, 2008). One such attachment point is the basal foot, a subdistal appendage that extends off the cylindrical wall of the basal body in the direction of ciliary flow. Loss of the basal foot, as occurs in Odf2 mouse mutants, leads to ciliary disorientation (Kunimoto et al., 2012). The basal foot has long been known to be an attachment point for microtubules, and a more recent study describes a link to a specific population of tyrosinated microtubules that extend in a planar fashion to localized PCP components (Vladar et al., 2012). Disrupting microtubules, using nocodazole, disrupts both the establishment and maintenance of cilia orientation (Vladar et al., 2012; Werner et al., 2011). A second appendage attached to basal bodies is the striated rootlet, a structure located at the proximal base, and extending in a planar direction opposite to the basal foot and the direction of flow. The striated rootlet interacts with a subapical actin network and disruption of this network with drugs also leads to mispositioning and misorientation of basal bodies (Werner et al., 2011). These and other observations suggest that the planar orientation of cilia is driven by complex interactions between basal bodies and dynamic networks of microtubule- and actin-based filaments. How these networks form and are directed by PCP signaling or by hydrodynamic cues to dictate ciliary orientation remains largely unknown.
To identify factors that are required to initiate and maintain ciliary orientation, one approach is to examine in more detail the genes activated in cells when motile cilia form, with the idea that these encode products required for motile cilia function, including basal body positioning. As foxj1, a winged-helix transcription factor, is sufficient to induce genes required for motile cilium formation, we have used microarrays to identify foxj1 targets in the Xenopus larval skin (Stubbs et al., 2008), a model system for studying MCC differentiation (Werner and Mitchell, 2012). Here, we describe a gene upregulated by foxj1 during motile cilia formation that encodes a small uncharacterized coiled-coil protein, annotated as ccdc176, but which we have termed basal body orientation factor 1 (bbof1) for reasons discussed below. Bbof1 is conserved among vertebrates but has not yet been studied in any system. Here, we examine the phenotypes that occur in MCCs when bbof1 activity is reduced or misexpressed. The results from these phenotypes suggest that bbof1 functions as a novel cilia orientation factor that is not required to respond to PCP or flow-based orientation cues, but functions to establish and maintain basal body alignment.
MATERIALS AND METHODS
Xenopus embryos, microinjection and drug treatment
The embryos of Xenopus laevis were obtained by in vitro fertilization using standard protocols (Sive et al., 1998) and staged according to Nieuwkoop and Faber (Nieuwkdop and Faber, 1967). Embryos were typically injected at the two- to four-cell stage, targeting animal blastomeres, with 1-5 ng of capped synthetic mRNA or with 40-50 ng of morpholinos (Gene Tools) injected separately. Bbof1 morpholinos (supplementary material Table S2) were designed against the start of translation (bbof1-MOatg) or a splice donor site between exon 7 and intron 7 in the bbof1 pre-mRNA (bbof1-MOspl). The spag6 morpholino has been described previously (Mitchell et al., 2007). For drug treatment, stage 23 embryos were incubated at 16°C in 0.1×MMR containing 1 μM of nocadozole (Sigma-Aldrich) or 5μM of cytochalasin D prepared from a 1000× stock dissolved in 100% DMSO.
RNA transcripts
bbof1 (Unigene Xl.66678) was previously identified as a gene upregulated in animal caps by foxj1 (Stubbs et al., 2008). A bbof1 cDNA was PCR amplified from a stage 17 library using the primers listed in supplementary material Table S2, cloned, sequenced and inserted into pCS2-GFP-N1 or into pCS2-MT to add GFP or 6 myc tags, respectively, to the N terminus. Tsga10 (unigene Xl.23696) was identified previously (Stubbs et al., 2012), amplified by PCR from a stage 17 library using primers listed in supplementary material Table S2 and cloned in pCS2-GFP-N3 to add GFP to the N terminus. Templates for RNA encoding centrin4-RFP, clamp-GFP, membrane-localized RFP (mRFP) and Hyls1-GFP have been described previously along with the methods to linearize the templates and generate synthetic mRNAs in vitro (Stubbs et al., 2012).
In situ hybridization
Albino Xenopus embryos were injected at the two-cell stage with RNA encoding foxj1, along with nlacZ RNA as a tracer, or alone as a control (Stubbs et al., 2008). Injected embryos were fixed at stage 22, reacted with X-gal to reveal β-galactosidase activity, and then probed for the expression of bbof1 RNA, or α-tubulin RNA as a control, using digoxigenin-labeled probes and in situ hybridization using standard methods (Sive et al., 1998), except the RNase digestion step was omitted.
Total RNA preparation and quantitative RT-PCR
Xenopus embryos were injected at the two-cell stage with either RNA encoding the activated form of Notch (intracellular domain of Notch, ICD), or with ICD and foxj1 RNA (Sive et al., 1998). Animal caps were isolated at stage 10, incubated to the equivalent of stage 14, 18, 22 and 24, and then extracted for total RNA using proteinase K digestion, LiCl precipitation and further treatment with RNase-free DNase. Three μg of RNA was used as a template to generate cDNA using Superscript III reverse transcriptase, and then assayed in triplicate using real-time PCR in an ABI Prism 7900HT Thermal Cycler, based on primers for bbof1, α-tubulin or ubiquitously expressed ODC as a normalization control (supplementary material Table S2). Data analysis was performed with the program Applied Biosystems Sequence Detection System software.
Basal body number, cilia length, cilia beat frequency, flow rate and cilia orientation
Basal body number and density were measured in embryos injected at the two-cell stage with Hysl1-GFP and mRFP RNA to label the basal bodies and cell membranes, respectively, and then fixed at stage 30 with 3.7% formaldehyde and 0.25% glutaraldehyde in PBT solution (PBS with 0.1% TritonX-100) for 10 minutes (referred to as quickfix hereafter). Confocal microscopy was used to image six to eight MCCs from each of three to five embryos. Cilia length was measured in stage 29-30 embryos after fixation in 4% paraformaldehyde and staining with a mouse monoclonal against acetylated tubulin as described previously (Stubbs et al., 2008). Cilia beat frequency was measured as described previously (Werner et al., 2011). Briefly, MCCs on the flank of stage 30 embryos were imaged using a Nikon A1R laser scanning confocal microscope and a 60× oil plan-Apo objective. Beating of fluorescently labeled cilia was imaged using resonance scanning confocal microscopy at 240 frames per second over a 5 seconds time period. Images were acquired using Nikon Elements software. To determine flow velocity, we used a Leica 165FC and a DFC295 digital camera to visualize the displacement of 10 μm yellow green fluorescent 505/515 FluoSpheres polystyrene microspheres (Invitrogen) along the skin of live embryos. Flow movies were acquired using Leica Application Suite software and flow velocities were scored using Nikon Elements Software. Cilia orientation was measured by imaging embryos injected with RNAs encoding centrin-RFP to label basal bodies, and either GFP-tagged clamp or tsga10 to label the rootlet. Embryos were quickfixed, washed and mounted in PVA/DABCO. In some cases, embryos were also stained with phalloidin 647. Images were capture at 63×, using a Biorad Radiance or Zeiss LSM710 confocal microscope, and used to measure cilia orientation based on the relative position of clamp-GFP (Tsga10-GFP) to centrin-RFP. For each cell analyzed, the angle of basal body orientation was measured for all basal bodies (150-200/cell) using a Matlab program and circular statistics of these orientations was analyzed by CircStats package of program R as described previously (Lund and Agostinell, 2001; Mitchell et al., 2007).
Stage 16 explants and flow chamber
Ventral skin was explanted from stage 16 embryos onto coverslip glass coated with fibronectin and incubated in Danilchik’s for Amy (DFA) supplemented with 0.1% BSA, as described previously (Mitchell et al., 2007). The anterior-posterior axis of each explant was aligned to the long axis of the glass in a head-tail arrangement. After developing to the equivalent of stage 28, explants were placed into a flow chamber (0.1 cm × 2.5 cm × 5.0 cm) and subjected to a shear force of 1 dynes cm-2, similar to that produced by ciliary flow in a stage 29 tadpole (Mitchell et al., 2007).
Transmission electron microscopy, tomography and scanning electron microscopy (SEM)
Xenopus larvae were fixed in 2% glutaraldehyde in 75 mM sodium cacodylate buffer (pH 7.4) overnight, cut in half along the transverse plane, secondarily fixed in 1% OsO4 and 1% potassium ferrocyanide, and counterstained in 2% aqueous uranyl acetate. After acetone dehydration, samples were infiltrated with Spurr’s resin in multiple steps, with each step followed by 5 minutes in a Pelco BioWave Pro microwave tissue processor at 250 W with vacuum. Samples were cured at 60°C for 48 hours, and then 300 nm sections along both a transverse and frontal plane were taken on a Leica UC7 ultramicrotome and placed on formvar-coated parallel bar copper grids. A tomography tilt series was acquired using a Zeiss Libra 120 PLUS EF-TEM ranging from -60° to 60° in 1° increments, aligned using the fiducialess edge detection algorithms of DigiECT (Digisens, France), and reconstructed using 25 iterations of the Ordered-Subset Simultaneous Algebraic Reconstruction Technique (OS-SART). Scanning electron microscopy (SEM) was carried out on fixed Xenopus embryos dehydrated in 100% ethanol and placed into Teflon sample holders and processed in an automated critical point drier, which was set to perform 25 exchange cycles of CO2 at medium speed and 20% stirring. After drying, the embryos were carefully removed and adhered to double-sided carbon tabs on aluminum stubs. The mounted samples were then sputter coated for 25 seconds (Leica SCD500, Leica, Vienna) with ∼7 nm of platinum while being rotated. The samples were then imaged on a FE-SEM (Sigma VP, Carl Zeiss, Cambridge, UK) at 5 kV with Everhart-Thornley secondary electron detection for optimal contrast.
RESULTS
Identification of bbof1 as a foxJ1 regulated gene
We previously used Affymetrix arrays to identify genes that are upregulated when the ectopic motile cilia are induced in the Xenopus skin by foxj1 (Stubbs et al., 2008) (GEO Accession Number GSE12613). A probe set corresponding to unigene Xl.66678 showed a 120-fold increase in response to foxj1, implicating this gene in motile cilia formation. The 531 amino acid protein encoded by Xl.66678 is annotated as ccdc176 in Xenbase and conserved among vertebrate species, but has not been functionally characterized in any system (supplementary material Fig. S1). Based on our functional analysis described below, we propose a new name: basal body orientation factor 1 (bbof1). The bbof1 protein contains two coiled-coil motifs found in other structural proteins (supplementary material Fig. S1B) and common among proteins in the ciliome, but little direct homology with other proteins.
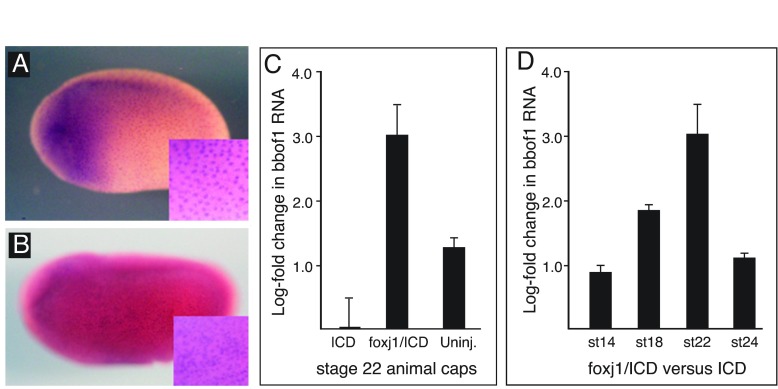
Expression of bbof1 in the X. laevis skin occurs in a spotty pattern that corresponds to other markers of MMCs, in terms of density and distribution within the skin along the embryonic axes (Fig. 1A). Consistent with the microarray results, injection of foxj1 RNA into embryos results in an upregulation and ectopic bbof1 RNA expression (Fig. 1B). The response of bbof1 expression to foxj1 was also measured in isolated animal cap assays using quantitative RT-PCR (Stubbs et al., 2008). In these assays, the expression of bbof1 was markedly downregulated when MCC differentiation was suppressed by injecting RNA encoding an activated form of Notch (Deblandre et al., 1999), ICD, but was induced three orders of magnitude in animal caps that were injected with both ICD and foxj1 RNAs (Fig. 1C). Notably, bbof1 expression in animal caps increased in response to foxj1 through the equivalent of stage 22 when MCCs undergo ciliogenesis, but was downregulated at later stages as MCCs generate flow (Fig. 1D). Thus, bbof1 is a foxj1-regulated gene activated in cells that form motile cilia, perhaps in a transient manner.
Fig. 1.
bbof1 is a foxj1-regulated gene. (A,B) Xenopus embryos were injected in one blastomere at the two-cell stage with foxj1 RNA (B), along with nlacz RNA as a tracer, fixed at stage 24, stained with X-gal to reveal the injected side, and probed for bbof1 RNA expression using in situ hybridization. Shown are representative images from the uninjected control embryo (A) and a foxj1 RNA-injected embryo (B), where anterior is oriented leftwards and dorsal upwards. A higher power image is shown in the inset. (C,D) Quantitative RT-PCR was used to quantify bbof1 RNA expression in animal caps isolated from embryos injected at the two-cell stage with ICD RNA, encoding an activated form of Notch, or with both foxj1 and ICD RNAs. At the indicated stage, total RNA was isolated and assayed in triplicate, using ODC RNA as a normalization control. The data are plotted with bbof1 levels in ICD-injected caps set at zero. Error bars indicate s.d.
Cilia in bbof1 morphants are motile but fail to produce flow
To examine the function of bbof1 in MCCs of the skin, we injected embryos at the two-cell stage with one of two bbof1 morpholinos: one designed to target the sequence around the start of translation in the bbof1 mRNA (bbof1-MOatg), or a second designed to target a splice junction in the bbof1 preRNA (bbof1-MOspl). As equivalent results were obtained with both morpholinos, we report those obtained with bbof1-MOatg in the main text, and those obtained with bbof1-MOspl in the supplementary data. Bbof1 morphants formed MCCs in the normal number (data not shown), cilia of normal length (supplementary material Fig. S2A) and cilia in the normal density (supplementary material Fig. S2B). In addition, the MCCs in bbof1 and control morphants appear similar when fixed and examined using SEM (supplementary material Fig. S2C-F). Together, these results indicate that bbof1 function is not required for the formation of MCCs or for ciliogenesis.
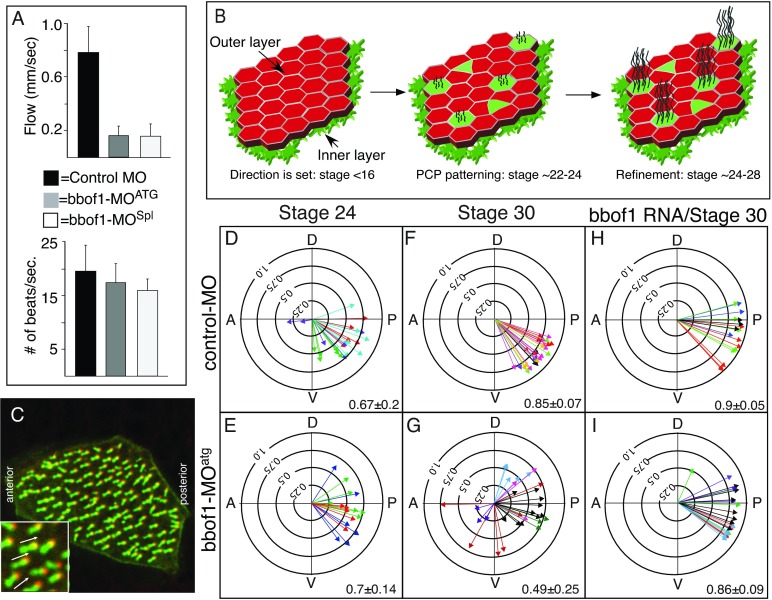
When small beads were used to measure flow produced by the skin, however, the flow generated by bbof1 morphants was considerably reduced compared with staged controls, suggesting that the cilia formed in bbof1 morphants were not functional (Fig. 2A). Strikingly, despite the low levels of measurable flow, the ciliary beat frequency of cilia in bbof1 morphants was statistically indistinguishable from that observed with control cilia (Fig. 2A). Cilia in bbof1 morphants are therefore motile but are unable to produce significant amounts of flow.
Fig. 2.
Cilia orientation in bbof1 morphants. (A) Cilia beat frequency and flow generation in control and bbof1 morphants at stage 28 based on data obtained from four embryos. Error bars indicate s.d. (B) Diagram illustrating the acquisition of cilia orientation in MCCs. Direction of flow is already set soon after gastrulation (before stage 16). As MCCs differentiate (stage 22-24), their orientation is still imprecise but initially biased in a posterior direction based on PCP signaling. As cilia beat, cilia orientation refines into a precise pattern (stage 24-28). (C) Shown is a confocal image of a MCC where the basal bodies are tagged with centrin4-RFP (red), and the rootlets with clamp-GFP (green), allowing one to measure the angular orientation of each basal body (inset at higher power). (D-I) Polar plots summarize cilia orientation in control or bbof1-MOatg morphants, as measured at stage 30 (F-I), stage 24 (D-E) or after rescue by injecting a full-length bbof1 RNA (H-I). Each arrow represents data for a single MCC, and arrow color represents MCCs taken from the same embryo. Arrow orientation represents the mean direction of basal bodies in relation to the embryonic axis (anteroposterior, A-P; dorsoventral, D-V); arrow length represents 1 minus the circular variation around that mean. Value in each panel equals the average arrow radius±s.d. for a given condition.
Cilia in bbof1 morphants are misoriented
The beating cilia in bbof1 morphants could conceivably fail to create a productive flow because they do not beat along the same planar axis. To address this possibility, we measured cilia orientation in bbof1 morphants using a previously described assay that employs two fusion proteins: centrin4-RFP, which labels the basal body, and clamp-GFP, which labels the rootlet (Mitchell et al., 2007; Park et al., 2008). As the rootlet projects from the basal body at an angle 180° opposite to the direction of flow, cilia orientation can be defined by a vector between the basal body labeled with centrin4-RFP and the extending rootlet labeled with clamp-GFP (Fig. 2C). We expressed the two fusion proteins in embryos by RNA injection, imaged MCCs in wild-type and morphant embryos by confocal microscopy, and scored the planar orientation of each basal body within individual MCCs relative to the embryonic axes (Fig. 2C). These data are shown in polar plots where each arrow represents a single MCC (Fig. 2D-I). Arrow direction denotes the mean planar orientation of basal bodies within that cell, and arrow length (R) denotes one minus the circular variation around that mean. Thus, arrow direction reveals the alignment of MCCs along the tissue planar axis, whereas arrow length reveals alignment of basal bodies within a cell’s planar axis, with longer arrows corresponding to more alignment.
As shown previously (Mitchell et al., 2007), MCCs in the skin of stage 30 control morphants are directed posteriorly (arrow orientation in Fig. 2F), with relatively little circular variation (R=0.85±0.07 degrees, long arrow length in Fig. 2F) around the mean direction. By contrast, cilia orientation in MCCs of bbof1-MOatg morphants, although mainly directed posteriorly, was much more variable (Fig. 2G, R=0.49±0.25) compared with the control (P<0.005). This cilia misalignment is due to a specific loss of bbof1, as proper alignment can be completely rescued by co-injecting with a full-length bbof1 RNA not targeted by the morpholino (Fig. 2I). Similar results were obtained with the second bbof1 splicing morpholino (supplementary material Fig. S3B,C). Together, these results indicate that when bbof1 function is reduced, cilia orientation is imprecise; this might account for the slow flow produced by bbof1 morphants.
bbof1 is not required for early ciliated cell patterning
Cilia orientation in the larval skin has been proposed to be established in several steps: a patterning step, presumably based on PCP signaling, that initially biases cilia orientiation posteriorly when MCCs first differentiate; followed by a refinement step that sharpens up orientation once cilia begin to beat and respond to flow (Fig. 2B) (Mitchell et al., 2007). To determine whether bbof1 is required for one or both of these steps, we scored cilia orientation in bbof1 morphants at stage 24, prior to the process of refinement. At early stages, the R values in bbof1 morphants are equivalent to that in control embryos (R=0.7±0.14 versus 0.67±0.2) and the mean arrow direction in morphants is generally biased posteriorly, as in controls (compare Fig. 2E and 2D). Bbof1, therefore, is unlikely to play a major role in the early patterning steps, although we cannot rule out the possibility that MCC orientation along a tissue axis (mean arrow direction) is slightly more variable in bbof1 morphants. Between stage 24 and 30, moreover, cilia alignment based on R values actually worsened in bbof1 morphants (compare Fig. 2G with 2E, P<0.005), indicating that the refinement step has not only failed but has gone awry. Similar results were obtained with the second bbof1 splicing morpholino (compare supplementary material Fig. S3B and S3A). Based on these phenotypes, bbof1 is likely to play a major role when cilia orientation undergoes the process of refinement.
bbof1 morphant cilia respond to flow
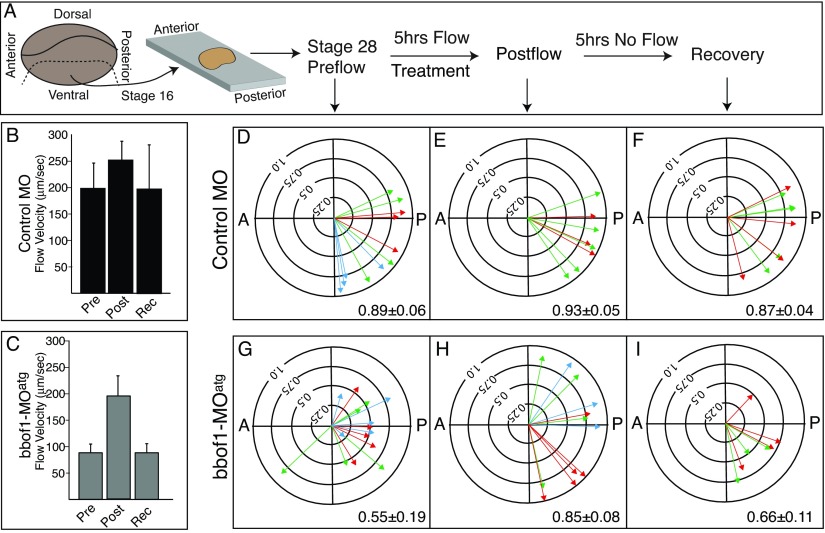
As the refinement process requires cilia to be motile to both produce and respond to flow (Mitchell et al., 2007), the cilia in bbof1 morphants could be unable to respond to flow, or simply fail to produce the flow needed for refinement, even though they are motile. To distinguish between these two possibilities, we used the experimental approach outlined in Fig. 3A to determine whether treatment with an external flow rescues the cilia orientation defect in bbof1 morphants. Ventral explants were isolated from embryos at stage 16 when the direction of flow along the anterior-posterior (AP) axis is already set, and allowed to attach to fibronectin-coated coverslips until the equivalent of stage 28. Attached explants were then placed in a flow chamber where they were subjected to an external flow of 1 dyne cm-2 directed in an AP direction for a period of 5 hours. The level of external flow used is similar to that generated by wild-type skin (Mitchell et al., 2007). Cilia orientation was measured by fixing explants before and after flow treatment, and imaging basal bodies using the assay described above (Fig. 3D-I).
Fig. 3.
bbof1 morphants respond to flow but cannot maintain a precise orientation. (A) The experimental design for treating ventral explants with an external flow is diagrammed. (B,C) Average ciliary flow produced by four explants from control (B) or bbof1 morphants (C) is shown, when measured pre-flow treatment (Pre), 0.5 hours post-flow treatment (Post) or after 5 hours of recovery (Rev). Error bars indicate ±s.d. (D-I) Cilia orientation in MCCs was measured and expressed in polar plots as described in the legend to Fig. 2, in explants before flow treatment (D,G), just after flow treatment (E,H) or after 5 hours of recovery (F,I). Arrow colors represent MCCs taken from the same explant. Each plot is oriented with anterior (cement gland) towards the left and posterior (proctodeum) towards the right. Value in each panel equals average arrow radius±s.d. for a given condition.
Prior to flow treatment, cilia orientation in explants was similar to that observed in embryos, both for control and bbof1-MOatg morphants. For example, MCCs in explants from bbof1-MOatg morphants were correctly oriented along the tissue axis, but failed to refine their orientation as in control explants (Fig. 3G, R=0.55±0.19 versus 3D, 0.89±0.06; P<0.001), just as in embryos (Fig. 2G, R=0.49±0.25 versus 2F, 0.85±0.07). Strikingly, after subjecting explants to flow, cilia orientation in bbof1-MOatg morphants improved significantly, to levels close to those in control explants (Fig. 3H, R=0.85±0.08 versus 3E, 0.93±0.05; P=0.016). By contrast, cilia orientation in untreated explants from either control or bbof1-MOatg morphants did not change significantly over the same time period (data not shown).
To determine whether flow treatment resulted in better ciliary flow, we measured the flow produced by the same explants before and after flow treatment (Fig. 3B,C). As in embryos, explants from bbof1-MOatg morphants produced weaker ciliary flow compared with controls (when tested prior to flow treatment), at the equivalent of stage 28 (Fig. 3B,C; Pre). After a 5-hour treatment with an external flow, however, these explants now generated significant ciliary flow rates, approaching those observed in control explants (Fig. 3B,C; Post). By contrast, the flow rate produced by untreated explants, either from control or bbof1-MOatg morphants was unchanged during the equivalent time frame (data not shown). For both the control and bbof1-MOatg morphant explants, the degree of ciliary flow correlated well with the degree of cilia orientation. Thus, an external flow can rescue both the cilia orientation defect in bbof1-MOatg morphants and, to a large extent, their ability to produce flow, indicating that the cilia in bbof1-MOatg morphants can respond to hydrodynamic cues to orient along the planar axis.
We next examined whether the response of cilia in bbof1-MOatg morphants remained stable by examining explants 5 hours after flow treatment (Fig. 3A). After 5 hours, the flow produced by explants of bbof1-MOatg morphants slowed to the same rate observed prior to flow treatment, whereas that in control explants remained high (Fig. 3B,C; Rec). Moreover, at 5 hours post-treatment, the cilia orientation in explants of bbof1-MOatg morphants became less precise and now resembled those in explants prior to treatment or that were untreated (compare Fig. 3I, R=0.66±0.11 with 3G, 0.55±0.19), whereas cilia in control explants remained precisely oriented (Fig. 3F, R=0.87±0.04). Similar results were obtained in explants injected with the second, bbof1-MOspl morpholino (supplementary material Fig. S3D-F). These findings suggest that the main defect in bbof1 morphants is an inability to maintain cilia orientation, even if orientation is imposed by an external flow.
bbof1 morphants fail to recover from disorientation
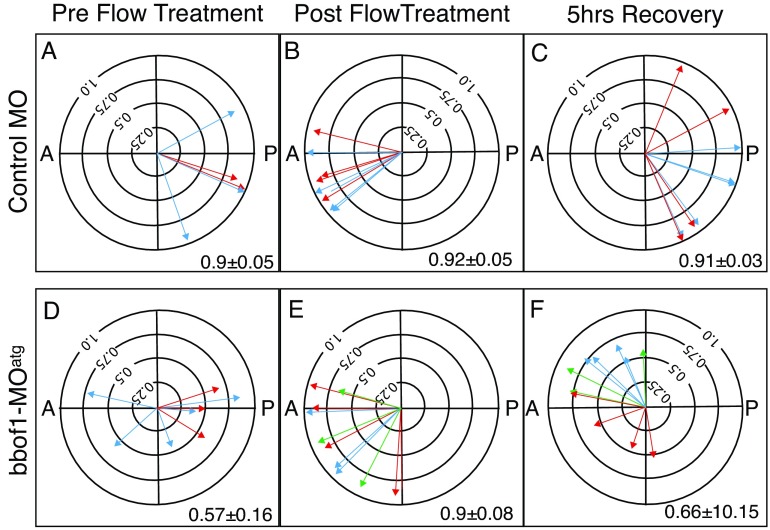
We also examine how bbof1 morphants respond to an external flow that is reversed 180° from the normal axis. The same experimental paradigm in Fig. 3A was followed, but the external flow was oriented from posterior to anterior. In control explants treated in this way, cilia initially reoriented in response to the direction of external flow and pointed anteriorly (Fig. 4B), but recovered after 5 hours to again point in a posterior direction (Fig. 4C). This response indicates that wild-type cilia continue to respond to flow, but that their orientation remains fixed and recovers once an external flow is removed. Strikingly, in bbof1 morphant explants, cilia also responded to the reversed external flow by pointing anteriorly (Fig. 6E), appearing similar to the cilia in control explants (R=0.92±0.05 versus 0.9±0.08, P=0.5). In contrast to the controls, however, 5 hours post-treatment, the cilia in bbof1 morphants became less precise, but also remained misoriented in an anterior direction (Fig. 4F). This result further supports the conclusion that the cilia in bbof1 morphants remain responsive to flow, but are unable to fix and maintain their orientation.
Fig. 4.
bbof1 morphants fail to recover from disorientation. Ventral explants from stage 16 control or bbof1 morphant embryos were subjected to an external flow and analyzed for cilia orientation, as described in Fig. 3A, except that the external flow was oriented from posterior to anterior. Plots are oriented with posterior towards the right and anterior leftwards, and the external flow was oriented from right to left.
Fig. 6.
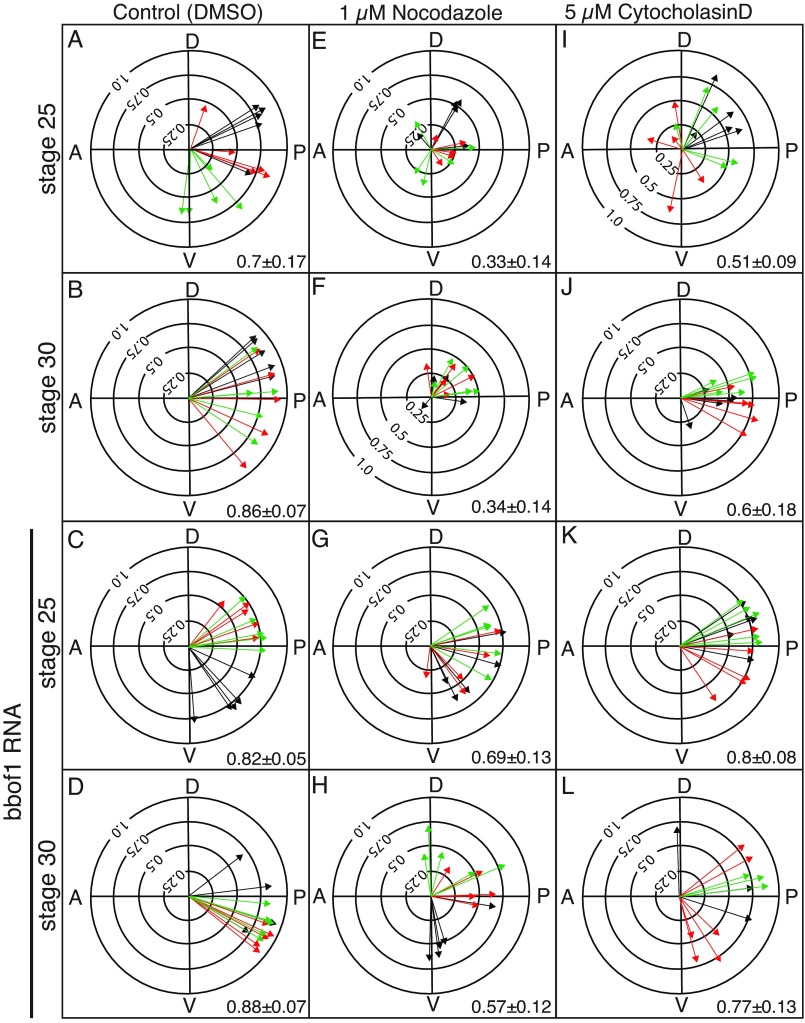
bbof1 misexpression promotes premature cilia orientation in the presence of nocodazole and cytochalasin D. (A-L) Embryos were injected at the two-cell stage with RNA encoding centrin4-RFP or clamp-GFP along with a full-length myc-tagged bbof1 RNA (lower panels as indicated). At stage 23, embryos were placed in 1 μM nocodazole (E-H) or 5 μM cytochalasin D (I-L), and then fixed and analyzed at stage 25 or 30, as indicated. Cilia orientation was analyzed and expressed in polar plots as described in the legend to Fig. 2. Value in each panel equals average arrow radius±s.d.
Misexpression of bbof1 RNA induces cilia orientation
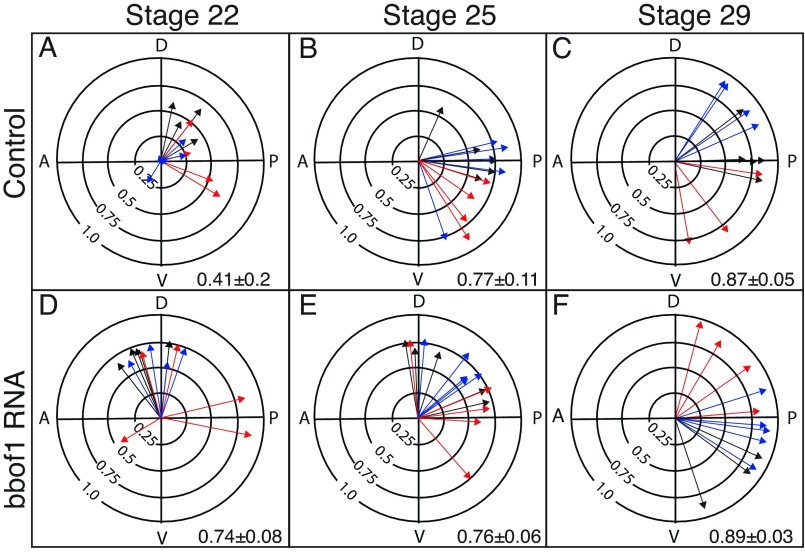
We next asked whether misexpression of bbof1 using RNA injection can promote cilia orientation, by examining injected and control embryos at stages when cilia orientation is still unrefined. Cilia orientation in control embryos at stage 22 is very imprecise (Fig. 5A, R=0.41±0.2), improves gradually at stage 25 (Fig. 5B, R=0.77±0.11) before reaching a final refined pattern at stage 29 (Fig. 5C, R=0.87±0.05). In embryos injected with bbof1 RNA, cilia orientation is already markedly refined at stage 22 (Fig. 5D, R=0.74±0.08) compared with control embryos (P=0.0002), indicating the bbof1 misexpression can promote premature cilia alignment. To confirm this phenotype, we also examined cilia orientation using a second tagged protein, tsga10-GFP, which independently marks rootlets (data not shown, supplementary material Fig. S4) and thus can be used along with centrin-RFP to score basal body orientation. In agreement with the result above, cilia orientation, as marked by tsga10-GFP, was significantly more precise at stage 23 in embryos that misexpress bbof1 compared with controls (supplementary material Fig. S4, R=0.76±0.12 versus 0.55±0.08, P=0.0004).
Fig. 5.
bbof1 misexpression promotes premature cilia orientation. (A-F) Embryos were injected at the two-cell stage with RNAs encoding centrin4-RFP and clamp-GFP (A-F), along with RNA encoding MT-bbof1 (D-F). At the indicated stage, embryos were fixed and cilia orientation was scored as described in the legend to Fig. 2. Value in each panel equals average the arrow radius±s.d.
Since bbof1 misexpression promotes cilia orientation at stages prior to pronounced ciliary flow, it likely acts independently of a flow-based mechanism. To examine this possibility further, we asked whether bbof1 misexpression still promotes cilia orientation when cilia motility is blocked using a second, previously described, morpholino directed against spag6 (spag6-MOatg) (Mitchell et al., 2007). In spag6 morphants, cilia orientation at early stages is similar to that of controls, but then fails to undergo further refinement into the final pattern, as shown previously (Fig. 4E-G) (Mitchell et al., 2007). Cilia orientation in spag6-MOatg morphants at stage 23 (supplementary material Fig. S4H, R=0.76±0.13), however, is similar to that in control embryos (Fig. 5D, R=0.74±0.08) after both are injected with bbof1 RNA, indicating that bbof1 promotes cilia alignment by a mechanism that is independent of flow.
Previous studies have shown that cilia alignment is lost when a network of apical microtubules is disrupted by treating embryos with 1 μM nocodazole during the period when cilia orientation is established (Fig. 6E,F) (Werner et al., 2011). In embryos injected with a full-length bbof1 RNA, and treated with nocodazole, however, cilia alignment markedly improved over that seen in drug-treated controls, when examined at either stage 25 (Fig. 6G versus 6E, P<0.0001) or stage 30 (Fig. 6H versus 6F, P<0.0001). bbof1 misexpression, therefore, promotes and maintains cilia orientation under conditions where orientation cues provided by microtubules are largely abrogated by nocodazole. A previous study has also shown that treating embryos with cytochalasin D disrupts a subapical actin network in MCCs that results in two phenotypes: basal bodies failing to distribute evenly within the apical domain (supplementary material Fig. S5B); and cilia failing to align properly (Fig. 6I,J) (Werner et al., 2011). In embryos injected with a full-length bbof1 RNA, and treated with cytochalasin D, cilia alignment was markedly improved over that seen in drug-treated controls (Fig. 6K versus 6I, P<0.0001 and Fig. 6L versus 6J, P=0.005), whereas the basal body clumping phenotype was largely unchanged (supplementary material Fig. S5C). Thus, bbof1 still promotes cilia alignment under conditions where three of the known orientation cues, namely flow, actin and microtubules, are disrupted.
bbof1-GFP localizes to filamentous structures adjacent to basal bodies
To assess where bbof1 localizes in MCCs, embryos were injected with RNA encoding a form of bbof1 tagged at the C terminus with GFP, along with RNA encoding centrin4-RFP to label basal bodies. In MCCs, bbof1-GFP localization was detected in the axoneme at both early (stage 22) and late stages (stage 29) of differentiation (Fig. 7A,D). In addition, bbof1-GFP also localized to centrioles in outer cells (data not shown), and to basal bodies in MCCs (Fig. 7B,C). Strikingly, although bbof1-GFP localization at centrioles (data not shown) and the axoneme (Fig. 7A,D) was evident at both stage 22 and stage 28, its localization to basal bodies was much more prominent at earlier stages and was largely lost by stage 29 (Fig. 7F). bbof1-GFP localized to the basal body in a polarized fashion, lying adjacent to centrin4-RFP towards the anterior side, suggesting close proximity to the straited rootlet (Fig. 7C). Consistent with this interpretation, bbof1-GFP colocalized with a striated rootlet marker, clamp-RFP (Fig. 7G,H). Although misexpression can lead to mislocalization, we tentatively conclude that bbof1-GFP localizes to the axoneme, and transiently to a polarized structure next to basal bodies, in close association with the striated rootlet. The latter localization raises the possibility that bbof1 acts as a bridging factor between appendages attached to the basal bodies, and polarized cytoskeletal elements involved in cilia orientation.
Fig. 7.
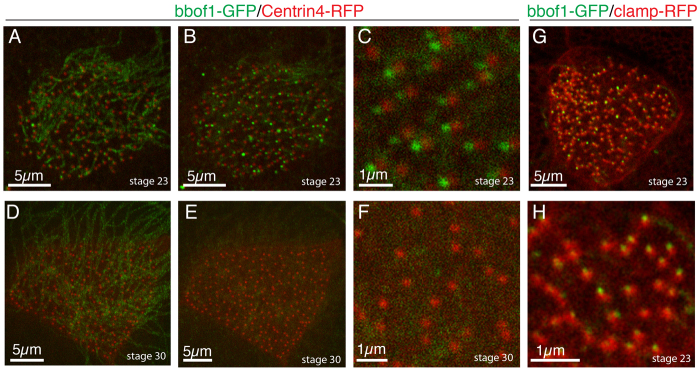
bbof1-GFP localizes to the axoneme and transiently to basal bodies. (A-H) Embryos were injected at the two-cell stage with RNAs encoding bbof1-GFP along with centrin4-RFP RNA (A-F) or clamp-RFP RNA (G-H). Embryos were fixed at the indicated stage and imaged by confocal microscopy. (A-F) Confocal slices at the level of the axoneme I (A,D), at the level of the basal bodies (B,E) or at a higher magnification (C,F). (G,H) bbof1-GFP along with clamp-RFP at low and high magnification as indicated by the scale bars. Each image is oriented with anterior towards the left and posterior towards the right.
Basal body structure in bbof1 morphants
We further examined the structure of the basal bodies in wild-type and bbof1 morphants using transmission electron microscopy and tomography. This approach allows one to capture enough depth (300 nm) to score basal bodies appendages, including the basal foot and rootlets, depending on their orientation. By imaging and scoring approximately 20 basal bodies from different cells under each condition, we find that the large striated rootlet and basal foot are present and appear normal in bbof1 morphants (supplementary material Fig. S6). Thus, the core features of basal bodies appear largely normal in bbof1 morphants, including the basal foot that was previously implicated in planar orientation (Kunimoto et al., 2012). The basal bodies in bbof1 morphants could have defects in more peripheral appendages or the way in which these appendages attach to cytoskeletal elements. For example, a thinner rootlet structure, which attaches on each side of the large rootlet, and projects deep into the cytoplasm in the direction of ciliary flow (supplementary material Fig. S6; Movie 1), is unrepresented in many of the tomographs of bbof1 morphants compared with wild type (supplementary material Table S1; Movie 2), and this might account in part for the cilia disorientation defects in bbof1 morphants. We also stained wild-type and morphant embryos with phalloidin to image a subapical actin network that has been shown to form as cilia orientation matures, creating a link between the rootlet and neighboring basal bodies (Werner et al., 2011). In bbof1 morphants, the apical actin network that surrounds each basal body appears similar to that in control embryos (supplementary material Fig. S7F versus S7B) but the organization of the subapical actin was largely disrupted, appearing similar to that in control embryos at early stages (supplementary material Fig. S7D versus S7H). Thus, bbof1 may act upstream or during the maturation of appendages that anchor basal bodies during cilia orientation.
DISCUSSION
The production of directed fluid flow by ciliated epithelia requires the alignment of basal bodies along a planar axis but the mechanisms involved remain poorly defined. In the Xenopus skin, basal body alignment in MCCs requires at least two steps: a patterning step in which the PCP pathway acts early to establish a posterior bias in orientation, followed by a refinement step when orientation becomes more precise based on a hydrodynamic process in which cilia produce flow and orient in response (Mitchell et al., 2007). Here, we define a third step required to establish cilia orientation based on mutant phenotypes for bbof1, a gene that is upregulated in cells with motile cilia by foxj1, and encodes a coiled-coil protein associated with the ciliary apparatus. We propose that bbof1 mediates a maturation step that stabilizes and aligns cilia orientation, following the response to PCP and hydrodynamic cues.
Bbof1 mediates ciliary orientation
The cilia in bbof1 morphants are normal in length and number, beat with a normal frequency, but are disoriented and fail to generate a productive flow. Cilia disorientation can cause flow to fail but conversely when ciliary flow is disrupted, cilia fail to refine and align, making the cause and effect underlying this phenotype a challenge to disentangle. To dissect this phenotype, we first examined bbof1 morphants at early stages, and found that the cilia orientation is similar to that in controls, suggesting that bbof1 morphants are normally patterned along the AP axis. This patterning is thought to depend on PCP cues that interact with a polarized microtubule network, and can be disrupted with nocodazole, resulting in severe disorientation phenotypes. That early cilia orientation is largely normal when bbof1 is knocked down strongly indicates that bbof1 is not required for AP patterning or initially directing cilia orientation along the embryonic axis.
Cilia orientation starts off normally in bbof1 morphants and fails to refine, actually worsening during the period when cilia alignment should become more precise. To dissect this phenotype, we subjected bbof1 morphants to an external flow, and found that cilia responded by aligning along a common axis. Once aligned, bbof1 morphants generated flow, indicating that the ability to respond and generate flow is not the primary defect that arises when bbof1 activity is lost. The bbof1 phenotype, therefore, differs markedly from that occurring when cilia are simply immotile, based on their ability to respond, align and produce flow. Strikingly, after entrainment, bbof1 mutants gradually lost alignment and the ability to generate flow, placing bbof1 at the nexus of establishing and maintaining alignment during the refinement process. Thus, the bbof1 phenotype reveals a new aspect of the orientation process occurring downstream of the PCP and flow-based cues where cilia orientation become fixed during a maturation and stabilization phase.
Misexpression of bbof1 also has the remarkable ability to drive cilia alignment, the first such gain-of-function phenotype observed for any factor implicated in cilia orientation. In this context, bbof1 can act in the absence of flow as well as under conditions where microtubules and actin filaments are disrupted by treatment with nocodazole or cytochalasin D, respectively, further distinguishing bbof1-mediated basal body alignment from those described previously. bbof1 promotes cilia alignment as early as stage 23, further suggesting that it acts before other polarized networks are thought to form. Bbof1 might act this early by simply aligning basal bodies to each other, or by connecting basal bodies to a polarized network as yet undescribed. Either mechanism could contribute to the maturation process.
Bbof1 localizes transiently in a polarized fashion to basal bodies
A bbof1-GFP fusion proteins localizes to the axoneme, as well as to a site adjacent to each basal body in close proximity to the large rootlet. The latter localization of bbof1 likely accounts for its role in cilia orientation, but we cannot rule out the possibility that bbof1 also functions in the axoneme. As is the case with odf2, which has been clearly shown to function both during basal body docking as part of the distal fibers and during cilia orientation as part of the basal foot, bbof1 could also contribute to several distinct parts of the ciliary apparatus (Kunimoto et al., 2012).
Bbof1-GFP localizes transiently to the basal body, raising the possibility that bbof1 mediates the maturation of a basal body attachment point around the time cilia orientation is established. The major appendages associated with basal bodies, namely the basal foot and the rootlets, appear largely normal in bbof1 mutants, but we cannot rule out defects in how these appendages link to specialized filament systems that not only include actin and microtubules, but also intermediate filaments (Gordon, 1982). The major defect observed in bbof1 morphants is the lack of a peripheral structure, the small rootlets and a failure to form a subapical actin network associated with basal body maturation. We favor the idea that these structures are likely to be secondary consequences of bbof1 loss of activity, because, for example, misexpression of bbof1 can drive premature cilia orientation but not the formation of subapical actin network (data not shown). Nonetheless, these observations suggest that bbof1 activity plays a crucial step in the maturation of cilia orientation, perhaps by setting in motion the formation of linkages that arise when the rootlet structures mature. A major component of the rootlet is a large coiled-coil protein, called rootletin (Yang et al., 2002), which has been gene targeted in the mouse, leading to defects in a variety of cilia subtypes, including those mediating mucociliary clearance in the lung (Yang et al., 2005). Analysis of this phenotype is complicated by the fact that the genetic lesion is not a complete null (Yang et al., 2006) and the effects on cilia orientation in these mutants have not been explored. Future studies will be needed to determine whether the stabilization of cilia alignment by bbof1 involves the large and perhaps small rootlet structures, and, if so, what polarized filament network involving these structures is established by bbof1 to maintain ciliary alignment.
Maturation during cilia orientation
Bbof1 further highlights the complexity of events that need to occur to align cilia in MCCs along a planar axis. Acquisition of cell polarity through PCP signaling is likely to be one of the first steps, but precise alignment of cilia within a MCC occurs gradually, including during a period when cilia beating contributes to the orientation process (Guirao et al., 2010; Mitchell et al., 2007; Vladar et al., 2012). As consequence, cilia orientation is likely to require flexibility as this refinement process takes place, followed by additional steps that presumably stabilize and lock in an orientation. Our results suggest that bbof1 mediates at least one step underlying maturation, operating as basal body-specific component induced by foxj1. Further analysis of gene expression associated with MCC differentiation is likely to be an important strategy to dissect additional basal body components required for cilia orientation in these cells.
Supplementary Material
Acknowledgments
The authors thank members of the Kintner lab for comments on the manuscript.
Footnotes
Funding
M.S.J. and J.A.J.F. gratefully acknowledge financial support from the Waitt Advanced Biophotonics Center, National Cancer Institute P30 Cancer Center Support Grant [CA014195-40] and National Institute of Neurological Disorders and Stroke P30 Neuroscience Center Core Grant [NS072031-02A1]. This work was supported by a National Institutes of Health grant [GM076507] to C.K. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
B.J.M. and J.S. carried out the initial screen that isolated bbof1 and tentatively localized it to the basal body in MCCs. M.E.W. and B.J.M. carried out the experiments shown in Fig. 2A and Fig. S2A,B. M.S.J. and J.A.J.F. carried out the SEM (Fig. S2) and TEM tomography analysis of wild type and bbof1 morphants. Y.-H.C. carried out the remainder of the work, including flow treatments of wild type and morphant explants in collaboration with J.L. and S.C. C.K. wrote the paper and aided in data analysis.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.096727/-/DC1
References
- Bayly R., Axelrod J. D. (2011). Pointing in the right direction: new developments in the field of planar cell polarity. Nat. Rev. Genet. 12, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblandre G. A., Wettstein D. A., Koyano-Nakagawa N., Kintner C. (1999). A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development 126, 4715–4728 [DOI] [PubMed] [Google Scholar]
- Gordon R. E. (1982). Three-dimensional organization of microtubules and microfilaments of the basal body apparatus of ciliated respiratory epithelium. Cell Motil. 2, 385–391 [DOI] [PubMed] [Google Scholar]
- Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J. M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y. G., et al. (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–350 [DOI] [PubMed] [Google Scholar]
- Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A., et al. (2012). Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189–200 [DOI] [PubMed] [Google Scholar]
- Lund U., Agostinell C. (2001). Circular Statistics. River Edge, NJ: World Scientific; [Google Scholar]
- Marshall W. F. (2008). Basal bodies platforms for building cilia. Curr. Top. Dev. Biol. 85, 1–22 [DOI] [PubMed] [Google Scholar]
- Marshall W. F., Kintner C. (2008). Cilia orientation and the fluid mechanics of development. Curr. Opin. Cell Biol. 20, 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B., Jacobs R., Li J., Chien S., Kintner C. (2007). A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97–101 [DOI] [PubMed] [Google Scholar]
- Mitchell B., Stubbs J. L., Huisman F., Taborek P., Yu C., Kintner C. (2009). The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr. Biol. 19, 924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose T., Kraus Y., Houliston E. (2012). A conserved function for Strabismus in establishing planar cell polarity in the ciliated ectoderm during cnidarian larval development. Development 139, 4374–4382 [DOI] [PubMed] [Google Scholar]
- Nieuwkdop P.B., Faber J. (1967). Normal Table of Xenopus Laevis. Amsterdam: North Holland Publishing; [Google Scholar]
- Park T. J., Mitchell B. J., Abitua P. B., Kintner C., Wallingford J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H., Grainger R. M., Harland R. M. (1998). The Early Development Of Xenopus Laevis: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Press; [Google Scholar]
- Stubbs J. L., Oishi I., Izpisúa Belmonte J. C., Kintner C. (2008). The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 40, 1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J. L., Vladar E. K., Axelrod J. D., Kintner C. (2012). Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 14, 140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar E. K., Bayly R. D., Sangoram A. M., Scott M. P., Axelrod J. D. (2012). Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 22, 2203–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B., Mitchell B. (2011). Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 25, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. E., Mitchell B. J. (2012). Understanding ciliated epithelia: the power of Xenopus. Genesis 50, 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. E., Hwang P., Huisman F., Taborek P., Yu C. C., Mitchell B. J. (2011). Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 195, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Yue G., Adamian M., Bulgakov O., Li T. (2002). Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J. Cell Biol. 159, 431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Gao J., Adamian M., Wen X. H., Pawlyk B., Zhang L., Sanderson M. J., Zuo J., Makino C. L., Li T. (2005). The ciliary rootlet maintains long-term stability of sensory cilia. Mol. Cell. Biol. 25, 4129–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Adamian M., Li T. (2006). Rootletin interacts with C-Nap1 and may function as a physical linker between the pair of centrioles/basal bodies in cells. Mol. Biol. Cell 17, 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A. (2007). Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.