Abstract
The group I members of the Nm23 (non-metastatic) gene family encode nucleoside diphosphate kinases (NDPKs) that have been implicated in the regulation of cell migration, proliferation and differentiation. Despite their developmental and medical significance, the molecular functions of these NDPKs remain ill defined. To minimize confounding effects of functional compensation between closely related Nm23 family members, we studied ndk-1, the sole Caenorhabditis elegans ortholog of group I NDPKs, and focused on its role in Ras/mitogen-activated protein kinase (MAPK)-mediated signaling events during development. ndk-1 inactivation leads to a protruding vulva phenotype and affects vulval cell fate specification through the Ras/MAPK cascade. ndk-1 mutant worms show severe reduction of activated, diphosphorylated MAPK in somatic tissues, indicative of compromised Ras/MAPK signaling. A genetic epistasis analysis using the vulval induction system revealed that NDK-1 acts downstream of LIN-45/Raf, but upstream of MPK-1/MAPK, at the level of the kinase suppressors of ras (KSR-1/2). KSR proteins act as scaffolds facilitating Ras signaling events by tethering signaling components, and we suggest that NDK-1 modulates KSR activity through direct physical interaction. Our study reveals that C. elegans NDK-1/Nm23 influences differentiation by enhancing the level of Ras/MAPK signaling. These results might help to better understand how dysregulated Nm23 in humans contributes to tumorigenesis.
Keywords: C. elegans, KSR scaffolds, Nm23/NDPK, NME, Ras signaling
INTRODUCTION
The human NME family (legacy nomenclature Nm23) consists of ten proteins, which are classified into two groups based on their sequence homology and nucleoside diphosphate kinase (NDPK) activity (Desvignes et al., 2009). Four members belonging to group I (NM23- H1 to -H4, now known as NME1-4) show high sequence similarity and a NDPK activity: group I NDPKs transfer phosphate to cognate dinucleotides through a highly conserved phosphohistidine intermediate (Steeg et al., 2003). The group II proteins of the Nm23 family (NM23-H5 to -H10, now known as NME5-9 and RP2) are more divergent and not necessarily active kinases. A group I NDPK, NM23-H1, was the first identified metastasis inhibitor (Steeg et al., 1988), with reduced levels or absence primarily detected in breast cancer (Heimann and Hellman, 2000). However, NM23-H1 was found to be overexpressed in ovarian, prostate and pancreatic cancers, suggesting a prominent, contributing role in tumor development (Harłozińska et al., 1996; Igawa et al., 1994; Andolfo et al., 2011; Takadate et al., 2012). Despite its significance in tumorigenesis, little is known about the developmental roles of NM23-H1. In Drosophila, deletion of the sole fly group I NDPK homolog abnormal wing discs (awd) induces imaginal disc cell death during larval development (Biggs et al., 1990). Mutations in awd also disrupt epithelial tubule morphogenesis during fly tracheal embryogenesis (Dammai et al., 2003).
Nucleoside diphosphates might not be the sole recipients of the high-energy phosphate transferred from H118 (histidine 118 residue) in group I members. In mammalian cells, NM23-H2 (NME2) can relay its high-energy phosphate to histidines located in various target proteins. For example, histidine phosphorylation of the beta subunit of heterotrimeric G proteins by NDPK-H2 augments cyclic AMP formation in the heart (Hippe et al., 2009) and phosphohistidine modification of the ATP-citrate lyase by NM23-H1 (NME1) is needed for its enzymatic activity (Wagner and Vu, 1995).
Besides the above-described biochemical and developmental activities, NDPKs have also been considered to act as, or to modify the activity of other, scaffold proteins. Recently, evidence was presented that NDPK-H2 is required as a scaffold that links heterotrimeric G proteins to caveolins (Hippe et al., 2011). Apparently, this complex regulates G protein content at the plasma membrane, thereby influencing cardiac contractility. NDPKs can locally enrich GTP and thus may control endocytosis through the function of the GTPase dynamin (Dammai et al., 2003) and small G proteins such as Rac (Rochdi et al., 2004). Furthermore, based on studies on human cell lines, NM23-H1 has been suggested to interact with the kinase suppressor of ras 1 (KSR1) scaffold protein (Hartsough et al., 2002; Salerno et al., 2005). In cell lines, phosphorylation of KSR1 by NM23-H1 leads to attenuation of Ras/ERK signaling (Hartsough et al., 2002). These diverse molecular functions of NDPKs might explain the documented pleiotropic effects of NDPK overexpression or deletion across species. There are also examples of important roles for NDPK in cell migration, growth and differentiation (Lee et al., 2009; Mochizuki et al., 2009), which might explain why NDPKs have been repeatedly implicated in various cancers while also appearing to act in apparently unrelated signaling processes.
To understand the mechanisms underpinning such diverse functions, we used the nematode Caenorhabditis elegans as a tractable genetic model whose genome encodes only a single mammalian group I NDPK ortholog (Bilitou et al., 2009), which we named NDK-1 (nucleoside diphosphate kinase-1). To pin down the function of NDK-1, we focused on the vulva and studied defects associated with the morphogenesis of this organ in nematodes defective for NDK-1. In addition, we used the well-characterized vulva induction system to place NDK-1 function into Ras/MAPK signaling. The vulva of the C. elegans hermaphrodite develops from a subset of six multipotent epidermal cells called vulval precursor cells (VPCs), consecutively termed P3.p to P8.p (Sternberg, 2005). An inductive signal conferred by an epidermal growth factor (EGF) ligand expressed from the gonadal anchor cell (AC) activates the Ras/MAPK pathway in P(5-7).p cells, causing them to adopt specific vulval cell fates. P6.p, the VPC closest to the AC, adopts the primary vulval fate, while P5.p and P7.p, the two adjacent VPCs to P6.p, adopt the secondary vulval fate as a result of lateral signaling, which is mediated by the LIN-12/Notch pathway (Greenwald, 2005). By contrast, P3.p, P4.p and P8.p, the VPCs farthest from the AC, receive only a basal level of Ras activation, thereby expressing the non-induced tertiary fate. Constitutive activation of the Ras/MAPK pathway leads to ectopic induction of the primary and secondary fate in the latter cells (P3.p, P4.p and P8.p), resulting in a multivulva (Muv) phenotype. Conversely, lack of Ras signaling causes a vulvaless (Vul) phenotype (none of the VPCs adopts an induced vulval fate).
In this study, we report that ndk-1 is an essential gene. Moreover, ndk-1 loss-of-function mutants show defects in the morphogenesis of the vulva. We demonstrate that NDK-1 augments - rather than attenuates, as in the case of human cell lines - the extent of Ras/MAPK signaling. We find that the extent of Ras/MAPK-dependent vulva induction is reduced in ndk-1 mutants, and that NDK-1 enhances Ras/MAPK signaling downstream of Raf at the level of the KSR scaffold proteins, probably through direct physical interaction. We also provide evidence that NDK-1 affects further developmental and physiological events that rely on Ras/MAPK signaling.
MATERIALS AND METHODS
Nematode strains and alleles
The wild-type C. elegans strain corresponds to var. Bristol (N2). The following mutant strains were used: EJ810 F25H2.5(ok314)/hT2[dpy-18(h662)]I; +/hT2[bli-4(e937)]III; TTV1 ndk-1(ok314) I/hT2[bli-4(e937) let-?(q782) qIs48](I;III); UP749 ksr-2(dx27)I/hT2[bli-4(e937) let-?(q782) qIs48](I;III); BC12969 dpy-5(e907)I; sIs12774[rCesF52H2.5::GFP + pCeh361]; TTV4 ksr-2(dx27)ndk-1(ok314)/hT2[bli-4(e937) let-?(q782) qIs48](I;III), TTV2 eluEx1[NDK-1::gfp; unc-119(+)];unc-119(ed3)III; TTV3 eluSi1[NDK-1::GFP + cb-unc-119(+)]III; PS1839 let-23(sa62)II; MT2131 lin-31(n1053)II; MT2124 let-60(n1046)IV; MT4698 let-60(n1700)IV; CB1275 lin-1(e1275)IV; NH2466 ayIs4 (egl-17::gfp)I; dpy-20(e1282)IV; kuIs57 col-10::lin-45(gf); dpy-20(e1282)IV; SD806 gaIS36[Hs::mpk-1(wt), Hs::mek]; him5(e1490)V; MT8677 ksr-1(n2526)X; PS3352 syIs50 (cdh-3::GFP)X; dpy-20(e1282)IV.
Microscopy
Animals were examined with an Olympus BX51 epifluorescence microscope equipped with DIC optics, F-View camera (Soft Imaging System, 10×), Olympus PlanApo 60×/1.40 Oil Ph3 ∞/0.17 objective, Olympus U-RFL-T Hg burner, and filters ex HQ470/40, DM Q495LP, em HQ515/30. Images were processed using analySIS PRO 3.2 software (Soft Imaging System). Scoring the number of divided versus non-divided Pn.px and Pn.pxx cells was performed at the L3 stage.
RNA interference (RNAi) and transgene construction
For RNAi via ingested double-stranded RNAs, an XhoI-digested fragment of the full-length cDNA corresponding to F25H2.5 (yk1105e04) was cloned into pPD129.36 (a gift from A. Fire), and the construct obtained was transformed into HT115 (DE3) bacteria. RNAi experiments were performed at 25°C. To generate a translational fusion F25H2.5::GFP reporter, the following primers (5′-3′) were used: Promoter plus ORF, TTGGGGCGCGCCATCGGCGATCGCCTTCGCTCTCCATCGGCTTCG and TTTTGGGCCCATTATTAATTAACTCAGGCCGGCCATCAGCGGCCTTCGTAGACCCATGAGTTGATGAATGG; 3′UTR, TTGGGGCGCGCCATCGGCGATCGCATCTGCGGCCGCATCTGGCCGGCCTGAGTTAATTAATAAACTGATTGCTTATGTCTCCCG and TTTTGGGCCCGAACGATTTTCCAAGGTTCCAAC. The amplified fragments were digested with AscI/NotI and FseI/ApaI, respectively, and cloned into pRH21 (Hofmann et al., 2002). Subsequently, transgenic worms (strain TTV2) were generated by biolistic transformation using the unc-119(ed3) mutant genetic background and the unc-119(+) gene as a cotransformation marker. A single-copy integrated version was generated by cloning the insert into the MosSCI vector PCFJ151 (Addgene) (AflII/SpeI sites) and injection-based transformation.
Western blot
Activated MAPK displays both a germline-specific band and a somatic band (Ohmachi et al., 2002). About 350 late L4 staged worms were picked and subjected to lysis. Probing for ERK and diphosphorylated ERK was performed using rabbit anti-ERK (K-23, Santa Cruz) and mouse anti-ERK (MAPK-YT, Sigma) antibodies at 1:2000 dilution. Mouse anti-tubulin antibodies (DM1A, Sigma; 1:4000) were used as loading controls. HRP-conjugated anti-rabbit (Jackson Immunochemicals; 1:10,000) and anti-mouse (DakoCytomation; 1:2000) were used as secondary antibodies.
MBP pulldown
ndk-1 and ksr-2 inserts were generated from cDNAs by PCR using the following primers (5′-3′): ndk-1, CAGAAGATCTATGAGCAACACTGAGAGAACC and ATAGTTTAGCGGCCGCTTTCGTAGACCCATGAGTTG; ksr-2, CTGAGGATCCATGAGCGATGAAAAGAAGAAAAAGC and ATAGTTTAGCGGCCGCTAAACAGTGGATTCTCGTGTGC; murine Ksr1, CAGAGATCTATGGATAGAGCGGCGTTGCG and ATAGTTTAGCGGCCGCTCATCTTTGGATTACCGGACTCC. ndk-1 was ligated into a modified pET vector (Novagen) and the GST::NDK-1::His6 protein was expressed in E. coli strain Rosetta pLysS. ksr-2 was ligated into a modified pFastBac vector (Invitrogen). MBP::KSR-2::His6 and murine MBP::KSR1 proteins were expressed in the Sf9 cell line using the BAC-to-BAC baculovirus expression system. NDK-1 was purified using Ni affinity and GST chromatography techniques. The MBP pulldown assay was performed in 200 μl total volume of 20 mM Tris buffer (pH 8) containing 1 mM EDTA, 0.1% IGEPAL, 5 mM β-mercaptoethanol, 2 mM benzamidine, 0.25 mM PMSF, 0.15 mM NaCl. Each binding reaction contained 5 μM NDK-1 and 30 μl MBP resin (New England Biolabs) saturated with MBP::KSR::His6. The reaction mixture was incubated at room temperature for 20 minutes, washed three times, then protein samples were analyzed by western blot using an anti-GST antibody (Cell Signaling). Control binding reaction contained MBP resin saturated with MBP protein only.
RESULTS
ndk-1, the sole Caenorhabditis elegans ortholog of group I NDPKs, is an essential gene
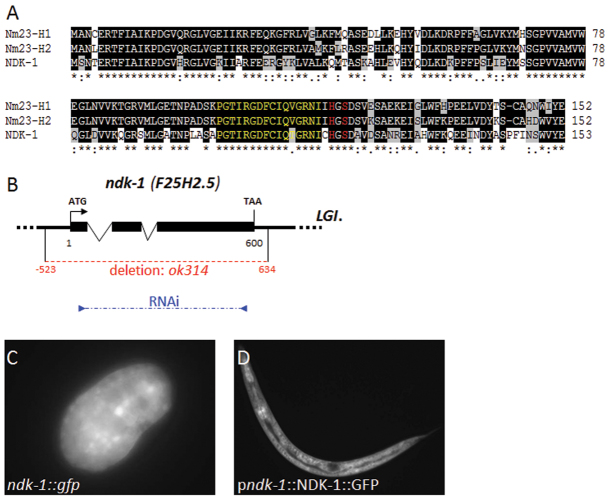
The C. elegans genome contains three open reading frames (ORFs), F25H2.5, F55A3.6 and Y48G8AL.15, which show high sequence similarity to NDPKs (Bilitou et al., 2009). F25H2.5 is the most closely related to group I NDPKs. The other two nematode NDPK paralogs, F55A3.6 and Y48G8AL.15, are more divergent and belong to group II NDPKs. Here, we focused on F25H2.5, which shows 65% sequence identity and 85% overall similarity to NM23-H1 and NM23-H2 (Fig. 1A). NM23-H1 amino acids His118 and Ser120, which are reported to be essential for kinase activity (Steeg et al., 2003) and NDPK multimer formation (Mocan et al., 2007), respectively, are conserved in the worm protein. A 16 amino acid stretch N-terminal to the catalytic site at His118 is also highly conserved (indicated by yellow lettering in Fig. 1A). Based on this sequence conservation, we named the F25H2.5 gene ndk-1 (C. elegans ortholog of nucleoside diphosphate kinase-1). Sequence analysis of the ndk-1 full-length cDNA clone yk1105 confirmed the exon-intron structure predicted by WormBase (Fig. 1B).
Fig. 1.
C. elegans ndk-1 is an essential gene. (A) Sequence alignment of human NM23-H1 (NME1), NM23-H2 (NME2) and C. elegans NDK-1 (F25H2.5) proteins. Identical and similar residues are highlighted with black and gray shading, respectively. NDK-1 shows 85% overall similarity to both NM23-H1 and -H2 isoforms. Amino acids His118 and Ser120 (red), which are essential for kinase activity and NDPK multimer formation, respectively, are conserved in these proteins. A 16 amino acid stretch N-terminal to the catalytic site at His118 (yellow) is also highly conserved. (B) Genomic structure of the ndk-1 gene. Sequencing of the ndk-1 full-length cDNA clone yk1105 confirmed the exon-intron structure predicted by WormBase. The dotted red line shows the position of the ok314 deletion, which includes the complete ORF of ndk-1, a 523 bp 5′ regulatory region and 34 bp of the 3′UTR. The position of primers used for generating the ndk-1 RNAi clone is indicated in blue. (C) Ubiquitous expression of a transcriptional ndk-1::gfp reporter in a comma stage C. elegans embryo. (D) Expression of a functional translational fusion NDK-1::GFP reporter in an L2 stage larva.
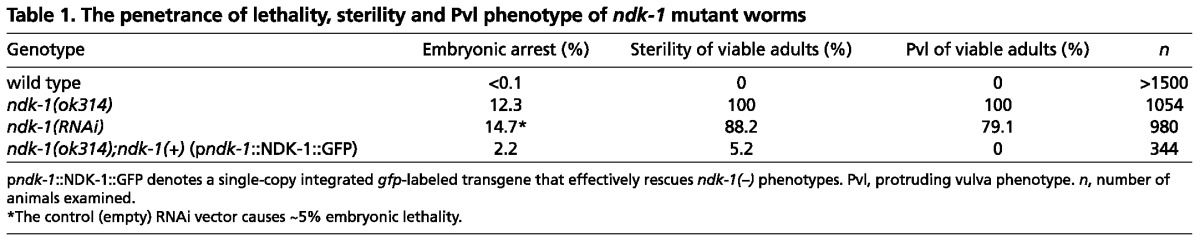
To assess the function of ndk-1 in development, we analyzed the ok314 1157 bp deletion allele of ndk-1, which removes the entire ndk-1 ORF, as well as 523 bp of upstream and 34 bp of downstream sequences (Fig. 1B). We backcrossed the ok314 null allele five times to eliminate unlinked mutations and balanced it with hT2qIs48 in order to allow for stable propagation of the ndk-1(ok314) mutation. Analysis of the progeny of ok314/+ heterozygotes revealed that nearly 12% of ok314 homozygotes die as embryos, while the remainder develop into sterile adults that display a protruding vulva phenotype (Pvl) (Table 1). In addition, we observed low penetrance Clear (Clr) and Constipation (Con) phenotypes in mutant adults (see below). The Clr phenotype was often coupled with a fluid-filled-like appearance to the affected worms. To confirm that this pleiotropic mutant phenotype associated with ok314 is indeed caused by this deletion, we depleted NDK-1 by RNAi. Many RNAi-treated worms were sterile and had a Pvl phenotype, and the remainder produced a reduced number of progeny (Table 1). An integrated single-copy, gfp-labeled ndk-1(+) transgene (pndk-1::NDK-1::GFP; see Materials and methods) was able to rescue the mutant ndk-1(-) phenotypes (Table 1). This rescue, together with the convergence of deletion and RNAi phenotypes, indicates that NDK-1 is required to form a properly developed vulva and for normal germline development. The weaker RNAi phenocopy is likely to be due to only partial depletion of NDK-1.
Table 1.
The penetrance of lethality, sterility and Pvl phenotype of ndk-1 mutant worms
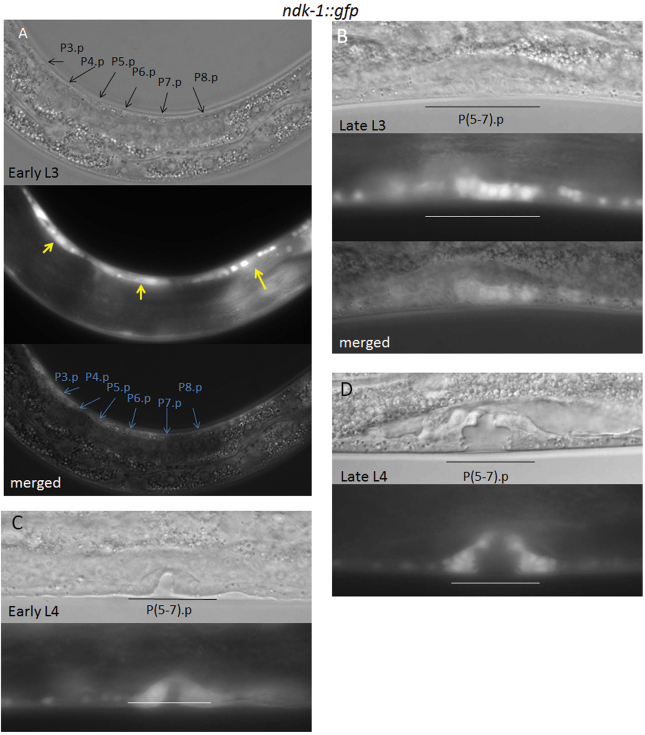
NDK-1 expression established by the aforementioned GFP fusion as well as by a transcriptional reporter construct converged, and expression was observed in different developmental stages, from embryonic through all larval stages to adulthood (Fig. 1C,D). During larval development, GFP expression was detected in the hypodermis, the intestine and body wall muscles, as well as in neurons residing in the ventral nerve cord, the head and the tail (Fig. 1D). The ndk-1::gfp reporter was ubiquitously expressed in the developing vulva tissue from mid-L3 larval stage until adulthood (Fig. 2, Fig. 3D).
Fig. 2.
ndk-1 is expressed in the developing vulval tissue. (A) Expression of the ndk-1::gfp reporter in an early L3 larva. The position of vulval precursor cells (VPCs) is indicated by black and light blue arrows on the DIC and merged images, respectively. At this developmental stage, NDK-1 reporter expression is not detected in VPCs. Instead, hypodermal seam cells show reporter expression (yellow arrows). Note that many cells from the ventral nerve cord are also fluorescent, possibly obscuring a lower level of expression in VPCs. (B) In late L3, NDK-1 accumulates in the VPC descendants. P(5-7).pxx cells are underlined. (A,B) Top, DIC image; middle, the corresponding fluorescence image; bottom, merge. (C) ndk-1 expression in the developing vulval tissue at the early L4 larval stage. (D) The expression of ndk-1 in the vulval cells of a late L4 stage larva. (C,D) Top, DIC image; bottom, the corresponding fluorescence image.
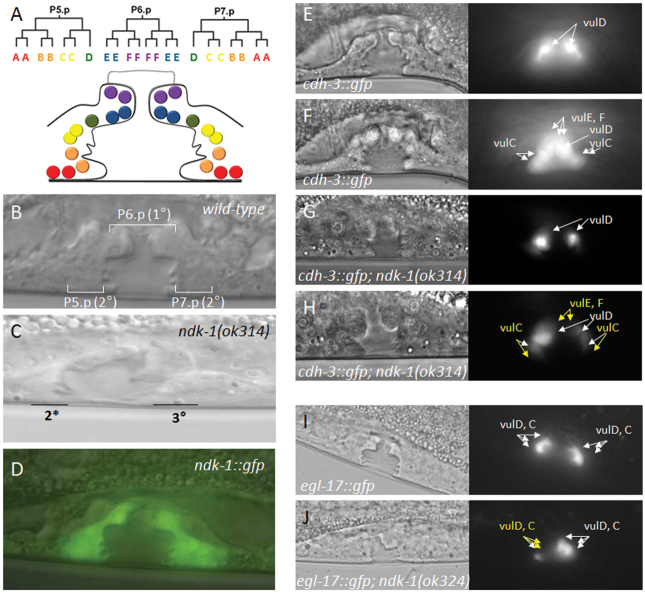
Fig. 3.
ndk-1(ok314) mutants display defects in vulval development. (A) The wild-type, mid-L4 stage vulva is composed of 22 cells, which can be grouped into seven cell types (vulA, B1, B2, C, D, E and F). These cells are the great-granddaughters of P5.p-P7.p VPCs (blue and violet circles, primary lineages; red, orange, yellow and green circles, secondary lineages). (B,C) The vulva structure of a wild-type (N2) mid-L4 stage larva (B) and an ndk-1(ok314) mutant mid-L4 stage larva (C). The mutant vulval structure is not symmetrical. (D) A transcriptional ndk-1::gfp reporter is expressed in the developing vulva of an L4 stage larva. (E-H) cdh-3::gfp is a marker for vulD, C, E and F cells (white arrows) at the L4 stage. Each figure consists of a fluorescent image (right) of cdh-3::gfp expression and a DIC image (left) of the corresponding focal plane. (E,F) cdh-3::gfp expression in a wild-type worm in two different focal planes: the focal plane of vulD cells (E) and that of vulC, E and F cells (F). (G,H) In an ndk-1(ok314) homozygote, vulD cells are present (G, white arrows), but vulE and F cells are missing (H, yellow arrows). (I,J) Expression pattern of the Ras/MAPK pathway target gene egl-17 in a wild-type mid-stage L4 larva (I) and in an ok314 mutant L4 stage larva (J). Both comprise a fluorescent image (right) of egl-17::gfp expression and a DIC image (left) of the corresponding focal plane. The ndk-1 mutant shows reduced expression of egl-17::gfp, indicating that Ras/MAPK signaling is inhibited in the ndk-1(-) mutant background; white arrows indicate correctly specified vulval cells, yellow arrows show the position of non-fluorescent vulval cells that would normally express egl-17.
ndk-1(-) mutants display defects in vulval development
We first analyzed the Pvl phenotype associated with ndk-1(ok314) mutants. The Pvl phenotype is characterized by the eversion of the vulval tissue and the formation of a single protrusion, and can be the consequence of misspecification of vulval cell fates, impaired vulval morphogenesis (Eisenmann and Kim, 2000) or partial cell cycle defects (O’Connell et al., 1998). The gross vulval morphology of homozygous ndk-1(ok314) mutants showed an aberration of the characteristic ‘christmas tree’ structure at the L4 larval stage; the vulval structure at this stage was not symmetrical, which was likely to be due to misspecification of certain vulval cell types (Fig. 3A,C), as indicated by the misexpression of vulval cell fate markers (see below).
During C. elegans vulval development, the induced VPCs P(5-7).p undergo three rounds of cell division in response to the inductive signal and generate distinct cell types: vulA, vulB1, vulB2, vulC and vulD cells are descendants of P5.p and P7.p and are referred to as secondary lineages, whereas vulE and vulF cells are great-granddaughters of P6.p and referred to as primary cells (Fig. 3A). At the mid-L4 larval stage, the developing vulva consists of 22 cells, which subsequently migrate and undergo cell fusion events to eventually form the adult vulval structure. To analyze the nature of the Pvl phenotype of ndk-1(ok314) mutant hermaphrodites, we monitored vulval cell types using specific markers for individual vulval cell fates (Fig. 3E-H) (Inoue et al., 2002). cdh-3 (cadherin family) encodes a cadherin-related protein, and cdh-3::gfp can serve as a marker for vulC, vulD, vulE and vulF cells (Fig. 3E,F). We found that among the cells conferring secondary fates, cdh-3::gfp expression in both vulD and vulC cell types was, with variable penetrance, missing in the mutant vulva. The lack of cdh-3::gfp expression in vulD was detected in 9% of ndk-1(-) mutants, whereas missing vulC cells were observed in 36% of cases (n=98). cdh-3::gfp expression was also missing in vulE cells in 36% of the mutants, and in vulF cells in 18%, showing that ndk-1(ok314) animals also lack cells belonging to primary lineages (Fig. 3G,H). egl-17 (egg-laying defective) encodes an fibroblast growth factor (FGF) ligand and is expressed specifically in vulC and vulD at the mid-L4 larval stage (Fig. 3I). In 96% of the ndk-1(ok314) hermaphrodites (n=145), vulC and/or vulD cells failed to express the egl-17::gfp reporter (Fig. 3J).
We also considered that the failure of cell type-specific gene expression, especially during late stage vulva morphogenesis, might be an indirect consequence of a reduced number of cell cycles occurring during vulva development. We thus counted the number of vulva cells in ndk-1(ok314) mutants, and found an average of 20±1 cells (n=98) compared with the 22 cells observed in the wild type. We thus conclude that reduced cell cycle progression might also contribute to the reduced cell type-specific gene expression, but is unlikely to solely account for this defect.
EGL-17/FGF is a downstream target of EGFR/Ras/MAPK signaling during vulval development (Cui and Han, 2003). Its accumulation in the P6.p descendants (daughters/granddaughters), in which signaling normally occurs, can therefore serve as a marker for Ras-dependent vulval fate induction (Burdine et al., 1998). This prompted us to monitor EGL-17::GFP accumulation in the P6p.xx granddaughters at the late L3 stage. We found markedly reduced levels or even the absence of EGL-17 in these cells in the ndk-1(-) mutant background, as compared with the wild-type genetic background (Fig. 4A-C). From these data, we conclude that NDK-1 may be necessary for the proper adoption of vulval cell fates, consistent with the strong NDK-1 expression observed starting from the late L3 stage (Fig. 2).
Fig. 4.
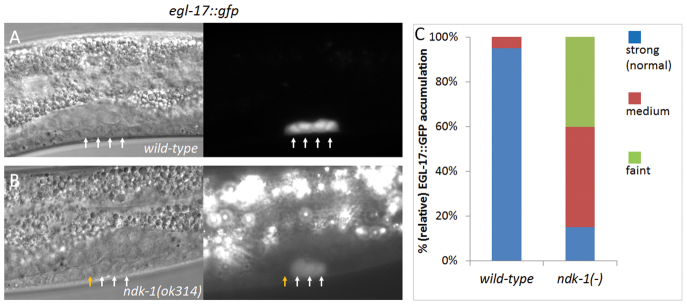
EGL-17::GFP expression is much reduced in P6p.xx cells of ndk-1(-) mutants. The accumulation of EGL-17/FGF, a marker for Ras-dependent vulval fate induction (egl-17 is an EGFR/Ras/MAPK signaling target), in the P6.p granddaughters, in which signaling actually occurs. (A) EGL-17::GFP expression in a wild-type L3 larva. The four P6.p descendants (P6p.xx, arrows) strongly accumulate EGL-17. (B) EGL-17::GFP expression in a ndk-1(-) mutant L3 larva. The expression intensity is much reduced compared with the wild type (the image was taken with a much longer exposure time than that used for the control, as indicated by the much brighter background of gut autofluorescence). In addition, one of the P6.p granddaughters (the yellow arrow) fails to accumulate EGL-17 in this specimen. (A,B) DIC images are on the left, the corresponding fluorescent images on the right. (C) Quantification of expression intensity of EGL-17::GFP in P6p.xx cells. n=20; for comparing levels of faint expression between wild type and ndk-1(-): P<0.001, unpaired Student’s t-test.
NDK-1 functions downstream of, or in parallel to, LIN-45 and upstream of MEK-2 and MPK-1 to affect vulval development
The observation that transcriptional induction of a known Ras/MAPK target, EGL-17/FGF, is alleviated in ndk-1(-) mutants, prompted us to examine whether ndk-1 might generally affect Ras/MAPK signaling. The C. elegans vulva induction pathway provides a unique experimental system, as differences in the level of MAPK signaling can be measured by scoring for the formation of a reduced or enhanced number of vulval structures.
The single vulva-like structure in ndk-1(ok314) worms indicates that a basal level of Ras/MAPK signaling needed for vulva induction still occurs in these mutants. We thus analyzed double-mutant combinations to see whether, in these genetically sensitized backgrounds, the extent of Ras/MAPK signaling is modified in vivo. We performed a genetic epistasis analysis and examined the phenotypes of ndk-1(ok314) mutants combined with various mutants defective in different steps of the Ras/MAPK signaling pathway. First, the let-23(n1045) hypomorphic mutant, which affects an EGF receptor, was crossed with ndk-1(ok314). At 15°C, let-23(n1045) single-mutant hermaphrodites are Vul (with 96% penetrance), whereas ndk-1(ok314) single-mutant adults exhibit a Pvl phenotype. We found that ndk-1(ok314);let-23(n1045) double-mutant adults have a fully penetrant Vul phenotype that affects 100% of the adults analyzed (the average number of induced VPCs in the double mutants was 0.09; n=11), consistent with the hypothesis that Ras/MAPK signaling might be further attenuated in the ndk-1(-) background. In addition, 43% of ndk-1(-);let-23(-) double mutants (n=35) were unable to develop into adults as they arrested development at earlier (L1-L3) larval stages. By contrast, larval lethality was not observed in either single mutant. This genetic interaction suggests that the ndk-1(ok314) mutation enhances the severity of the Let-23(n1045) phenotype (Vul adults) towards a Let-23 null phenotype [rod-like L1 arrest (Yochem et al., 1997)], and represents additional evidence for reduced signaling efficiency in ndk-1(-) mutants.
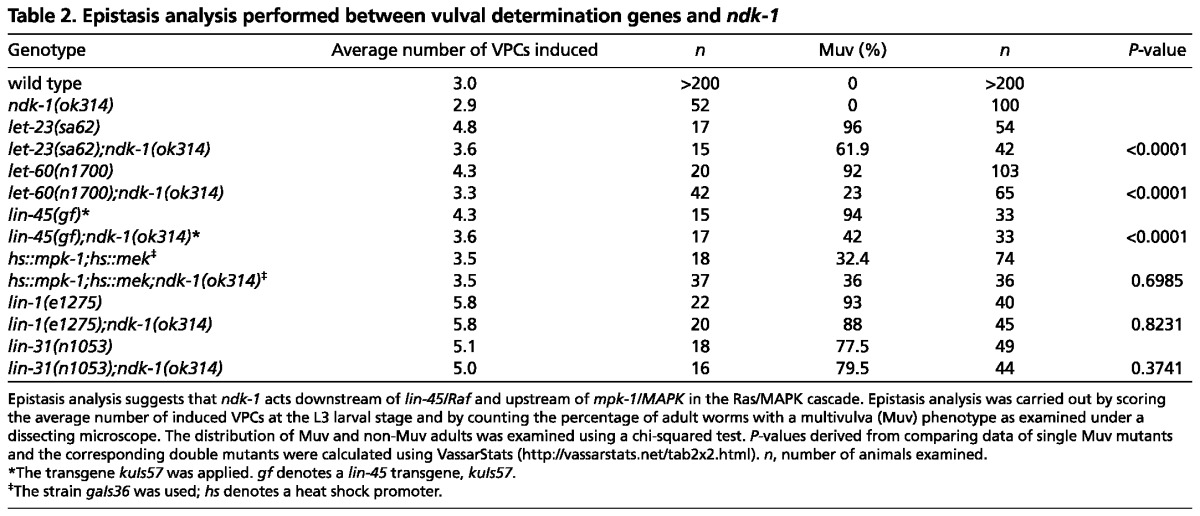
To further verify this notion and to start establishing epistatic relationships, we next crossed ndk-1(-) mutants with animals in which Ras/MAPK signaling is hyperactivated. The ndk-1 mutation ok314 significantly reduced the number of excessive vulval protrusions (the Muv multivulva phenotype) in let-23/EGFR and let-60/RAS gain-of-function mutants (Table 2). These data suggest that ndk-1 acts downstream of let-23 and let-60 to enhance Ras/MAPK signaling. To more precisely place the function of NDK-1 in the Ras/MAPK signaling pathway, we also tested its epistatic relationship with lin-45/Raf. lin-45 overexpression conferred by the transgene kuIs57 leads to a Muv phenotype (Yoder et al., 2004). We found that this phenotype is significantly reduced by the ndk-1(-) mutation, showing that NDK-1 functions downstream of LIN-45 in the vulva induction pathway (Table 2). By contrast, the excessive vulva induction conferred by overexpression of downstream components of the pathway, such as mek-2/MEK and mpk-1/MAPK, could not be suppressed by the ndk-1(ok314) deletion (Table 2). Thus, NDK-1 is likely to function upstream of MEK-2 and MPK-1. Consistently, the ectopic vulva formation conferred by deleting lin-1 and lin-31, which encode MPK-1 targets, was not suppressed by the ndk-1 deletion (Table 2). The results of this epistasis analysis were further confirmed by scoring the average number of induced VPCs in the single Muv mutants and the corresponding double mutants (Table 2). In summary, these experiments suggest that NDK-1 is required for the full activation of Ras/MPK-1 signaling but not for Ras/MPK-1 signaling per se.
Table 2.
Epistasis analysis performed between vulval determination genes and ndk-1
The epistasis analysis described above, together with the reduced expression of the LIN-1/LIN-31 target egl-17/FGF (Cui and Han, 2003), indicates that the extent of Ras/MAPK signaling might be dampened in ndk-1(ok314) mutants (Fig. 3I,J, Fig. 4). To confirm this, we directly measured the level of activated, threonine and tyrosine phosphorylated MAPK in C. elegans extracts using phospho-specific MAPK antibodies. C. elegans MPK-1/MAPK is expressed in two isoforms: a larger isoform that is expressed in the germline and a smaller isoform that is restricted to somatic tissues (Fig. 5) (Ohmachi et al., 2002). We found expression of the germline isoform to be missing in the ndk-1(ok314) mutant, consistent with the largely stunted germline development observed in ndk-1(ok314) homozygotes. The dramatic reduction of activated MAPK in the soma demonstrates that MAPK signaling, but not the basal protein level, is reduced in all somatic tissues of animals defective for NDK-1 (Fig. 5, middle panel).
Fig. 5.
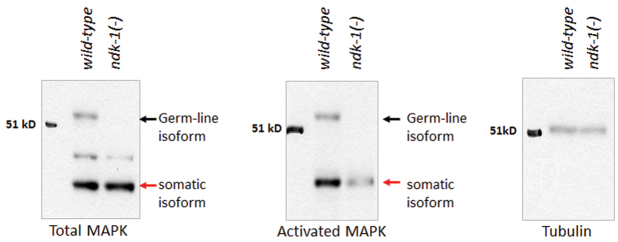
Ras/MAPK signaling is inhibited in the ndk-1(-) mutant background. Analysis of total and diphosphorylated MAPK levels of wild-type and ndk-1(ok314) worms by western blotting. Black arrows indicate the germline-specific (55 kDa) isoform of MAPK; red arrows show the somatic (45 kDa) isoform of MAPK. As a control for loading, the blot was reprobed using anti-tubulin antibody. The mutant germline completely lacks MAPK. The level of total MAPK in the soma is comparable in mutant and wild-type worms. However, the level of activated MAPK is severely reduced in ndk-1(ok314) mutants.
The Ksr-1 and Ksr-2 phenotypes are exacerbated in the ndk-1(ok314) background
Human NM23-H1 was found to interact with KSR1 in vitro, suggesting that NM23-H1 affects Ras/MAPK signaling through modulating KSR activity (Hartsough et al., 2002; Salerno et al., 2005). KSR1 is a conserved scaffold protein that is required for signaling through the Ras/MEK signaling module, but the function of the NM23-KSR1 interaction has not been determined in vivo. In addition, unlike data obtained from human cell cultures, genetic results presented here demonstrate a stimulatory effect of NDK-1 on Ras/MAPK signaling in somatic tissues, and we wondered whether NDK-1 might act by modulating the activity of KSR proteins. The C. elegans genome encodes two KSR paralogs, KSR-1 and KSR-2, which function redundantly in most Ras-related processes (Kornfeld et al., 1995; Sundaram and Han, 1995; Ohmachi et al., 2002; Sundaram et al., 1996). KSR-1 and KSR-2 share 26% and 31% sequence identity with murine KSR1 (Ohmachi et al., 2002). Our genetic results indicated that ndk-1 occupies a similar epistasis position in the Ras/MAPK signaling pathway as that reported for KSRs, which function downstream of lin-45 and upstream of mek-2 and mpk-1 to affect vulval development (Table 2) (Yoder et al., 2004). Therefore, we assessed genetic interactions between ndk-1 and the two worm KSR paralogs.
ndk-1(ok314);ksr-1(n2526) double-mutant animals developed into sterile adults showing a Pvl phenotype, as it is the case for the ndk-1(ok314) single mutant. However, the 33% penetrant egg-laying defective (Egl) phenotype associated with the ksr-1 mutation was enhanced to 77% in the ndk-1(ok314)/+ heterozygous ksr-1(n2526) strain (n=125; Table 3). The Egl phenotype of ksr-1(n2526) single mutants is due to a defect in sex myoblast migration, a process that also relies on Ras/MAPK signaling (Sundaram et al., 1996). By contrast, vulval development in ksr-1(n2526) single mutants appears normal. The enhanced Egl phenotype observed in ndk-1(ok314)/+;ksr-1(n2526) worms might therefore arise either from abnormal sex myoblast migration or from defects associated with the morphogenesis of the vulva. To address this issue, we checked vulval morphology in the F1 progeny of ndk-1(ok314)/+;ksr-1(n2526) hermaphrodites. Abnormal vulval structures were observed in 60% of L3-L4 stage worms (n=20; data not shown). Thus, given that only 25% of these animals are expected to be homozygous for the ndk-1(ok314) mutation, the high penetrance of abnormal vulval structures suggests that the vulva development of ndk-1(ok314)/+;ksr-1(n2526) worms is abnormal [note that ksr-1(n2526) mutants form a wild-type-like ‘christmas tree’ in L4]. To gain a deeper insight into the vulva development of ndk-1(ok314)/+;ksr-1(n2526) worms, we counted the number of induced VPCs in these animals (the average number of induced VPCs was 2.83; n=12) and found that they possess a mild induction defect similar to ndk-1(-) single mutants. Thus, the enhanced egg-laying defect might be due to a combination of a defective vulva and abnormal sex myoblast migration. Irrespectively, we convincingly show a genetic interaction between ndk-1 and ksr-1.
Table 3.
ndk-1 shows a genetic interaction with both ksr-1 and ksr-2
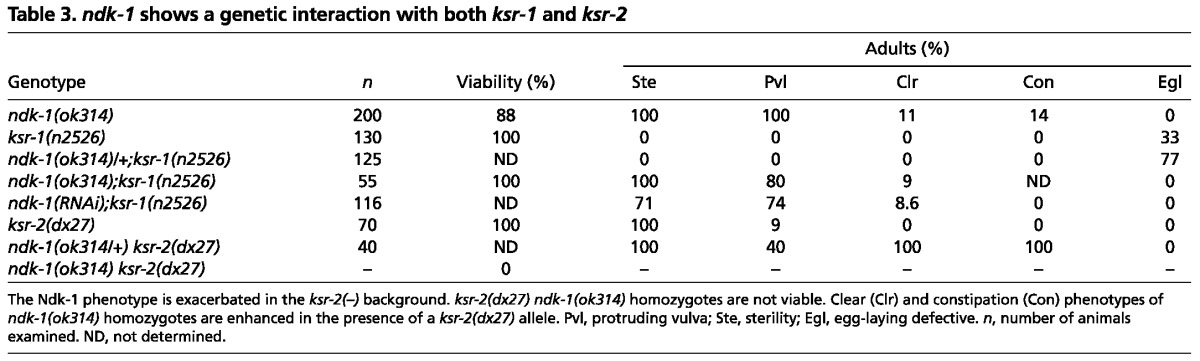
We next focused on possible genetic interactions between ndk-1 and ksr-2. Given that both genes are located on chromosome I, we generated a cis-heterozygous ndk-1(ok314) ksr-2(dx27)/++ strain balanced with hT2qIs48, which carries gfp as a marker [ndk-1(ok314)ksr-2(dx27)/hT2qIs48]. This strain segregates GFP-positive larvae and adults only, indicating that (non-GFP) ndk-1 ksr-2 homozygous double-mutant worms are embryonic lethal (Table 3).
To further assess genetic interactions between ksr-2 and ndk-1, we analyzed ndk-1(ok314) ksr-2(dx27)/+ ksr-2(dx27) worms, expecting that Ndk-1 phenotypes might be enhanced. In addition to sterility and the Pvl phenotype already observed in the ndk-1(ok314) single mutant, these animals also showed 100% penetrant clear (Clr) and constipation (Con) phenotypes (Fig. 6A, Table 3). Clr and Con phenotypes were also observed in ndk-1(ok314) single mutants, although with a low penetrance (Table 3). The Clr phenotype can be associated with defects in protein degradation in muscles (Szewczyk and Jacobson, 2003) and is reported to result from excessive FGFR/Ras/MAPK signaling (Kokel et al., 1998). However, decreased EGFR/Ras/MAPK activity in the excretory duct can also result in arrested Clr animals (Yochem et al., 1997; Abdus-Saboor et al., 2011). The Con phenotype is also linked to excessive Ras/MAPK signaling as implicated in the defense mechanism against pathogens (Nicholas and Hodgkin, 2004). However, a reduced level of Ras/MAPK signaling can also lead to a Con phenotype (Nicholas and Hodgkin, 2004). Nevertheless, these genetic interactions indicate that NDK-1 might modulate KSR activity, which in turn affects Ras/MAPK signaling.
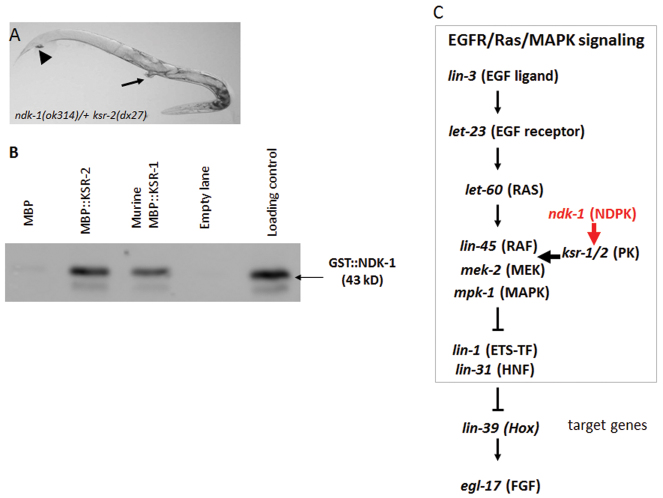
Fig. 6.
NDK-1 interacts with KSRs. (A) ndk-1(ok314) ksr-2(dx27)/+ ksr-2(dx27) animals are clear, have a protruding vulva (arrow) and display a constipation (arrowhead) phenotype. (B) In vitro pulldown assay shows that NDK-1 binds to KSR-2 and to murine KSR1. Western blot was performed with anti-GST antibody. Lane 1, resin-bound MBP and GST::NDK-1 as a negative control; lane 2, MBP::KSR-2 and GST::NDK-1; lane 3, MBP::KSR1 (mouse protein) and GST::NDK-1; lane 5, GST::NDK-1 as positive control for loading. (C) Epistatic position of ndk-1 in Ras/MAPK signaling. ndk-1 acts downstream of lin-45/Raf and upstream of mpk-1/MAPK in the Ras/MAPK pathway during vulval development. We propose that NDK-1 activates the Ras/MAPK cascade through modification of the activity of KSR scaffolds.
To assess whether modulation of KSR activity by NDK-1 might be mediated by a direct interaction, we tried to establish whether KSR and NDK-1 proteins can directly interact. Given that we are working in an in vivo system in which key biochemical interactions are predicted to occur in only a subset of cells, we focused on in vitro interactions using pulldown experiments with recombinant GST::NDK-1 and MBP::KSR fusion proteins. We managed to express C. elegans KSR-2, but could not express worm KSR-1 and therefore used mouse KSR1. These pulldown experiments demonstrate that both murine KSR1 and worm KSR-2 interact with NDK-1 (Fig. 6B). Thus, the increased level of Ras/MAPK signaling conferred by NDK-1 might reflect direct modulation of KSR activity by NDK-1.
DISCUSSION
In this study we characterized ndk-1, the C. elegans ortholog of human NM23-H1/H2 (NME1/2). ndk-1 knockouts display sterility and a protruding vulva. By analyzing the vulva phenotype, we demonstrated that both primary and secondary vulval cell fates are affected in ndk-1(-) mutant hermaphrodites, raising the hypothesis that ndk-1 influences the execution and/or stage-specific gene expression of several vulval cell types. In addition, we assessed whether ndk-1 modifies Ras/MAPK signaling. We initially focused on the inductive (Ras) signaling pathway, which is needed to initiate vulva development at the early L3 stage (Sternberg, 2005). Epistasis analysis performed with ndk-1 and Ras pathway mutants demonstrated that ndk-1 acts downstream of, or in parallel to, lin-45/Raf, and upstream of mek-2/MEK and mpk-1/MAPK to affect the formation of the vulva tissue. NDK-1 deficiency suppressed the extent of multiple vulva formation conferred by let-23(gf), let-60(gf) and lin-45(gf) gain-of-function (gf) mutants (Table 2). Conversely, the ndk-1(ok314) mutation, although not itself causing a reduction in the number of vulva structures formed, nevertheless results in a reduced level of MAPK signaling. The level of egl-17/FGF, a known MAPK target (Cui and Han, 2003), is reduced in ndk-1 mutants in the developing vulval tissue (Fig. 3I,J, Fig. 4), similar to the global levels of activated MAPK (Fig. 5). In summary, NDK-1 is necessary for proper MAPK activation in somatic tissues.
We noted that our ndk-1 reporter only showed strong expression from the late L3 stage onwards. We speculate that the effects of NDK-1 in the vulva induction pathway only require a very low level of expression that is not detectable by our reporter. The vulva induction system provides the unique possibility to uncover and characterize modulators of MAPK signaling; even so single mutants similar to ndk-1, such as sur-6/PP2A or par-1, display only mild (e.g. Pvl) or no vulval phenotype on their own (Sieburth et al., 1999; Hurd and Kemphues, 2003; Yoder et al., 2004).
As NDPKs have been associated with diverse biological processes in other model systems, it is not surprising that NDK-1 also has multiple functions in the worm. Besides modulating Ras/MAPK signaling, NDK-1 may also act in later processes during vulval development, such as cell fate execution, as strong NDK-1 expression can be detected from the late L3 stage onwards, stages at which fate patterning of the VPC daughter cells occurs. Defects in the secondary vulval lineages of ndk-1(-) mutants (Fig. 3) raised the question of whether NDK-1 plays a role in LIN-12/Notch-mediated lateral signaling, which is essential for secondary cell fate specification (Greenwald, 2005). However, depleting NDK-1 in animals with hyperactive lin-12 function did not alter vulval induction, as compared with lin-12(gf) mutants (data not shown).
KSRs act as scaffold proteins in the Ras/MAPK cascade and assemble Raf/MEK complexes, thereby facilitating MAPK activation (Kolch, 2005). C. elegans KSR-1 and KSR-2 function redundantly in most Ras-related processes in somatic tissues, such as the vulva tissue, the excretory system and the male spicules, whereas KSR-2 acts alone in the germline to ensure meiotic progression (Ohmachi et al., 2002). Since ndk-1 and the KSR paralogs operate at the same level in the Ras/MAPK pathway, and they all contribute to the process of MAPK activation, we generated ndk-1(-);ksr-1(-) and ndk-1(-)ksr-2(-) double knockouts in order to test potential genetic interactions between ndk-1 and the KSR genes. We conclude from these results that NDK-1 may act together with both KSRs during development, as depletion of either ksr-1 or ksr-2 in the ndk-1(-) background results in enhancement of the single-mutant phenotypes. In the progeny of ndk-1(-)/+;ksr-1(-) animals we saw enhancement of the single ksr-1(-) mutant phenotype (Egl). By contrast, in ndk-1(-)/+ ksr-2(-) worms, characteristics of ndk-1, such as the Clr and Con phenotypes, as well as embryonic lethality are exacerbated. Embryonic lethality of homozygous ndk-1(-) mutants (50%) becomes fully penetrant in the ksr-2 mutant background.
ksr-1 and ksr-2 were reported to act redundantly in the development of the male spicule and in the formation of the excretory duct (Ohmachi et al., 2002). We observed that ndk-1(ok314) males exhibit an abnormal tail without rays (100%, n=36), suggesting that NDK-1 also participates in male tail development. However, ndk-1 mutants display no L1 larval lethality, a Ras phenotype reported to be linked to the loss of the excretory duct cell (Yochem et al., 1997). A possible explanation is that excretory duct cell specification might be mediated redundantly by the worm NDPK paralogs or that the maternal contribution of NDK-1 is sufficient for this developmental event to occur. Alternatively, MAPK signaling needed for excretory duct function might not require NDK-1. Analysis of ndk-1(-)/+ ksr-2(-) animals revealed the enhancement of Clr and Con phenotypes. At present we cannot explain why the ndk-1 mutation enhances the Clr phenotype in a ksr-2(-) mutant background. The cause of the 10% penetrant Clr phenotype in ndk-1(-) single mutants and its enhancement by ksr-2(-) will need further investigation.
Human NM23-H1 was shown to bind to KSR1 in tissue culture models (Hartsough et al., 2002; Salerno et al., 2005; Tso et al., 2013), and NM23-H1 overexpression in those cells resulted in reduced levels of MAPK signaling, the importance of which has not been demonstrated in vivo. Another study also suggested that increased levels of NM23-H2 blocks the ERK pathway (Lee et al., 2009). Here, we provide a compelling case that NDK-1 affects Ras/MAPK signaling at the level of KSRs.
In contrast to previous studies on mammalian cell lines, we found that NDK-1 promotes rather than suppresses Ras/MAPK signaling. Given that NDK-1 directly interacts with KSR-2, and possibly with KSR-1, we consider it likely that NDK-1 modifies the function of KSR scaffolds. We postulate that this modification alters KSR activity but is not absolutely essential for its function, as the ndk-1(ok314) mutation moderately decreases activated somatic MAPK levels and vulval induction in Ras hyperactive genetic backgrounds. It was previously reported that mouse KSR1 harbors 14-3-3 domain binding sites (Ser297 and Ser392, numbered according to the mouse KSR1 sequence) that are involved in KSR scaffold regulation in mammals (Müller et al., 2001). Comparison of mammalian and worm KSR sequences, however, reveals that Ser297 and Ser392 are not evolutionary conserved among different KSR proteins (Ohmachi et al., 2002). Overall, these findings suggest that KSR scaffold proteins could play diverse and context-dependent physiological roles in regulatory circuits controlling MAPK activation. Further studies are needed to dissect the molecular mechanism by which worm NDK-1 mediates the regulation of MAPK activity.
Earlier studies demonstrated that Nm23 proteins regulate neuronal differentiation in Xenopus and in cell lines (Mochizuki et al., 2009). Studies on knockout mice have shown that Nm23-M1 (Nme1) mutant females cannot feed their pups due to growth retardation of the mammary glands and defects in the final step of mammary duct maturation of the nipple leading to duct obstruction (Deplagne et al., 2011). These genetic data are consistent with the prevailing idea that Nm23 proteins are involved in cell growth and differentiation. Our present data demonstrate that NDK-1/NM23-H1 controls differentiation through enhancing Ras/MAPK signaling. Activation of Ras/MAPK signaling by NDK-1/NDPK might be relevant in ovarian, prostate and pancreatic cancers in which Nm23 proteins are overexpressed (Harłozińska et al., 1996; Igawa et al., 1994; Andolfo et al., 2011; Takadate et al., 2012).
Acknowledgments
We thank M. V. Sundaram and Y. Kohara for cDNA clones; M. Han, A. Hajnal and the Caenorhabditis Genetics Center (which is funded by the National Institutes of Health) for nematode strains; T. Korcsmáros for preparing Fig. 2D; and Cs. Verasztó for statistical analysis.
Footnotes
Funding
This work was supported by the OTKA Hungarian Scientific Research Fund [PD75477 to K.T.-V.] T.V. is supported by OTKA [K109349]. A.M. is supported by the Wellcome Trust [069150]. A.G. is a Wellcome Trust Senior Research Fellow [0909444/Z/09/Z]. N.M. and E.P. were supported by Parkinson’s UK [H-0709] and CRUK [C11852/A8052] PhD fellowships. K.T.-V., T.V. and A.R. are grantees of the János Bolyai Scholarship of the Hungarian Academy of Sciences. A.R. is a Wellcome Trust International Senior Research Fellow [081665/Z/06/Z]. The work was also supported by the ‘Lendület’ grant from the Hungarian Academy of Sciences to A.R. Deposited in PMC for immediate release.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
K.T.-V., A.M., A.G. and T.V. designed research; N.M., L.F., E.P., A.A., T.V. and K.T.-V. performed research; A.R., A.G. and K.T.V. analyzed data; K.T.-V., A.M., A.G. and T.V. wrote the paper.
References
- Abdus-Saboor I., Mancuso V. P., Murray J. I., Palozola K., Norris C., Hall D. H., Howell K., Huang K., Sundaram M. V. (2011). Notch and Ras promote sequential steps of excretory tube development in C. elegans. Development 138, 3545–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo I., De Martino D., Liguori L., Petrosino G., Troncone G., Tata N., Galasso A., Roma C., Chiancone F., Zarrilli S., et al. (2011). Correlation of NM23-H1 cytoplasmic expression with metastatic stage in human prostate cancer tissue. Naunyn Schmiedebergs Arch. Pharmacol. 384, 489–498 [DOI] [PubMed] [Google Scholar]
- Biggs J., Hersperger E., Steeg P. S., Liotta L. A., Shearn A. (1990). A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell 63, 933–940 [DOI] [PubMed] [Google Scholar]
- Bilitou A., Watson J., Gartner A., Ohnuma S. (2009). The NM23 family in development. Mol. Cell. Biochem. 329, 17–33 [DOI] [PubMed] [Google Scholar]
- Burdine R. D., Branda C. S., Stern M. J. (1998). EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125, 1083–1093 [DOI] [PubMed] [Google Scholar]
- Cui M., Han M. (2003). Cis regulatory requirements for vulval cell-specific expression of the Caenorhabditis elegans fibroblast growth factor gene egl-17. Dev. Biol. 257, 104–116 [DOI] [PubMed] [Google Scholar]
- Dammai V., Adryan B., Lavenburg K. R., Hsu T. (2003). Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev. 17, 2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplagne C., Peuchant E., Moranvillier I., Dubus P., Dabernat S. (2011). The anti-metastatic nm23-1 gene is needed for the final step of mammary duct maturation of the mouse nipple. PLoS ONE 6, e18645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes T., Pontarotti P., Fauvel C., Bobe J. (2009). Nme protein family evolutionary history, a vertebrate perspective. BMC Evol. Biol. 9, 256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann D. M., Kim S. K. (2000). Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics 156, 1097–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. (2005). LIN-12/Notch signaling in C. elegans. In WormBook: The Online Review of C. elegans Biology (ed. The C. elegans Research Community). doi/10.1895/wormbook.1.6.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Harłozińska A., Bar J. K., Gerber J. (1996). nm23 expression in tissue sections and tumor effusion cells of ovarian neoplasms. Int. J. Cancer 69, 415–419 [DOI] [PubMed] [Google Scholar]
- Hartsough M. T., Morrison D. K., Salerno M., Palmieri D., Ouatas T., Mair M., Patrick J., Steeg P. S. (2002). Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. J. Biol. Chem. 277, 32389–32399 [DOI] [PubMed] [Google Scholar]
- Heimann R., Hellman S. (2000). Individual characterisation of the metastatic capacity of human breast carcinoma. Eur. J. Cancer 36 13 Spec No, 1631–1639 [DOI] [PubMed] [Google Scholar]
- Hippe H. J., Wolf N. M., Abu-Taha I., Mehringer R., Just S., Lutz S., Niroomand F., Postel E. H., Katus H. A., Rottbauer W., et al. (2009). The interaction of nucleoside diphosphate kinase B with Gbetagamma dimers controls heterotrimeric G protein function. Proc. Natl. Acad. Sci. USA 106, 16269–16274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H. J., Wolf N. M., Abu-Taha H. I., Lutz S., Le Lay S., Just S., Rottbauer W., Katus H. A., Wieland T. (2011). Nucleoside diphosphate kinase B is required for the formation of heterotrimeric G protein containing caveolae. Naunyn Schmiedebergs Arch. Pharmacol. 384, 461–472 [DOI] [PubMed] [Google Scholar]
- Hofmann E. R., Milstein S., Boulton S. J., Ye M., Hofmann J. J., Stergiou L., Gartner A., Vidal M., Hengartner M. O. (2002). Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 12, 908–918 [DOI] [PubMed] [Google Scholar]
- Hurd D. D., Kemphues K. J. (2003). PAR-1 is required for morphogenesis of the Caenorhabditis elegans vulva. Dev. Biol. 253, 54–65 [DOI] [PubMed] [Google Scholar]
- Igawa M., Rukstalis D. B., Tanabe T., Chodak G. W. (1994). High levels of nm23 expression are related to cell proliferation in human prostate cancer. Cancer Res. 54, 1313–1318 [PubMed] [Google Scholar]
- Inoue T., Sherwood D. R., Aspöck G., Butler J. A., Gupta B. P., Kirouac M., Wang M., Lee P. Y., Kramer J. M., Hope I., et al. (2002). Gene expression markers for Caenorhabditis elegans vulval cells. Mech. Dev. 119 Suppl 1, S203–S209 [DOI] [PubMed] [Google Scholar]
- Kokel M., Borland C. Z., DeLong L., Horvitz H. R., Stern M. J. (1998). clr-1 encodes a receptor tyrosine phosphatase that negatively regulates an FGF receptor signaling pathway in Caenorhabditis elegans. Genes Dev. 12, 1425–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W. (2005). Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6, 827–837 [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Hom D. B., Horvitz H. R. (1995). The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell 83, 903–913 [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Jeong W. J., Oh J. W., Choi K. Y. (2009). NM23H2 inhibits EGF- and Ras-induced proliferation of NIH3T3 cells by blocking the ERK pathway. Cancer Lett. 275, 221–226 [DOI] [PubMed] [Google Scholar]
- Mocan I., Georgescauld F., Gonin P., Thoraval D., Cervoni L., Giartosio A., Dabernat-Arnaud S., Crouzet M., Lacombe M. L., Lascu I. (2007). Protein phosphorylation corrects the folding defect of the neuroblastoma (S120G) mutant of human nucleoside diphosphate kinase A/Nm23-H1. Biochem. J. 403, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Bilitou A., Waters C. T., Hussain K., Zollo M., Ohnuma S. (2009). Xenopus NM23-X4 regulates retinal gliogenesis through interaction with p27Xic1. Neural Dev. 4, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J., Ory S., Copeland T., Piwnica-Worms H., Morrison D. K. (2001). C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8, 983–993 [DOI] [PubMed] [Google Scholar]
- Nicholas H. R., Hodgkin J. (2004). The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 14, 1256–1261 [DOI] [PubMed] [Google Scholar]
- O’Connell K. F., Leys C. M., White J. G. (1998). A genetic screen for temperature-sensitive cell-division mutants of Caenorhabditis elegans. Genetics 149, 1303–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmachi M., Rocheleau C. E., Church D., Lambie E., Schedl T., Sundaram M. V. (2002). C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr. Biol. 12, 427–433 [DOI] [PubMed] [Google Scholar]
- Rochdi M. D., Laroche G., Dupré E., Giguère P., Lebel A., Watier V., Hamelin E., Lépine M. C., Dupuis G., Parent J. L. (2004). Nm23-H2 interacts with a G protein-coupled receptor to regulate its endocytosis through an Rac1-dependent mechanism. J. Biol. Chem. 279, 18981–18989 [DOI] [PubMed] [Google Scholar]
- Salerno M., Palmieri D., Bouadis A., Halverson D., Steeg P. S. (2005). Nm23-H1 metastasis suppressor expression level influences the binding properties, stability, and function of the kinase suppressor of Ras1 (KSR1) Erk scaffold in breast carcinoma cells. Mol. Cell. Biol. 25, 1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D. S., Sundaram M., Howard R. M., Han M. (1999). A PP2A regulatory subunit positively regulates Ras-mediated signaling during Caenorhabditis elegans vulval induction. Genes Dev. 13, 2562–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. (1988). Evidence for a novel gene associated with low tumor metastatic potential. J. Natl. Cancer Inst. 80, 200–204 [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Palmieri D., Ouatas T., Salerno M. (2003). Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Lett. 190, 1–12 [DOI] [PubMed] [Google Scholar]
- Sternberg P. W. (2005). Vulval development. WormBook: The Online Review of C. elegans Biology (ed. The C. elegans Research Community). doi/10.1895/wormbook.1.6.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Sundaram M., Han M. (1995). The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell 83, 889–901 [DOI] [PubMed] [Google Scholar]
- Sundaram M., Yochem J., Han M. (1996). A Ras-mediated signal transduction pathway is involved in the control of sex myoblast migration in Caenorhabditis elegans. Development 122, 2823–2833 [DOI] [PubMed] [Google Scholar]
- Szewczyk N. J., Jacobson L. A. (2003). Activated EGL-15 FGF receptor promotes protein degradation in muscles of Caenorhabditis elegans. EMBO J. 22, 5058–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadate T., Onogawa T., Fujii K., Motoi F., Mikami S., Fukuda T., Kihara M., Suzuki T., Takemura T., Minowa T., et al. (2012). Nm23/nucleoside diphosphate kinase-A as a potent prognostic marker in invasive pancreatic ductal carcinoma identified by proteomic analysis of laser micro-dissected formalin-fixed paraffin-embedded tissue. Clin. Proteomics 9, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso P. H., Wang Y., Yung L. Y., Tong Y., Lee M. M., Wong Y. H. (2013). RGS19 inhibits Ras signaling through Nm23H1/2-mediated phosphorylation of the kinase suppressor of Ras. Cell. Signal. 25, 1064–1074 [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Vu N. D. (1995). Phosphorylation of ATP-citrate lyase by nucleoside diphosphate kinase. J. Biol. Chem. 270, 21758–21764 [DOI] [PubMed] [Google Scholar]
- Yochem J., Sundaram M., Han M. (1997). Ras is required for a limited number of cell fates and not for general proliferation in Caenorhabditis elegans. Mol. Cell. Biol. 17, 2716–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder J. H., Chong H., Guan K. L., Han M. (2004). Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 23, 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]