Abstract
Prolonged fixation of cells and tissues in 10% neutral buffered formalin (NBF) may decrease immunorecognition in some antigen-antibody pairs. Short fixation in 10% NBF followed by transfer to 70% ethanol has been used to overcome these effects, but the effects of this transfer on immunorecognition have not been explored adequately. We used two cell lines, DU145 (prostate cancer) and SKOV3 (ovarian cancer), grew them on coverslips and fixed them with 10% NBF at room temperature for 5 min and 12, 15, 18, 36, 108 and 180 h. Aliquots of the same cells were fixed in 10% NBF for 12 h, then transferred to 70% ethanol for 3, 6, 24, 96 and 168 h. Immunostaining with PCNA, Ki67-MIB-1, cytokeratins AE1/AE3 and EGFr was done concomitantly. In both cell lines, immunorecognition decreased between 18 and 36 h of fixation in 10% NBF for PCNA, Ki67-MIB-1 and cytokeratins AE1/AE3. By 108 to 180 h of 10% NBF exposure, there was complete loss of immunorecognition of PCNA and extensive loss of Ki67-MIB-1 and cytokeratins AE1/AE3. The effects on EGFr immunorecognition were less. Transfer to 70% ethanol after fixation for 12 h in 10% NBF preserved immunorecognition of the antibodies.
Keywords: cytokeratins AE1/AE3, EGFr, 70% ethanol, 10% formalin, immunohistochemistry, Ki67-MIB-1, PCNA
Ten percent neutral buffered formalin (NBF) is used almost universally for diagnostic pathology because of its ability to preserve consistently the morphological details of cells and tissues (Grizzle et al. 2008, Grizzle 2009).
The reaction of 10% NBF with proteins in cells and tissues is thought to occur by three reactions. In the first reaction, formaldehyde reacts rapidly primarily with the amino and thiol groups of amino acids to form hydroxymethyl derivatives (Pearse 1968). Subsequently, in the case of primary amino groups, some hydroxymethyl groups may undergo a condensation reaction to form an imine, also called a Schiff base (Metz et al. 2004). This second reaction occurs more slowly. These two initial reactions may influence immunostaining based on the current approach to fixation, which typically limits fixation to less than 24 h for a thin (e.g., 3 mm) piece of tissue. As the time of exposure to 10% NBF increases, a third reaction occurs in which the imine reacts with other side chains including glutamine, asparagine, tryptophan, histidine, arginine, cysteine and tyrosine in a cross-linking fashion (Metz et al. 2004). These molecular changes may mask some antigen epitopes, which would reduce immunostaining (Arnold et al. 1996). The relatively slow penetration of formaldehyde (< 1 mm/h) affects the fixation reactions in tissues. In addition, processing tissues fixed in 10% NBF to paraffin also affects immunorecognition (Grizzle et al. 2008, Otali et al. 2009).
Fixation by 10% NBF for longer than 48 h has been studied extensively, but less is known concerning fixation in 10% NBF for shorter periods, e.g., < 12 h. Also, the effects of various concentrations of ethanol following initial fixation in 10% NBF, has not been studied adequately (Otali et al. 2009). It has been reported anecdotally and generally accepted that transferring thin tissues and cells that have been fixed in 10% NBF for more than 24 h to 70% ethanol prevents further loss of immunorecognition of some epitopes. There are few studies in the literature, however, to support this belief and to our knowledge, there is no study of the optimal time for transferring tissues from 10% NBF to 70% ethanol (Leung et al. 2011).
Ethanol is a dehydrating agent, but it also may act on the hydroxymethyl adducts that have not been cross-linked to catalyze the formation of reactive imines by removing the hydrogen atom from the nitrogen of the original amine end group and the hydroxyl group from the 10% NBF adduct (Dapson 2007).
Immunorecognition by monoclonal and polyclonal antibodies likely depends on many factors including how both the epitopes/antigens and the antibody identifying the epitopes react; this is indicated by the differential effects of fixation in 10% NBF and/or other cross-linking fixatives that may modify the epitope (Arnold et al. 1996). Other less commonly considered effects of cross-linking fixatives that may affect immunorecognition include steric hindrance of epitope-antibody reactions resulting from cross-linking and local chemical effects caused by reactive hydroxymethyl groups and/or imines.
Previously, we reported that fixation interacts with tissue processing to decrease immunorecognition (Otali et al. 2009). In that study, cells grown on microscope slides were processed to paraffin as a model to study the interaction of fixation by 10% NBF with cumulative processing steps to paraffin. In the study reported here, cells were grown on coverslips, because this approach frequently is used in research and constitutes a simplified model for enhancing our understanding of the potential short term effects of 10% NBF fixation and transfer to 70% ethanol.
We grew cells on coverslips specifically to evaluate whether transfer of cells from 10% NBF to 70% ethanol decreases the loss of immunorecognition caused by fixation in 10% NBF. Our study also was designed to determine the optimal time for transfer of cells from 10% NBF to 70% ethanol and how various times in ethanol affect immunorecognition. The results suggest that there is a clear benefit to transferring cells, and potentially tissues, from 10% NBF to 70% ethanol prior to immunohistochemical analysis.
Material and methods
The effects of fixation of cells by 10% NBF for 5 min, 12, 15, 18, 36, 108 and 180 h were compared with fixation for 12 h in 10% NBF followed by transferring the cells to 70% ethanol for 3, 6, 24, 96 and 168 h so that the total time in the two solutions would be equivalent (Table 1). Fixation for 5 min was considered minimal, because “no fixation” usually resulted in detachment of cells from the coverslips during immunostaining.
Table 1.
Duration of fixation in 10% NBF compared to fixation for 12 h in 10% NBF followed by transfer to 70% ethanol
| Fixation in 10% NBF (h) | Fixation in 10% NBF for 12 h and transfer to 70% ethanol (h) |
|---|---|
| 0.083 | – |
| 12 | – |
| 15 | 12 + 3 |
| 18 | 12 + 6 |
| 36 | 12 + 24 |
| 108 | 12 + 96 |
| 180 | 12 + 168 |
Two cell lines were used: DU145 (prostate cancer) and SKOV3 (ovarian cancer) obtained from American Type Culture Collection (ATCC). The cell lines were maintained in RPMI 1640 and DMEM, respectively, with 10% fetal calf serum plus supplements, MEM vitamin solution (Gibco, Grand Island, NY) 1-glutamine (Gibco), antibiotic-antimycotic solution of penicillin, streptomycin and amphotericin B (Gibco) in an incubator with 5% CO2 at 37° C.
DU145 or SKOV3 cell lines were trypsinized, re-suspended in their respective media and plated uniformly on 22 × 22 mm sterile coverslips in six-well plates at a cell concentration of 150,000/ml for DU145 and 175,000/ml for SKOV3. Plating was carried out over several days in a decreasing time schedule. When confluence reached about 70%, usually after two days, the cells on the coverslips were rinsed quickly twice in PBS, pH 7.4, and either fixed in 10% NBF alone (Richard Allan Scientific, Kalamazoo, MI) for 5 min, 12, 15, 18, 36, 108 or 180 h or fixed in 10% NBF for 12 h, then placed in 70% ethanol (AAPER Alcohol and Chemical Co. Shelbyville, KY) for 3, 6, 24, 96 or 168 h (Table 1). Fixation was carried out at room temperature and all fixation times were synchronized to enable immunostaining of all experimental variations to be performed at the same time.
When the designated end point of fixation had been reached, the fixed cells on the coverslips were rinsed in Tris buffer, pH 7.6, for 10 min and permeabilized. For the permeabilization step, the cells on the coverslips were dehydrated through graded concentrations of ethanol, i.e., 70, 95%, and absolute ethanol for 2 min at each concentration, treated with acetone (Fisher Scientific, Fairlawn, NJ) for 15 sec, then rehydrated through graded concentrations of ethanol, i.e., absolute, 95, and 70%, before washing in Tris buffer for 2 min (Rodriguez-Burford et al. 2002). Endogenous peroxidase was quenched by exposure to 3% aqueous H2O2, for 5 min and rinsing with Tris buffer. To reduce nonspecific staining, 3% goat serum was added to the cells on the coverslips for 1 h at room temperature. The cells on the coverslips were stained with a monoclonal antibody to the proliferative nuclear marker, PCNA (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:18,000, a monoclonal antibody to the proliferative biomarker, Ki67, clone MIB-1 (Bio-genex, San Ramon, CA) diluted 1:60, monoclonal antibodies to anti-keratins AE1/AE3 (Boehinger Mannheim Corp., Indianapolis, IN) 5 μg/ml, diluted 1: 40 and a monoclonal antibody to EGFr (Zymed, San Francisco, CA) 3 μg/ml, diluted 1:5. Dilutions of antibodies were in phosphate buffer EDTA (PBE), pH 7.6. These relatively dilute concentrations of antibodies were chosen so that staining was relatively weak to make assessment of effects on immunorecognition easier. For each antibody and cell line, a control was included in which the primary antibody was replaced with 3% goat serum. Next, the cells on the coverslips were rinsed with Tris buffer, pH 7.6, and incubated with multispecies biotinylated goat anti-mouse/rabbit secondary antibody for 10 min (Signet, Dedham, MA) and HRP-conjugated streptavidin for 5 min (Signet). Color was developed with diaminobenzidine (DAB) for 7 min (Bio-genex, San Ramon, CA) to produce an insoluble chromogen. The cells on the coverslips were rinsed with deionized water, counterstained with Mayer’s hematoxylin (Sigma-Aldrich, St. Louis, MO) for 1 min 15 sec, blued in tap water, dehydrated though graded concentrations of ethanols: 70, 95%, and absolute ethanol, before clearing in thee changes in xylene (Fisher Scientific, Fairlawn, NJ). The cells on the coverslips were mounted on microscope slides using Permount (Fisher Scientific). Immunostaining for each experiment was repeated independently three times.
Blinded evaluations were performed by a board certified diagnostic pathologist (W.E.G.). The immunostaining for a specific cell line for all three experiments under each condition was evaluated during the same session. Two parameters were evaluated: percentage of cells stained and immunostaining score as described in (Grizzle et al. 1998, Otali et al. 2009). Briefly, the intensity of nuclear staining was determined in 1.0 increments from 0 for no staining to 4 for strongest staining. The proportion of cells stained at each intensity level was estimated and multiplied by the staining intensity. The total immunostaining score is the sum of the products of the proportion of cells stained at each staining intensity multiplied by the staining intensity, e.g., 40% of cells staining at intensity of 2 would yield 0.4 × 2 = 0.8 and 60% of cells staining at 3 would yield 0.6 × 3 = 1.8. Adding the components of the immunostaining score for this example would be 2.6. Similarly, 100% of cells with no staining would give a total immunostaining score of 1×0 = 0, and 100% of cells staining at maximum intensity would give an immunostaining score of 1 × 4 = 4, thus immunostaining scores range from 0 to 4. Five random fields were evaluated for each variable for each experiment and the values obtained from the three replicate independent experiments were used to calculate means and standard deviations. Where appropriate, intracellular localization was evaluated separately, e.g., EGFr cellular membrane staining was evaluated separately from cytoplasmic staining for EGFr. Because of the pattern of staining of Ki67-MIB-1 at low concentrations, only staining of mitotic cells was evaluated.
Because our hypothesis was based on prior studies that indicated that the percentage of cells stained and the immunostaining score would decrease with longer fixation in 10% NBF, right-tailed pair-wise t-tests were performed to assess the differences in immunostaining between experimental time periods. We considered the difference statistically significant if p ≤ 0.01. As expected, we observed a general trend toward a decreasing immunostaining score with increasing duration of fixation in 10% NBF. In addition, preliminary data indicated that transfer of specimens into 70% ethanol would minimize the decreased immunorecognition caused by fixation in 10% NBF; thus, right-tailed tests also were performed for these data, which also followed the trends observed in the preliminary studies.
Results
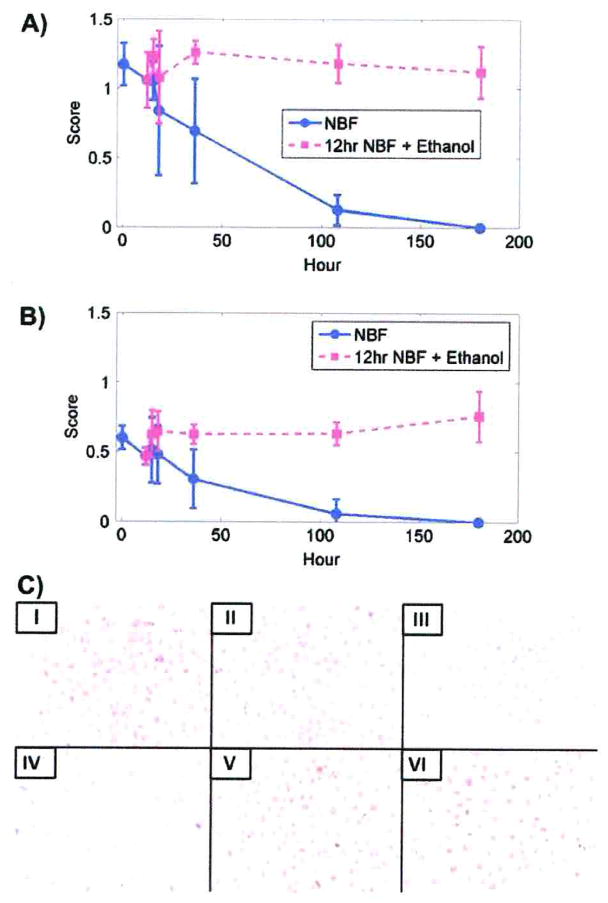
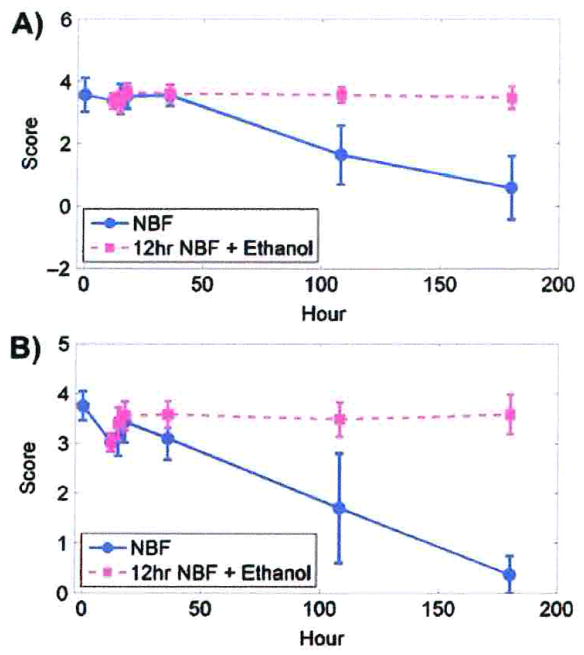
Immunostaining scores were more sensitive than the percentage of cells stained. For both DU145 and SKOV3 cells, as the duration of fixation in 10% NBF increased, immunostaining scores decreased so that by 180 h there was no observable PCNA staining (Fig. 1A, B).
Fig. 1.
Immunostaining scores for PCNA. A) PCNA staining in DU145 cells after fixation in 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol at comparable total exposure times. B) PCNA staining for SKOV3 cells after fixation in 10% NBF at times matched with fixation for 12 h in 10% NBF followed by transfer to 70% ethanol for comparable total times of exposure. Data from both cell lines are from three replicate experiments. Error bars are standard deviations. C) DU145 cells after staining for PCNA. × 200. I, II, III, IV, V and VI represent 5 min, 12 h, 36 h, 180 h 10% NBF, and 12 h in 10% NBF + 24 h in 70% ethanol, and 12 h in 10% NBF + 168 h 70% ethanol, respectively.
After fixation by 10% NBF for 5 min, the PCNA immunostaining scores differed from each subsequent experimental time period for both DU145 and SKOV3 cells (Table 2). A statistically significant decrease (p < 0.01) was observed after 18 h in DU145 cells. In SKOV3 cells, a statistically significant decrease (p < 0.01) occurred after 36 h.
Table 2.
P-values of t-tests between any sample pairs for immunostaining score for PCNA in DU145 and SKOV3 cells fixed in 10% NBF
| Time (h) in 10% NBF | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| DU145 | 0.083 | – | 0.05 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | 0.46 | 0.05 | <0.01 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.05 | <0.01 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.18 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 | |
| SKOV3 | 0.083 | – | <0.01 | 0.09 | 0.02 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | 0.77 | 0.59 | <0.01 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.34 | <0.01 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.02 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | 0.02 |
Data are from three replicate experiments.
Immunostaining scores for cells that were fixed for 12 h in 10% NBF followed by 70% ethanol for various periods showed no statistically significant decreases in immunostaining throughout the 180 h of the study for either DU145 or SKOV3 cells (Table 3).
Table 3.
P-values of t-tests for immunostaining score between 12 h of 10% NBF fixation or 10% NBF for 12 h followed by transfer to 70% ethanol at experimental time points for DU145 and SKOV3 after cells
| 12 (h) in 10% NBF + EtOH (h) | 12 + 0 | 12 + 3 | 12 + 6 | 12 + 24 | 12 + 96 | 12 + 168 | |
|---|---|---|---|---|---|---|---|
| PCNA | |||||||
| DU145 | 12 + 0 | – | 1.0 | 0.58 | 1.0 | 0.97 | 0.80 |
| SKOV3 | 12 + 0 | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Cytokeratins AE1/AE3 | |||||||
| DU145 | 12 + 0 | – | 0.20 | 0.18 | 0.72 | 0.07 | 0.25 |
| SKOV3 | 12 + 0 | – | 0.12 | 0.52 | 0.77 | 0.86 | 0.37 |
| EGFr cytoplasmic | |||||||
| DU145 | 12 + 0 | – | 0.96 | 0.99 | 0.98 | 0.99 | 0.81 |
| SKOV3 | 12 + 0 | – | 1.0 | 0.99 | 1.0 | 0.98 | 1.0 |
| EGFr membrane | |||||||
| DU145 | 12 + 0 | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| SKOV3 | 12 + 0 | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Ki67-MIB-1 | |||||||
| DU145 | 12 + 0 | – | 0.36 | 1.0 | 0.99 | 0.98 | 0.82 |
| SKOV3 | 12 + 0 | – | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Data are from three replicate experiments.
With regard to the percentage of cells stained, there was no statistically significant decrease in staining in either DU145 or SKOV3 cells until after more than 36 h fixation in 10% NBF (p < 0.01; Table 4). There were no cells that were stained for PCNA in either cell line after fixation for 180 h. Figure 1C (IV) shows no DU145 cells stained for PCNA after fixation in 10% NBF for 180 h.
Table 4.
P-values of t-tests for percentage of DU145 and SKOV3 cells staining for PCNA, cytokeratins AE1/AE3 and EGFr; values are comparisons between 10% NBF fixation for 5 min and each subsequent experimental time point
| Time in 10% NBF (h) | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| PCNA | ||||||||
| DU145 | 0.083 | – | 0.94 | 0.92 | 0.50 | 0.10 | <0.01 | <0.01 |
| SKOV3 | 0.083 | – | 0.96 | 0.86 | 0.65 | 0.03 | <0.01 | <0.01 |
| Cytokeratins AE1/AE3 | ||||||||
| DU145 | 0.083 | – | 0.72 | 0.33 | <0.01 | <0.01 | <0.01 | <0.01 |
| SKOV3 | 0.083 | – | 0.80 | 0.66 | 0.97 | <0.01 | <0.01 | <0.01 |
| EGFr cytoplasmic | ||||||||
| DU145 | 0.083 | – | 0.57 | 0.43 | 0.64 | 0.56 | 0.08 | <0.01 |
| SKOV3 | 0.083 | – | 0.85 | 0.73 | 0.50 | 1.0 | 0.97 | 0.98 |
| EGFr membrane | ||||||||
| DU145 | 0.083 | – | 0.04 | 0.32 | <0.01 | <0.01 | <0.01 | 0.01 |
| SKOV3 | 0.083 | – | 0.31 | 0.02 | 0.02 | 0.08 | <0.01 | 0.01 |
Data are from three replicate experiments.
There were no statistically significant differences in percentages of DU145 and SKOV3 cell staining for all periods of fixation in 10% NBF for 12 h followed by transfer to 70% ethanol (Table 5).
Table 5.
P-values of t-tests for percentage of DU145 and SKOV3 cells staining; values are comparison between 10% NBF fixation for 12 h with 10% NBF fixation plus subsequent experimental time points in 70% ethanol
| Time in 10% NBF (h) + EtOH (h) | 12 + 0 | 12 + 3 | 12 + 6 | 12 + 24 | 12 + 96 | 12 + 168 | |
|---|---|---|---|---|---|---|---|
| PCNA | |||||||
| DU145 | 12 + 0 | – | 0.98 | 0.79 | 0.68 | 1.0 | 0.89 |
| SKOV3 | 12 + 0 | – | 0.89 | 1.0 | 1.0 | 1.0 | 1.0 |
| Cytokeratins AE1/AE3 | |||||||
| DU145 | 12 + 0 | – | 0.01 | 0.22 | 0.88 | 0.53 | 0.88 |
| SKOV3 | 12 + 0 | – | 0.16 | 0.35 | 0.95 | 0.98 | 1.0 |
| EGFr cytoplasmic | |||||||
| DU145 | 12 + 0 | – | 1.0 | 1.0 | 0.84 | 0.94 | 1.0 |
| SKOV3 | 12 + 0 | – | 1.0 | 0.99 | 1.0 | 0.98 | 1.0 |
| EGFr membrane | 12 + 0 | – | 0.63 | 0.99 | 0.98 | 1.0 | 0.92 |
| 12 + 0 | – | 0.94 | 0.91 | 1.0 | 0.99 | 1.0 | |
Data are from three replicate experiments.
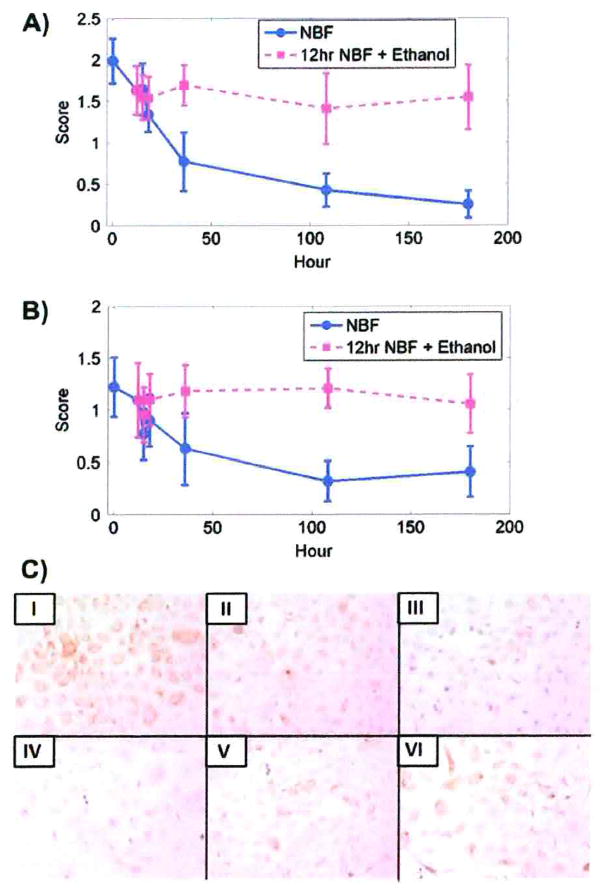
A statistically significant decrease (p < 0.01) in immunostaining scores for all periods was observed for cytokeratins AE1/AE3 in DU145 after fixation for 5 min in 10% NBF compared to other fixation times as shown in Table 6. For SKOV3 cells, a statistically significant decrease (p <0.01) in immunostaining scores was observed after ≥ 12 h fixation in 10% NBF (Table 6). Figure 2A, B demonstrate that in both DU145 and SKOV3 cells, the extent of the decrease in immunostaining scores for cytokeratins AE1/AE3 could be important for interpreting staining after fixation for approximately 36 h in 10% NBF. Figure 2C (IV) shows DU145 cells with significantly reduced staining for cytokeratins AE1/AE3 after fixation for 180 h in 10% NBF.
Table 6.
P-values of t-tests between any sample pairs for immunostaining score for cytokeratins AE1/AE3 in DU145 and SKOV3 cells fixed in 10% NBF
| Time (h) in 10% NBF | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| DU145 | 0.083 | – | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | 0.55 | <0.01 | <0.01 | <0.01 | <0.01 | |
| 15 | – | – | – | <0.01 | <0.01 | <0.01 | <0.01 | |
| 18 | – | – | – | – | <0.01 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 | |
| SKOV3 | 0.083 | – | 0.15 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | <0.01 | 0.05 | <0.01 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.93 | 0.10 | <0.01 | <0.01 | |
| 18 | – | – | – | – | <0.01 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | 0.02 | |
| 108 | – | – | – | – | – | – | 0.86 |
Data are from three replicate experiments.
Fig. 2.
Immunostaining scores for cytokeratins AE1/AE3. A) Immunostaining for cytokeratins AE1/AE3 in DU145 cells after fixation in 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol for comparable total times of exposure. B) Immunostaining for cytokeratins AE1/AE3 in SKOV3 cells after fixation in 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol for comparable total times of exposure. Data from both cell lines are from three replicate experiments. Error bars are standard deviations. C) Immunostaining of DU145 cells for cytokeratins AE1/AE3. I, II, III, IV, V and VI represent 5 min, 12 h, 36 h, 180 h in 10% NBF, and 12 h in 10% NBF + 24 h in 70% ethanol and 12 h in 10% NBF + 168 h in 70% ethanol, respectively. × 200.
After fixation for 12 h in 10% NBF and transfer to 70% ethanol, there were no statistically significant changes in immunostaining scores for DU145 or SKOV3 between 12 h and any subsequent times (Table 3).
Compared to fixation for 5 min in 10% NBF, there was a statistically significant decline (p < 0.01) in the number of DU145 cells stained for cytokeratins AE1/AE3 after fixation for > 18 h in 10% NBF (Table 4); the number of stained SKOV3 cells decreased after 36 h (p < 0.01) (Table 4).
There were no significant differences in the percent of DU145 and SKOV3 cells stained for cytokeratins AE1/AE3 after fixation in 10% NBF for 12 h followed by transfer to 70% ethanol (Table 5).
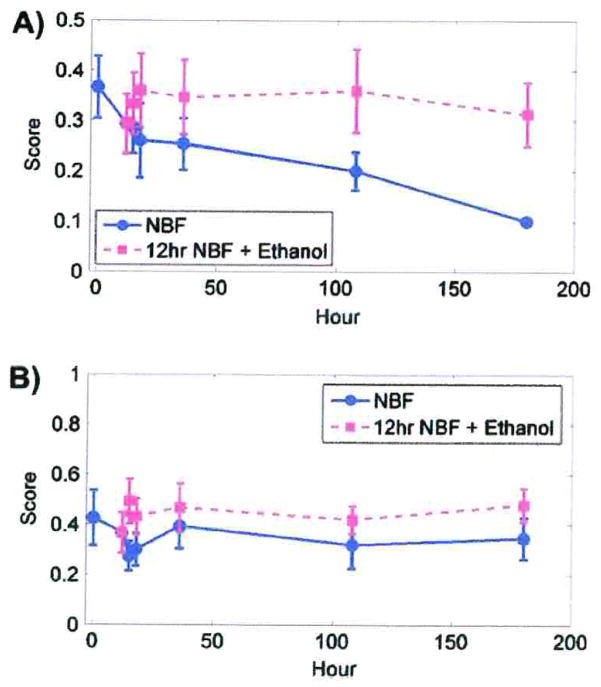
Comparing cytoplasmic immunostaining scores for EGFr in DU145 cells after fixation for 5 min in 10% NBF with other periods of fixation, statistically significant decreases (p < 0.01) were observed at 12 h and subsequent times (Table 7). Although there were statistically significant changes in cytoplasmic immunostaining scores for EGFr in SKOV3 (Table 7), the relative differences were too small to be of practical importance (Fig. 3B).
Table 7.
P-values of t-tests between any sample pairs for cytoplasmic immunostaining score of EGFr in DU145 and SKOV3 cells fixed in 10% NBF
| Time (h) in 10% NBF | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| DU145 | 0.083 | – | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | 0.37 | 0.09 | 0.03 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.13 | 0.04 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.39 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 | |
| SKOV3 | 0.083 | – | 0.05 | <0.01 | <0.01 | 0.18 | <0.01 | 0.02 |
| 12 | – | – | <0.01 | 0.01 | 0.80 | 0.08 | 0.26 | |
| 15 | – | – | – | 0.87 | 1.0 | 0.94 | 1.0 | |
| 18 | – | – | – | – | 1.0 | 0.75 | 0.95 | |
| 36 | – | – | – | – | – | 0.02 | 0.07 | |
| 108 | – | – | – | – | – | – | 0.79 |
Data are from three replicate experiments.
Fig. 3.
Cytoplasmic immunostaining scores for EGFr. A) DU145 cells after fixation in 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol at comparable total times of exposure. B) SKOV3 cells after fixation in 10% NBF at times matched with fixation for 12 h in 10% NBF followed by transfer to 70% ethanol at comparable total times of exposure. Data from both cell lines are from three replicate experiments. Error bars are standard deviations.
Cytoplasmic immunostaining scores for EGFr after fixation for 12 h in 10% NBF compared to fixation for 12 h in 10% NBF followed by experimental periods in 70% ethanol showed no statistically significant differences for either DU145 or SKOV3 cells (Table 3).
Comparing the percentages of DU145 cells stained for cytoplasmic EGFr after fixation in 10% NBF for 5 min with other periods, a statistically significant difference was observed only at 180 h exposure to 10% NBF (p<0.01) (Table 4).
The percentage of cells stained for cytoplasmic EGFr in SKOV3 cells after fixation for 5 min in 10% NBF was not statistically different compared to any of the other experimental periods (Table 4).
The percentage of cells with cytoplasmic staining for EGFr after fixation for 12 h in 10% NBF compared to fixation for 12 h in 10% NBF followed by experimental time periods in 70% ethanol showed no statistically significant differences for either DU145 or SKOV3 (Table 5).
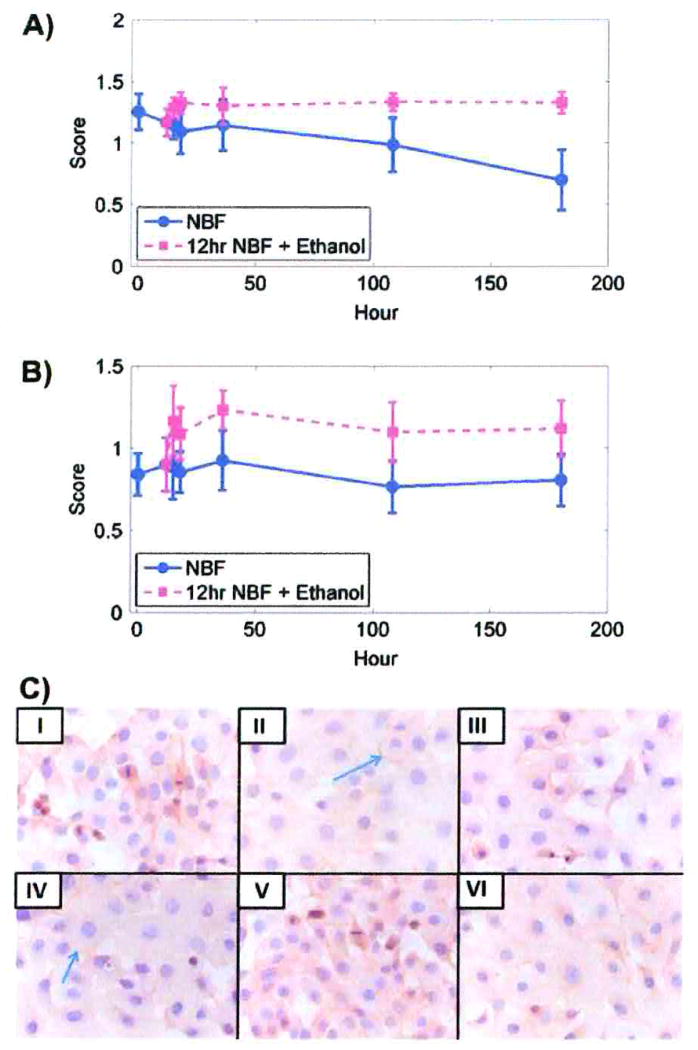
Membrane immunostaining scores for EGFr staining in DU145 cells after fixation for 5 min in 10% NBF compared to the subsequent experimental time periods showed statistically significant differences (p < 0.01) at 18 h and after ≥ 36 h fixation (Table 8). No statistically significant differences in SKOV3 cells were observed at any of the time intervals. (Table 8) compared to 10% NBF fixation for 5 min.
Table 8.
P-values of t-tests between any sample pairs of membrane immunostaining score for EGFr in DU145 and SKOV3 cells fixed in 10% NBF
| Time (h) in 10% NBF | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| DU145 | 0.083 | – | 0.04 | 0.02 | <0.01 | 0.06 | <0.01 | <0.01 |
| 12 | – | – | 0.31 | 0.09 | 0.37 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.17 | 0.50 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.77 | 0.08 | <0.01 | |
| 36 | – | – | – | – | – | 0.03 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 | |
| SKOV3 | 0.083 | – | 0.86 | 0.78 | 0.61 | 0.93 | 0.09 | 0.27 |
| 12 | – | – | 0.42 | 0.19 | 0.66 | 0.02 | 0.06 | |
| 15 | – | – | – | 0.29 | 0.72 | 0.04 | 0.11 | |
| 18 | – | – | – | – | 0.90 | 0.05 | 0.19 | |
| 36 | – | – | – | – | – | <0.01 | 0.03 | |
| 108 | – | – | – | – | – | – | 0.75 |
Data are from three replicate experiments.
Membrane immunostaining scores for EGFr for DU145 or SKOV3 cells after fixation for 12 h in 10% NBF followed by transfer to 70% ethanol showed no statistically significant differences at any of the experimental time periods (Fig. 4A, B; Table 3). Figure 4C shows DU145 cells at selected times after fixation by 10% NBF and fixation for 12 h in 10% NBF followed by transfer to 70% ethanol.
Fig. 4.
Membrane immunostaining scores for EGFr. A) DU145 cells after fixation with 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol at comparable total times of exposure. B) SKOV3 cells after fixation in 10% NBF at times matched with fixation in 10% NBF for 12 h followed by transfer to 70% ethanol for comparable total times of exposure. Data from both cell lines are from three replicate experiments. Error bars are standard deviations. C) DU145 cells after staining for EGFr. I, II, III, IV, V and VI represent 5 min, 12 h, 36 h, and 180 h in 10% NBF, and 12 h in 10% NBF + 24 h in 70% ethanol and 12 h in 10% NBF + 168 h in 70% ethanol, respectively. Blue arrows show membrane staining. × 400.
The percentage of both DU145 and SKOV3 cells stained were significantly decreased in DU145 cells after 18 h and in SKOV3 cells after 108 h; however, these statistical changes were too small to be of experimental importance (Table 4). Transfer of both DU145 and SKOV3 to 70% ethanol preserved immunorecognition (Table 5).
After fixation in 10% NBF for 5 min, staining of mitoses in DU145 cells with the MIB-1 antibody to Ki67 showed a statistically significant decrease (p < 0.01) after 108 or 180 h (Table 8); however, in SKOV3 cells, staining at all subsequent experimental time points were variable (Table 8). Figure 5A, B shows that a significant decrease in MIB-1 staining occurs in DU145 and SKOV3 cells after 108 h and 36 h, respectively, compared to fixation for 12 h in 10% NBF followed by transfer to 70% ethanol for the same amount of time. No statistically significant differences were observed for either DU145 or SKOV3 cells fixed in 10% NBF for 12 h followed by transfer to 70% ethanol for the experimental time periods, (Table 3).
Fig. 5.
Ki67-MIB-1 immunostaining scores. A) Immunostaining scores of DU145 cells after fixation in 10% NBF compared to comparable times for 12 h in 10% NBF followed by transfer to 70% ethanol. B) Immunostaining scores for SKOV3 cells after fixation in 10% NBF compared to comparable times for 12 h in 10% NBF followed by 70% ethanol. These data are from three replicate experiments. Error bars are standard deviations.
Discussion
Cells and tissues fixed initially in 10% NBF sometimes may be transferred to 70% ethanol to reduce the loss of immunorecognition that may occur with longer fixation times. Our study demonstrates that this approach may improve immunodetection of some antigens, although the benefits vary with the antigen-antibody pair, and the cell lines and tissues investigated.
We used cell lines grown on coverslips as a model to evaluate the effects of fixation by 10% NBF compared to 10% NBF followed by transfer to 70% ethanol to study the effects of fixation on immunohistochemistry of solid tissue.
Both the proportion of cells stained and immunostaining scores of the cells studied were used to assess the effects of fixation (Grizzle et al. 1998). As expected, the immunostaining score was more sensitive for identifying the effects of fixation on immunorecognition than the percentage of cells stained (Grizzle et al. 1998, Poczatek et al. 1999).
These results agree with previous reports that immunorecognition of specific antibodies of some specific antigens decreases after fixation in 10% NBF and that this decrease is related directly to the duration of fixation (Otali et al. 2009). Frequently, a statistically significant decrease in immunorecognition after fixation for only 12 h in 10% NBF was observed; however, the decrease usually was not large enough to be important for interpretation of staining until after fixation in 10% NBF for 18 h. The extent and time varied with the cell line and antigen-antibody pair. For EGFr, there was less variation in pattern and intensity of immunostaining compared to other antibody-antigen combinations after fixation in 10% NBF or fixation for 12 h in 10% NBF followed by transfer to 70% ethanol.
For all other antigen-antibody pairs studied, transfer from 10% NBF to 70% ethanol after 12 h resulted in improved immunorecognition. The results show the optimal time for transfer to 70% ethanol is between 18 and 36 h of fixation in 10% NBF. This varied slightly with the antigen-antibody pair and the cell line. Our study supports the general approach of transferring cells grown on coverslips, and by analogy tissues, from 10% NBF to 70% ethanol to preserve immunorecognition.
Previously, using a “cell model” of fixation and tissue processing, it was demonstrated that exposure of cells fixed initially by 10% NBF followed by 70% ethanol preserved immunorecognition (Otali et al. 2009). Transfer to 70% ethanol may be useful for immunohistochemistry, because ethanol may facilitate the penetration of antibodies into cells and tissues (Farmilo and Stead 2001). Documented reports on the role that ethanol plays in fixation of cells and tissues and the extent of the effects of ethanol on immunohistochemistry are conflicting. Clearly, 70% ethanol acts as a dehydrating agent for cells and tissues. Some reports indicate that the effects of ethanol on fixation depend on the type of tissue. Ethanol, by extracting lipids, may variably affect tissue antigenicity of specific antigens. For example, in mice, ethanol treatment markedly reduced the detection of Gb3 in normal kidney tissue, but only minimally in neurons. This variation was attributed to differences in the lipid composition of the tissues (Kolling et al. 2008).
Improved immunohistochemical staining of mammary cancers following fixation in ethanol or alcoholic formalin has been reported for keratins and p53 compared to fixation with 10% NBF (Arnold et al. 1996). A study that compared the expression of p185erbB-2 in ethanol fixed cell blocks and fine needle aspirates of formalin fixed breast tissue in paraffin blocks reported variable results, although no empirical evidence was presented (Williams et al. 2009).
Fowler et al. (2008) compared the structural properties of RNase A after fixation in 10% formalin, 10% NBF plus ethanol dehydration, or 100% ethanol without prior fixation in 10% NBF. These investigators reported that fixation in 10% NBF for one week did not alter significantly the secondary structure of RNase A. They reported that unfixed RNase A incubated in 100% ethanol for the same period recovered its native structure after ethanol was removed and the RNase A was reconstituted in phosphate buffer. When formaldehyde fixed RNase A was incubated in ethanol for 1 week, then re-hydrated in phosphate buffer, there was a significant decrease in near UV light spectral band intensity, which indicated that the changes were not reversible. Both native and formalin fixed RNase A have been reported to undergo structural transition from the α and β conformation to nearly all β conformation as ethanol concentration was increased from 80 to 100%. Fowler et al. (2008) suggested that exposure to ethanol after formalin fixation causes protein aggregation, which likely stabilizes methylene-bridge crosslinks, hydrogen bonds and van der Waals interactions; this interpretation does not support the preservation of immunorecognition that was observed in these studies.
The permeabilization step during immunostaining required the cells on the coverslips be taken through a series of increasing concentrations of ethanol to acetone, then reversed. This method was used because it gave better immunostaining than other alternatives, e.g., using only acetone or using Triton X-100 and subsequently washing in Tris buffer. This approach of permeabilizing cells (Rodriguez-Burford et al. 2002) is unlikely to have biased experimental observations or interpretations.
Immunorecognition of PCNA, cytokeratins AE1/AE3, and Ki67-MIB-1 decreased significantly after fixation in 10% NBF > 12 h; however, changes in immunorecognition were not large enough to make a practical difference until after exposure to 10% NBF for 18 h. The immunorecognition of EGFr was affected less by fixation in 10% NBF. By contrast, fixation for 12 h with 10% NBF followed by transfer to 70% ethanol between 3 and 168 h prevented significant loss of immunostaining for any of the antigens studied.
Although the results of our study apply most directly to immunostaining of cells, the approach also could be a preliminary model to test whether the immunorecognition by tissues fixed in 10% NBF might benefit from transfer to 70% ethanol prior to processing to paraffin. This study is underway.
To evaluate immunohistochemical staining, the staining score, which considers both the proportion of cells stained and their intensity of staining, is more sensitive than using only the percentage of cells stained for identifying subtle changes in immunostaining as described previously (Poczatek et al. 1999).
Table 9.
P-values of t-tests between any sample pairs of immunostaining for Ki67-MIB-1 in mitotic DU145 cells fixed 10% NBF
| Time in 10% NBF (h) | 0.083 | 12 | 15 | 18 | 36 | 108 | 180 | |
|---|---|---|---|---|---|---|---|---|
| DU145 | 0.083 | – | 0.12 | 0.26 | 0.43 | 0.48 | <0.01 | <0.01 |
| 12 | – | – | 0.68 | 0.89 | 0.94 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.70 | 0.77 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.58 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 | |
| SKOV3 | 0.083 | – | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 |
| 12 | – | – | 0.83 | 1.0 | 0.75 | <0.01 | <0.01 | |
| 15 | – | – | – | 0.98 | 0.43 | <0.01 | <0.01 | |
| 18 | – | – | – | – | 0.02 | <0.01 | <0.01 | |
| 36 | – | – | – | – | – | <0.01 | <0.01 | |
| 108 | – | – | – | – | – | – | <0.01 |
Data are from three replicate experiments.
Acknowledgments
Supported in part by the following grants: the Susan G. Komen Breast Cancer Foundation (BCTR0600484), the Cooperative Human Tissue Network (5U01CA44968), the UAB Pancreatic (2P50CA101955) and Breast (5P50CA089019) SPORES, the DOD Grant (W81XWH-10-1-0543), the UAB Comprehensive Cancer Center Core Support Grant (P30CA13148) and the U54 MSM/TU/UAB Comprehensive Cancer Center Partnership (2U54CA118948, 2U54CA118623).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immuniohistochemical demonstration of specific antigens. Biotech & Histochem. 1996;71:224–230. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- Dapson RW. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech & Histochem. 2007;82:133–140. doi: 10.1080/10520290701567916. [DOI] [PubMed] [Google Scholar]
- Farmilo AJ, Stead RH. Handbook of Immunochemical Staining Methods. 3. DAKO Corp; Carpentaria, CA: 2001. Fixation; pp. 18–22. [Google Scholar]
- Fowler CB, O’Leary TJ, Mason JT. Modeling formalin fixation and histological processing with ribonuclease A: effects of ethanol dehydration on reversal of formaldehyde cross-links. Lab Invest. 2008;88:785–791. doi: 10.1038/labinvest.2008.43. [DOI] [PubMed] [Google Scholar]
- Grizzle EW, Fredenburgh LJ, Russell MB. Theory and Practice of Histology Techniques. 6. Churchill Livingstone Elsevier; Philadelphia, PA: 2008. Fixation of Tissues; pp. 53–74. [Google Scholar]
- Grizzle EW, Myers RB, Manne U, Srivastava S. Methods in Molecular Medicine. Vol 14: Tumor marker protocols. Human Press; Totowa, NJ: 1998. Factors affecting immunohistochemical evaluation of biomarkers in prostatic and colorectal neoplasia; pp. 161–179. [Google Scholar]
- Grizzle WE. Special symposium: fixation and tissue processing models. Biotech & Histochem. 2009;84:185–193. doi: 10.3109/10520290903039052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling GL, Obata F, Gross LK, Obrig TG. Immunohistologic techniques for detecting the glycolipid Gb(3) in the mouse kidney and nervous system. Histochem Cell Biol. 2008;130:157–164. doi: 10.1007/s00418-008-0417-8. [DOI] [PubMed] [Google Scholar]
- Leung C, Churg A, Wright LJ, Elliott MW. Effects of isopropanol storage time on histochemical and immunohistochemical stains in lung tissue. J Histotechnol. 2011;34:132–137. [Google Scholar]
- Metz B, Kersten GF, Hoogerhout P, Brugghe HF, Timmermans HA, de Jong A, Meiring H, ten Hove J, Hennink WE, Crommelin DJ, Jiskoot W. Identification of formaldehyde-induced modifications in proteins: reactions with model peptides. J Biol Chem. 2004;279:6235–6243. doi: 10.1074/jbc.M310752200. [DOI] [PubMed] [Google Scholar]
- Otali D, Stockard CR, Oelschlager DK, Wan W, Manne U, Watts SA, Grizzle WE. Combined effects of formalin fixation and tissue processing on immunorecognition. Biotech & Histochem. 2009;84:223–247. doi: 10.3109/10520290903039094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry, Theoretical and Applied. 3. Vol. 1. Little Brown; Boston: 1968. The chemistry of fixation; pp. 70–105. [Google Scholar]
- Poczatek RB, Myers RB, Manne U, Oelschlager DK, Weiss HL, Bostwick DG, Grizzle WE. Ep-Cam levels in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. J Urol. 1999;162:1462–1466. [PubMed] [Google Scholar]
- Roudrigez-Buford C, Barnes MN, Oelschlager DK, Myers RB, Talley LI, Patridge EE, Grizzle WE. Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on ovarian carcinoma cell lines: Preclinical evaluation of NSAIDs as chemopreventive agents. Clin Cancer Res. 2002;8:202–209. [PubMed] [Google Scholar]
- Williams SL, Birdsong GG, Cohen C, Siddiqui MT. Immunohistochemical detection of estrogen and progesterone receptor and HER2 expression in breast carcinomas: comparison of cell block and tissue block preparations. Int J Clin Exp Pathol. 2009;2:476–480. [PMC free article] [PubMed] [Google Scholar]