Abstract
Class I phosphoinositide 3-kinase (PI3K) is a lipid kinase playing key roles in many signaling pathways regulating cell survival and growth. Besides its important role in signal transduction, PI3K is also involved in actin and membrane reorganization such as protrusion, adhesion, phagocytosis and macropinocytosis. Receptor-mediated endocytosis is initiated by plasma membrane reorganization creating buds that then mature to small vesicles. Whereas most of endocytic mechanisms involve actin polymerization, PI3K requirement has not been clearly investigated. Our study identifies class I PI3K as a key player in clathrin-independent endocytosis of the interleukin 2 receptor (IL-2R) in contrast to the clathrin-dependent entry of transferrin (Tf). IL-2R is a cytokine receptor, inducing several signaling cascades such as PI3K, that are essential for the immune response. We have shown previously that IL-2R can be internalized with or without IL-2 and this process requires dynamin, actin and their regulators cortactin, N-WASP, Rac1 and the kinases Pak. Our recent work reveals that PI3K regulates Rac1 during IL-2R uptake in two ways: via its catalytic activity (p110) and via its regulatory factor (p85). Indeed, the catalytic activity of PI3K is required for both constitutive and IL-2 induced uptake of cytokine receptors, in lymphocytes as well as in epithelial cells. Interestingly, Vav2, a Rac1 GTPase exchange factor (GEF) induced upon PI3K activation, is specifically involved and recruited during IL-2R uptake. The second action of PI3K is via its regulatory subunit, p85, which binds activated Rac1 and IL-2R; this interaction being enhanced upon IL-2 treatment. Thus, PI3K regulates both the activation of Rac1 and its recruitment during IL-2R endocytosis. Finally, our results identify a link between cytokine receptors signaling and clathrin-independent endocytosis.
Keywords: cytokine, receptor-mediated endocytosis, Rac1, signal transduction, PI3K, plasma membrane
There are three classes of PI3K, class I encompasses a catalytic subunit (p110) and a regulatory one p85, p50 or p55.1 Activation of class I PI3K can occur via many receptors tyrosine kinase (EGFR, PDGFR) or by receptor having no kinase activity but transmitting signal such as cytokine receptors (IL-2R), antigen receptors or adhesion molecules.2 The receptor-mediated activation of PI3K produces PI(3,4,5)P3 at the plasma membrane that act as a secondary signal, which bind to and regulate downstream protein effectors. For instance, PI(3,4,5)P3 leads to the activation of AKT, a kinase inducing cell growth and survival2 and to the recruitment of several regulators of Rho GTPase leading to actin reorganization.3 Thus, class I PI3K activity regulates the plasma membrane linked actin network and signaling. Moreover, the regulatory subunit p85 interacts with many proteins.4 In particular, p85 BH domain (also called RhoGAP-homology region) was shown to bind Rab5 and consequently regulate the vesicle fusion to early endosomes.5,6 Therefore, p85 is also involved in intracellular trafficking. In consequence, PI3K is a multifunctional factor implicated in cell signaling, intracellular trafficking and actin-membrane reorganization.
Several mechanisms of endocytosis exist in mammals, they allow cells to internalize part of their plasma membrane, enclosing a wide variety of material and contribute to many essential functions.7 The importance of actin cytoskeleton in the formation of small vesicles (100 nm) from the plasma membrane has been only recently uncovered.8 This might be the reason why the role of PI3K has not been deeply studied. In the most well-known mechanism of endocytosis dependent on clathrin, many factors involved in the vesicle formation are linked to actin.9 Thus, the actin cytoskeleton has been implicated in plasma membrane invagination to clathrin vesicle scission. Although poorly characterized, at least three clathrin-independent endocytosis pathways have been uncovered:10-12 the caveolae route used by viruses and bacterial toxins,13 the mechanism internalizing glycosylphosphatidylinositol-anchored proteins also called CLIC and the process used by cytokine receptors such as IL-2R.14,15 All of these pathways require the actin cytoskeleton and are regulated by Rho GTPases such as RhoA, Rac1 (IL-2R)14,16 and Cdc42 (CLIC).17
In the light of the recent implication of actin in early stages of endocytosis, we investigated the role of PI3K in the clathrin-dependent (Tf) and -independent entry (IL-2R). Our previous work identified several factors, cortactin, N-WASP, Arp2/3, F-actin and dynamin, involved in both pathways and linking the actin network to the pinchase enzyme dynamin.15,16 Furthermore, we have shown that Rac1 and its target the kinases Paks control cortactin-N-WASP association and thus the actin polymerization during the IL-2R internalization.16,18 By contrast Rac1-Pak cascade is not implicated in clathrin-dependent entry. Our study reveals that PI3K has a dual role on IL-2R endocytosis in contrast to Tf entry.19 First, the catalytic activity of class I PI3K and its target the Rac1GEF Vav2 are required for IL-2R uptake in contrast to the clathrin-dependent route. Coherently, at early stages of endocytosis, IL-2R is predominantly associated with membrane protrusions enriched in PI(3,4,5)P3.19 The second action of PI3K is via its regulatory subunit, p85, which binds to and recruits Rac1 during IL-2R internalization. Indeed, the BH domain of p85, which is not necessary for the regulation of the catalytic activity of PI3K, is involved in IL-2R entry in contrast to Tf uptake.19 Moreover, we observed an interaction and colocalization at the plasma membrane between p85, Rac1-GTP and IL-2R.19 Therefore, our data identify a new role for p85 in recruiting Rac1 during the formation of IL-2R-endocytic vesicle. Thus, our study demonstrates the concerted action of PI3K subunits in regulating Rac1 and actin during IL-2R endocytosis.19 Our model proposes that IL-2R interacting with p85 would activate PI3K activity leading to the production of PI(3,4,5)P3 and the induction of Vav2, the GEF inducing Rac1 (Fig. 1).19 Rac1-GTP would be then recruited to sites containing the IL-2R via its direct binding to p85. Finally, this would activate the Rac1-Pak1-N-WASP cascade and enhances the rate of actin polymerization during clathrin-independent endocytosis of IL-2R (Fig. 1).19
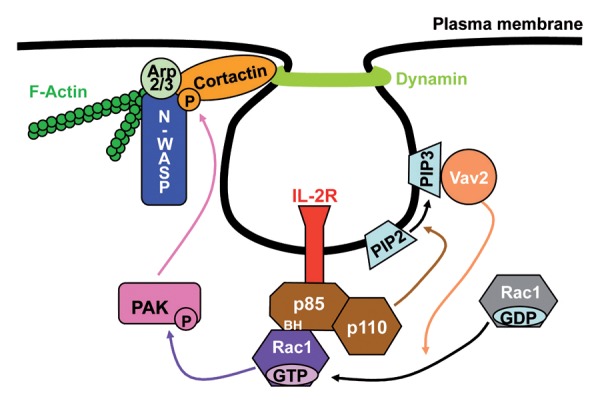
Figure 1. Multiple roles for PI3K in IL-2R endocytosis. First IL-2Rβ in association with p85 would activate the recruitment of PI3K, leading to the production of PI(3,4,5)P3. This would induce Vav2 that then activates Rac1. Activated Rac1 would be then recruited through the BH domain of p85. This in turn would lead to a local actin polymerization to the newborn vesicle via the activation of the Rac-Pak-N-WASP cascade. Figures extracted from reference 19.
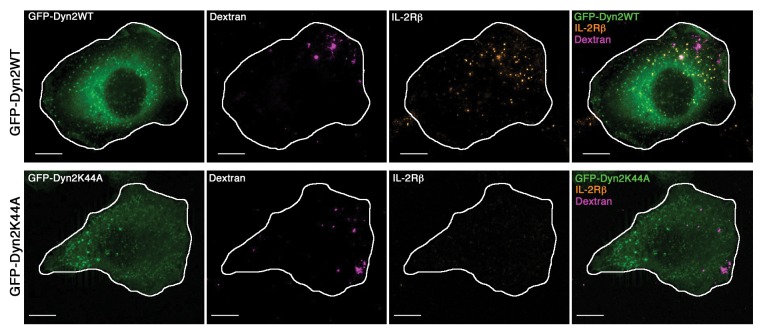
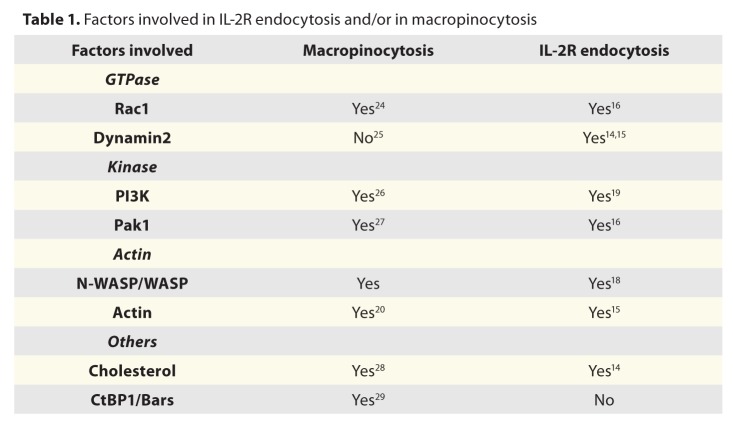
Class I PI3K was already involved in macropinocytosis a process generating big vesicles from plasma membrane (> 0.5 μm) and allowing a massive fluid uptake into the cell.20 This mechanism involves actin-mediated membrane ruffling at the plasma membrane that can be induced by growth factor stimulation (EGF, PDGF). This pathway requires several regulators and some are also involved in IL-2R entry (Table 1). Indeed, cholesterol PI3K, Rac1, Pak, N-WASP and actin are required for both endocytosis of IL-2R and macropinocytosis, rendering these two processes closely related. Thus, to compare these two routes we observed simultaneously the uptake of IL-2R and 500 kDa-Dextran (a marker of macropinocytosis).19 First, both cargos were not colocalized and they were entering inside different vesicle, much smaller for IL-2R than for dextran (Fig. 2).19 Moreover, we confirmed that macropinocytosis was not dependent on dynamin in contrast to IL-2R entry (Table 1, Fig. 2). Taken together clathrin-independent entry of IL-2R and macropinocytosis share common regulators such as PI3K but are distinct processes.
Figure 2. IL-2R endocytosis and macropinocytosis are two distincts mechanisms. Hep2β cells were transfected with either GFP tagged Dynamin2 WT (GFP-Dyn2WT) or with a dominant-negative form of dynamin2 (GFP-Dyn2K44A). To follow endocytosis, Hep2β cells were incubated with 500 kDa-Dextran (a marker of macropinocytosis) and Alexa-Fluor-conjugated anti-IL2R for 15 min at 37°C and fixed. A medial section is shown. The scale bar represents 10 μm. Figures extracted from reference 19.
Last point we would like to discuss is the link between endocytosis and signaling. The high affinity IL-2R is composed of three chains β (IL-2Rβ), shared in IL-2 and IL-15 receptors, γc (IL-2Rγ), shared in IL-2, -4, -7, -9, -15 and -21 receptors, and α chain (IL-2Rα).21 The essential role of cytokine receptors in immune response is linked to signaling cascades induced by the cytokine. The main signaling cascade induced by IL-2 is the pathway involving Janus kinases and signal transducers and activators of transcription leading to the activation of the mitogen activated protein kinase and PI3K pathways.21 In parallel, IL-2 induces the oligomerization of its receptor (IL-2Rα,β,γ) and its internalization. IL-2Rβ and IL-2Rγ are then sorted toward lysosomes where they are degraded.22 Therefore, endocytosis is a way to control the signaling cascade. It should be noticed that IL-2Rβ and IL-2Rγ could be internalized constitutively or in the presence of their ligand. The fact that IL-2-induced endocytosis is faster than the constitutive one suggested that a signaling factor might enhance endocytosis.23 Interestingly, our study reveals that PI3K is involved in both constitutive and IL-2-induced endocytosis of the IL-2R.19 Moreover our results show that IL-2 enhances p85α-IL-2R association in lymphocytes. Since PI3K is induced by IL-2 and is involved in IL-2R endocytosis, its enhanced association in the presence of IL-2 might contribute to the faster IL-2R internalization observed when IL-2 is present. Our work indicates that besides its important role in IL-2 signaling, PI3K allows better control of cell proliferation by contributing to IL-2R endocytosis.
In conclusion PI3K has multiple role in cytokine receptors trafficking and signaling: promoting its endocytosis by regulating the Rac1-mediated actin cytoskeleton reorganization at the plasma membrane, transducing the proliferative signal and controlling the signal by ensuring a negative feedback loop increasing IL-2R uptake and its degradation.
Table 1. Factors involved in IL-2R endocytosis and/or in macropinocytosis
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24243
References
- 1.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–62. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 3.Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell. 2009;101:13–29. doi: 10.1042/BC20080079. [DOI] [PubMed] [Google Scholar]
- 4.Mellor P, Furber LA, Nyarko JN, Anderson DH. Multiple roles for the p85α isoform in the regulation and function of PI3K signalling and receptor trafficking. Biochem J. 2012;441:23–37. doi: 10.1042/BJ20111164. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem. 2004;279:48607–14. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain MD, Oberg JC, Furber LA, Poland SF, Hawrysh AD, Knafelc SM, et al. Deregulation of Rab5 and Rab4 proteins in p85R274A-expressing cells alters PDGFR trafficking. Cell Signal. 2010;22:1562–75. doi: 10.1016/j.cellsig.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP. Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev. 2012;92:273–366. doi: 10.1152/physrev.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–8. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 9.Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–86. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 10.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–27. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–20. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–63. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 13.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–71. doi: 10.1016/S1097-2765(01)00212-X. [DOI] [PubMed] [Google Scholar]
- 15.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J Cell Biol. 2005;168:155–63. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9:356–62. doi: 10.1038/embor.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–23. doi: 10.1016/S1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 18.Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11:1079–91. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 19.Basquin C, Malardé V, Mellor P, Anderson DH, Meas-Yedid V, Olivo-Marin JC, et al. The signalling factor PI 3-kinase is a specific regulator of the clathrin-independent dynamin-dependent endocytosis of IL-2 receptors. J Cell Sci. 2013 doi: 10.1242/jcs.110932. In press. [DOI] [PubMed] [Google Scholar]
- 20.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–43. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 21.Gesbert F, Sauvonnet N, Dautry-Varsat A. Clathrin-independent endocytosis and signalling of interleukin 2 receptors IL-2R endocytosis and signalling. Curr Top Microbiol Immunol. 2004;286:119–48. [PubMed] [Google Scholar]
- 22.Hémar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor α, β, and γ chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hémar A, Lieb M, Subtil A, DiSanto JP, Dautry-Varsat A. Endocytosis of the β chain of interleukin-2 receptor requires neither interleukin-2 nor the γ chain. Eur J Immunol. 1994;24:1951–5. doi: 10.1002/eji.1830240902. [DOI] [PubMed] [Google Scholar]
- 24.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 25.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 26.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–60. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmawardhane S, Schürmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–52. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–62. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- 29.Liberali P, Kakkonen E, Turacchio G, Valente C, Spaar A, Perinetti G, et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 2008;27:970–81. doi: 10.1038/emboj.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]