Abstract
Cell migration is an important process involved in developmental events and in pathologies such as cancer. Cell migration can be classified into two types: individual and collective cell movements. Compared with individual migration, collective cell migration is less understood and has drawn increasing attention lately because of its emerging role in cancer spreading. We have recently established that Rab11 is absolutely required for spatial control of Rac1 activity through the control of cell-cell communication during collective movements (Ramel, et al. 2013). Moreover, we demonstrated that Rab11 acts through the control of Moesin activity. Here, we discuss how Rab11 and Moesin could cooperate to transfer forces from cell to cell in order to insure coordinated collective cell migration.
Keywords: collective cell migration, endocytosis, Rho GTPases, actin cytoskeleton, Drosophila
Cell migration is crucial during development, but it is also involved in pathologies such as inflammation or tumor metastasis.1,2 Different types of cell migration exist including single cell migration, amoeboid migration and collective cell migration. Individual cell migration has been extensively studied and greatly benefited from the in vitro culture of migratory cells. Collective cell migration is much less understood and has drawn increasing attention lately because of its emerging role in development and cancer spreading.3,4 The small GTPaseRac is a key regulator of actin dynamics and cell migration. However, the mechanisms regulating Rac in time and space during collective cell migration are poorly understood. Here, we discuss our recent evidence that endocytic trafficking coordinates Rac activity during collective cell movements.
Currently, few models have been applied to study collective cell migration. However, most of these in vivo models are very complex and cannot be easily genetically manipulated. For our study, we used Drosophila ovary. During Drosophila oogenesis, the so-called border cells perform a stereotypic migration between germ cells toward the oocyte to form the future sperm-entry point.5,6 As a simple and genetically tractable in vivo model, border cell migration provides a good platform for our understanding of collective cell movement.7 Four major signaling pathways have been identified by traditional genetic studies to regulate the different aspects of border cell migration: the JAK/STAT pathway, a global steroid-hormone pathway, cell-cell contact dependent Notch signaling, and Receptor Tyrosine Kinase (RTK) signaling. In addition to these four major signals, hedgehog8 and JNK9signaling pathways have been implicated in the control of border cell migration. The hedgehog pathway regulate different factors necessary for BC but its direct target has to be determined.8 JNK signaling is implicated in the control of cell-cell communication restricting Rac activity at the front of the cluster.9 However the molecular mechanism achieving this restriction of Rac was still largely misunderstood. Our recent study provides the first elements of the molecular basis regulating cell-cell communication during collective cell movements.10
Endocytosis has been considered for a long time as a way to simply attenuate signaling by receptor displacement from the plasma membrane. However, it is now appreciated as a major regulator of signaling outcome. In collective migration, endocytosis has been shown to restrict RTK signaling at the leading edge, either by controlling lateral diffusion of signaling molecules or by recycling RTKs to active regions of the plasma membrane at the leading edge.11-13 Our results demonstrate that Rac is also an important target of endocytosis during collective cell migration. With the use of advanced imaging techniques, we first determined that Rab11 is a key regulator of cell-cell communication during collective movements. Second, we outlined a new role for Rab11 in the regulation of Moesin activity and we demonstrated that a phospho-mimetic form of Moesin is able to rescue migration phenotypes induced by Rab11 loss of function. Finally, we showed that Moesin activation is necessary for normal cell-cell communication during collective cell migration.10 Thus, our work places Rab11 at the center of these processes and provides new insights into the role of endocytosis in the organization of individual cells in a coherent multicellular motile structure.
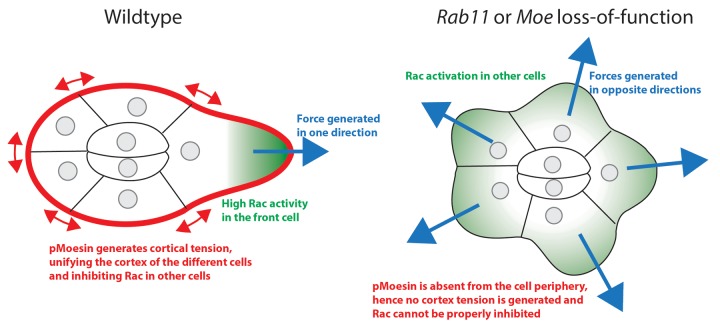
Indeed, one of the key findings of our study is the identification of Rab11 as a regulator of cell-cell communication in collective movements. Wang and colleagues have shown that cell-cell communication is required for proper restriction of Rac in the leading cell and that this is key for directionality of the cluster migration.9 The mechanism by which cells are able to sense the relative level of Rac activity in neighboring cells remains unclear. This study identifies both Rab11 and Moesin as major regulators of this process. Moesin plays a critical role in organizing the epithelial architecture and in the regulation of membrane tension through regulation of the acto-myosin cytoskeleton.14 Recently, it has been proposed that membrane tension could be a mechanism to restrict signaling to the leading edge of neutrophils.15 Indeed, Houk and colleagues have shown that plasma membrane tension is generated by the formation of protrusions at the leading edge. This tension is subsequently transmitted to the cell body to inhibit formation of other leading edges. The molecular basis of this mechanism is still unknown but it might be possible to parallel neutrophils and border cells. Indeed, during border cell migration, there is emission of one major protrusion at the leading edge. Moreover, photo-activation of Rac has been shown to induce formation of protrusions in the photo-activated cell and inhibit formation of protrusions in the other cells of the group. Formation of protrusions in the leading border cell might thus increase plasma membrane tension that is subsequently transmitted around the cluster to inhibit Rac signaling in other cells. Conversely, photoactivation of the dominant negative form of Rac in the leading cell leads to retraction of the protrusions and apparition of protrusions in other cells of the cluster suggesting that relaxing tension at the leading edge allows formation of protrusions in the other cells. We propose that Rab11 and Moesin could regulate this mechanical effect (Fig. 1), but it remains challenging to understand the molecular mechanism that controls the transmission of tension from one cell to a group of migrating cells.
Figure 1. Working model explaining the mechanism by which Rab11 and Moesin restrict Rac activity to the lead cell.
Acknowledgments
G.E. is supported by grants from the Canadian Institute for Health Research (CIHR) (MOP-114899), from the Cancer Research Society and from the Natural Sciences and Engineering Research Council of Canada. G.E. holds a Canada Research Chair (Tier II) in Vesicular Trafficking and Cell Signaling. D.R. is supported by Fonds de Recherche Québec Santé (FRQS). IRIC is supported in part by the Canadian Center of Excellence in Commercialization and Research (CECR), the Canada Foundation for Innovation (CFI) and by the Fonds de Recherche du Québec en Santé (FRQS).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24587
References
- 1.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Metastasis: alone or together? Curr Biol. 2009;19:R1121–3. doi: 10.1016/j.cub.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–42. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 5.Mazurkiewicz M, Kubrakiewicz J. Differentiation and diversification of follicular cells in polytrophic ovaries of crane flies (Diptera: Nematocera: Tipulomorpha and Trichoceridae) Tissue Cell. 2005;37:367–77. doi: 10.1016/j.tice.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 7.Prasad M, Wang X, He L, Montell DJ. Border cell migration: a model system for live imaging and genetic analysis of collective cell movement. Methods Mol Biol. 2011;769:277–86. doi: 10.1007/978-1-61779-207-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisbrecht ER, Sawant K, Su Y, Liu ZC, Silver DL, Burtscher A, et al. Genetic interaction screens identify a role for hedgehog signaling in Drosophila border cell migration. Dev Dyn. 2013 doi: 10.1002/dvdy.23926. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–7. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramel D, Wang X, Laflamme C, Montell DJ, Emery G. Rab11 regulates cell-cell communication during collective cell movements. Nat Cell Biol. 2013;15:317–24. doi: 10.1038/ncb2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assaker G, Ramel D, Wculek SK, González-Gaitán M, Emery G. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc Natl Acad Sci U S A. 2010;107:22558–63. doi: 10.1073/pnas.1010795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jékely G, Sung HH, Luque CM, Rørth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Laflamme C, Assaker G, Ramel D, Dorn JF, She D, Maddox PS, et al. Evi5 promotes collective cell migration through its Rab-GAP activity. J Cell Biol. 2012;198:57–67. doi: 10.1083/jcb.201112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]