Abstract
The Ras GTPases comprising three main isoforms H-, N- and K-Ras operate at the plasma membrane as molecular switches in essential signaling pathways. Active concentration of the minor phospholipid phosphatidylserine in the inner leaflet of the plasma membrane contributes to the electrostatic potential that is required for K-Ras anchoring to the plasma membrane. We recently observed that staurosporine and related analogs: 7-oxostaurosporine, UCN-01 and UCN-02, long known as relatively non-specific protein kinase inhibitors, block endosomal sorting and recycling of phosphatidylserine, resulting in redistribution of phosphatidylserine to endosomes and endomembranes with concomitant mislocalization of K-Ras. Staurosporines are therefore a new tool to study phosphatidylserine trafficking. We discuss whether the mechanism of action of UCN-01, an FDA-approved staurosporine analog used as an anti-cancer therapeutic, is related to effects on phosphatidylserine subcellular distribution. Given the high prevalence of expression of constitutively active K-Ras in human cancers, we ask whether inhibitors of phosphatidylserine trafficking may have important therapeutic applications.
Keywords: Ras GTPase, phosphatidylserine, lipid recycling, staurosporine, UCN-01
Ras proteins are small GTPases that regulate key pathways involved in cell growth, proliferation and differentiation. Ras proteins need to localize on the plasma membrane (PM) for its biological activity. Correct post-translational modification of the hypervariable region (HVR) at C-terminus of Ras is required for translocation to the inner leaflet of the PM.1-3 Constitutively active mutant K-Ras is expressed in pancreatic, lung and colon cancers, and is therefore a major clinical problem.4,5 Pharmacological agents that block K-Ras biological activity would have great clinical utility. Point mutations that prevent Ras post-translational processing block PM localization and abrogate all biological and oncogenic activity.2,6 Farnesyltransferase inhibitors (FTI), which block the first step of Ras post-translational modification should phenocopy this mode of Ras inhibition. However, in cells treated with FTIs K-Ras and N-Ras undergo alternative processing by geranylgeranyltransferase 1.7 Geranylgeranylated K-Ras and N-Ras localize normally to the PM, and are equipotent with farnesylated K- and N-Ras in transforming assays.8 Despite the clinical failure of FTIs for inhibiting K-Ras PM localization, the basic biological observation that preventing K-Ras PM localization abrogates transforming activity remains valid.2,6
In our recent study, we developed and utilized a high content assay to identify compounds that inhibit PM localization of K-Ras and that may thereby inhibit its biological activity. We screened a microbial extracts library composed of ~10,000 metabolites, and identified 21 extracts that mislocalized K-Ras and/or H-Ras from the PM. Two extracts that displaced both K- and H-Ras from the PM contained staurosporines and analogs; 7-oxostaurosporine (OSS), UCN-01 and UCN-02.9 We subsequently showed that staurosporines are more active against K-Ras than H-Ras. In staurosporine-treated cells, K-Ras is translocated to the ER, early endosomes, late endosomes/lysosomes and mitochondria, while H-Ras is redistributed predominantly to the Golgi. Although staurosporines are protein kinase C inhibitors and can induce apoptosis at high concentrations, we showed that the Ras mislocalization mechanism is independent of protein kinase C inhibition and induction of apoptosis.
Phosphatidylserine (PS) is an anionic phospholipid that is asymmetrically concentrated on the inner leaflet of the PM, and confers electrostatic potential to the PM.10 Using a PS binding probe, we showed that OSS disrupts the subcellular distribution of PS without externalizing PS to the outer leaflet of the PM, indicating OSS does not inhibit PM flippase nor activate scramblases. In addition, OSS treatment decreased not only the total amount of K-RasG12V and PS on the inner leaflet of the PM but also the nanoclustering of K-RasG12V and PS on the PM (Fig. 1A and B). OSS did not change H-RasG12V nanoclustering but did significantly reduce the amount of H-RasG12V associating with the PM (Fig. 1C). These are important observations, since perturbation of Ras nanocluster organization dysregulates Ras-mediated cellular signaling.11-13 Consistent with the nanoclustering and PM localization data, staurosporine treatment significantly decreased cell proliferation and MAPK signaling in K-Ras-transformed cells. Taken together, these and other data suggest that PS provides membrane affinity for K-RasG12V, facilitates the formation of K-RasG12V nanoclusters, and maintains H-RasG12V on the PM.
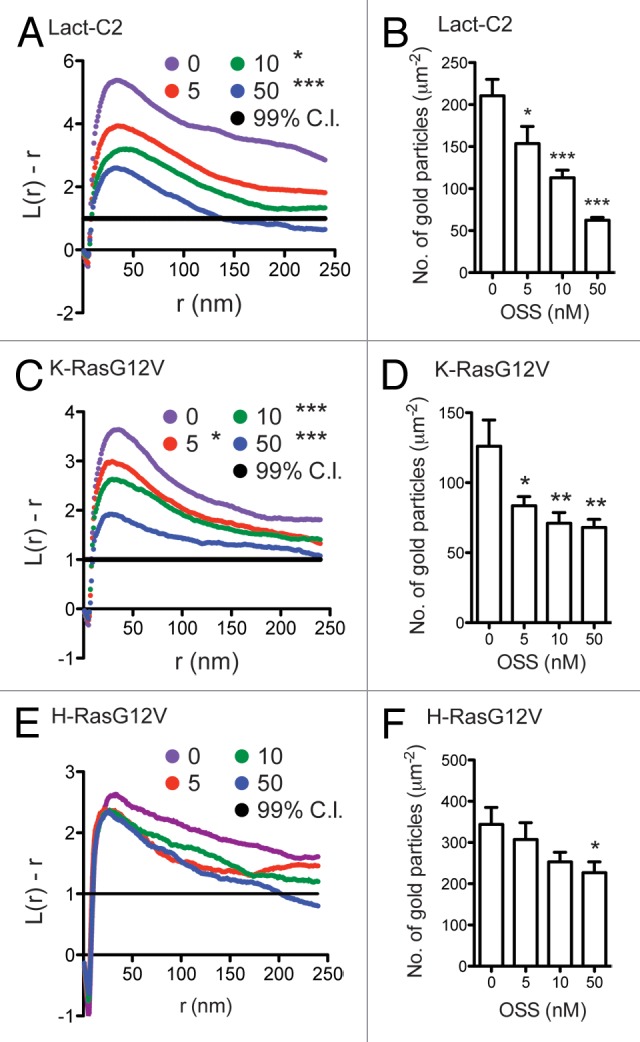
Figure 1. OSS reduces the total amounts and nanoclusterings of PS and K-RasG12V, but not H-RasG12V. Basal PM sheets were prepared from MDCK cells expressing mGFP-tagged Lact-C2, K-RasG12V or H-RasG12V were treated with OSS for 48h. PM sheets were labeled with anti-GFP antibody conjugated to 4.5nm-gold particles. Spatial mapping of the gold-labeled Lact-C2 (A), K-RasG12V (C) or H-RasG12V (E) were performed. The L(r)-r curve is weighted mean K-function (n ≥ 15), where values above the 99% confidence interval (C.I.) for a random pattern indicate clustering at that value of r. Significant differences between the L(r)-r curves of OSS-treated and control cells were analyzed using bootstrap tests (*, p < 0.05; ***, p < 0.001). (B, D and F) The graphs show the mean number of gold particles/μm2 (± S.E.M). Differences between OSS-treated and control cells were assessed using one-way ANOVA tests. Significant differences are indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (A–D) previously published in Figure 3B and C, and Figure 5G and H of Staurosporine disrupts phosphatidylserine trafficking and mislocalize Ras proteins. JBC 2012, 287:43573–43584.
We conducted PS add-back experiments to elicit the possible targets of staurosporines in PS trafficking. When OSS-treated cells were supplemented with exogenous PS, the intracellular mislocalized K-Ras were rapidly recruited to the PM. Intriguingly, the correction of K-Ras PM localization was transient. After 1 h of PS supplementation, K-Ras became more extensively mislocalized than immediately before PS addition. We interpret these results as indicating that staurosporines target endosomal sorting/recycling of PS. When exogenous PS reaches the inner leaflet of the PM, the increased PS concentration on the inner leaflet corrects PM binding of K-Ras on a time scale that matches PS delivery from the outer to the inner leaflet.14 PS then undergoes normal endocytic recycling14 such that in the absence of staurosporines, internalized PS is recycled back to the PM by vesicular pathways.10 However, when staurosporines continuously block the sorting/recycling of PS back to the PM, the internalized PS is instead redistributed to endosomes. Consequently, PS concentration on endomembranes now is greater than before the PS supplementation, accounting for the observed enhancement of K-Ras mislocalization in OSS-treated cells supplemented with exogenous PS.
Clinical studies involving UCN-01 (7-hydroxystaurosporine) have been completed or are currently active for a wide range of tumors.15-19 Although UCN-01 inhibits protein kinases and dysregulates cell cycling, the exact mechanism of its anti-tumor activity is still unclear.15 Our study suggests a new mechanism that merits further investigation. A review of the study designs for UCN-01 would also be appropriate to see if an adequate sample of K-Ras-positive tumors was included. The identification of additional compounds that target PS lipid trafficking is now required to validate whether reducing PM PS content is a viable strategy to therapeutically modify K-Ras signaling.
In summary, staurosporines significantly decrease the level of PS on the inner leaflet of the PM, and mislocalize Ras proteins from the PM. PS depletion on the PM results in a reduced nanoclustering of K-RasG12V, suggesting that PS is required for both PM binding of K-Ras as well as the maintenance of K-RasG12V nanoscale spatial organization. Staurosporines disrupt the endosomal recycling of PS. The exact molecular target of staurosporines in PS recycling process is currently unknown, but whatever the mechanism staurosporine is a new pharmacological tool to study the cellular trafficking of PS.
Materials and Methods
EM and spatial mapping of basal plasma membranes in polarized epithelial cells
Basal sheets of MDCK cells transiently expressing mGFP-H-RasG12V were prepared exactly as described.9 The prepared basal PM sheets on gold EM grids were washed and fixed, and the cytosolic leaflet was labeled with anti-GFP antibody conjugated to 4.5-nm gold particles as previously described.20,21 Digital images of the immunogold-labeled plasma membrane sheets were taken in a JEOL 1400 transmission EM. Intact 1 μm2 areas of the plasma membrane sheet were identified using ImageJ, and the x, y coordinates of the gold particles were determined.20,21 K-functions21 were calculated and standardized on the 99% confidence interval for complete spatial randomness. Bootstrap tests to examine differences between replicated point patterns were constructed exactly as described previously, and statistical significance evaluated against 1,000 bootstrap samples.13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24746
References
- 1.Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–9. doi: 10.1016/0092-8674(90)90294-O. [DOI] [PubMed] [Google Scholar]
- 2.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–77. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 3.Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–84. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 4.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines AT, Xu D, Der CJ. Inhibition of Ras for cancer treatment: the search continues. Future Med Chem. 2011;3:1787–808. doi: 10.4155/fmc.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–6. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 7.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–64. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 8.Cox AD, Hisaka MM, Buss JE, Der CJ. Specific isoprenoid modification is required for function of normal, but not oncogenic, Ras protein. Mol Cell Biol. 1992;12:2606–15. doi: 10.1128/mcb.12.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KJ, Park JH, Piggott AM, Salim AA, Gorfe AA, Parton RG, et al. Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J Biol Chem. 2012;287:43573–84. doi: 10.1074/jbc.M112.424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–27. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 11.Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol. 2007;9:905–14. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- 12.Cho KJ, Kasai RS, Park JH, Chigurupati S, Heidorn SJ, van der Hoeven D, et al. Raf inhibitors target ras spatiotemporal dynamics. Curr Biol. 2012;22:945–55. doi: 10.1016/j.cub.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 13.Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci USA. 2005;102:15500–5. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay JG, Koivusalo M, Ma X, Wohland T, Grinstein S. Phosphatidylserine dynamics in cellular membranes. Mol Biol Cell. 2012;23:2198–212. doi: 10.1091/mbc.E11-11-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotte SJ, Oza A, Winquist EW, Moore M, Chen EX, Brown S, et al. Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II Consortium study. Ann Oncol. 2006;17:334–40. doi: 10.1093/annonc/mdj076. [DOI] [PubMed] [Google Scholar]
- 16.Sausville EA, Arbuck SG, Messmann R, Headlee D, Bauer KS, Lush RM, et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol. 2001;19:2319–33. doi: 10.1200/JCO.2001.19.8.2319. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Christensen SD, Frankel PH, Margolin KA, Agarwala SS, Luu T, et al. A phase II study of cell cycle inhibitor UCN-01 in patients with metastatic melanoma: a California Cancer Consortium trial. Invest New Drugs. 2012;30:741–8. doi: 10.1007/s10637-010-9562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelman MJ, Bauer KS, Jr., Wu S, Smith R, Bisacia S, Dancey J. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clin Cancer Res. 2007;13:2667–74. doi: 10.1158/1078-0432.CCR-06-1832. [DOI] [PubMed] [Google Scholar]
- 19.Marti GE, Stetler-Stevenson M, Grant ND, White T, Figg WD, Tohnya T, et al. Phase I trial of 7-hydroxystaurosporine and fludararbine phosphate: in vivo evidence of 7-hydroxystaurosporine induced apoptosis in chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52:2284–92. doi: 10.3109/10428194.2011.589547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–70. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock JF, Prior IA. Electron microscopic imaging of Ras signaling domains. Methods. 2005;37:165–72. doi: 10.1016/j.ymeth.2005.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


