Abstract
Although early studies have suggested that the onco-protein Mdm2 is the primary E3 ubiquitin ligase for the p53 tumor suppressor, an increasing amount of data suggests that p53 ubiquitination and degradation are more complex than once thought. The discoveries of MdmX, HAUSP, ARF, COP1, Pirh2, and ARF-BP1 continue to uncover the multiple facets of this pathway. There is no question that Mdm2 plays a pivotal role in downregulating p53 activities in numerous cellular settings. Nevertheless, growing evidence challenges the conventional view that Mdm2 is essential for p53 turnover.
The p53 protein is known as a “guardian of the genome” because of its crucial role in coordinating cellular responses to genotoxic stress (Lane, 1992; Levine, 1997). The tumor suppression effects of p53 are mediated by a variety of mechanisms, including cell cycle arrest, apoptosis, and cellular senescence (Vogelstein et al., 2000). p53 is tightly regulated, such that its protein product usually exists in a latent form, and at low levels, in unstressed cells. However, the steady-state levels and transcriptional activity of p53 increase dramatically in cells that sustain various types of stress. Although the precise mechanisms of p53 activation are not fully understood, they are generally thought to involve post-translational modifications, including ubiquitination, acetylation, phosphorylation, sumoylation, neddylation, methylation, and glycosylation of the p53 polypeptide. Ubiquitination regulates a diverse spectrum of cellular processes by providing a specific signal for intracellular protein degradation as well as some degradation-independent functions. It is well accepted that the ubiquitin-proteasome pathway plays a major part in the scope of p53 regulation; however, it is becoming more apparent that the role of ubiquitination in the balance of p53 is not as simple as once thought.
Mdm2: An Oncogenic E3 Ligase for p53
p53 was first shown to be degraded through this pathway by the human papilloma virus E6-associated cellular protein E6AP (Scheffner et al., 1993). p53 is efficiently ubiquitinated and degraded by E6AP in the presence of HPV E6, providing one mechanism by which HPV can reduce p53 levels in order to replicate in a host cell. Soon after this finding, however, another cellular E3 ligase, mouse double-minute 2 protein (Mdm2), was discovered that could ubiquitinate and degrade p53 in the absence of exogenous factors (Haupt et al., 1997; Honda et al., 1997; Kubbutat et al., 1997). Mdm2, so called for its original discovery as one of three genes located on extrachromosomal amplifications in a spontaneously transformed murine cell line (3T3-DM) (Cahilly-Snyder et al., 1987; Fakharzadeh et al., 1991), was shown to downregulate p53 activities (Momand et al., 1992; Oliner et al., 1992). Mdm2 itself is a highly regulated protein under DNA damage conditions and was thought for quite some time to be the sole E3 ligase responsible for p53 degradation under normal physiologic conditions. Amplifications of the mdm2 gene in 7% of human tumors account for one mechanism of overexpression, and more recent data have shown that a naturally occurring polymorphism (SNP309) occurring within the mdm2 promoter leads to an increase in mdm2 mRNA and protein in human populations (Bond et al., 2004). The observed increase in transcription and translation is a common occurrence of the mdm2 gene in hematopoietic malignancies (Momand et al., 1998). Notably, the levels of Mdm2 in normal cells are also dynamically regulated; p53 is not only induced upon stress but also assists in its own maintenance by driving the gene expression of mdm2 in a negative feedback loop (Wu et al., 1993). The critical role of Mdm2 in degrading p53 is best illustrated by studies carried out in mice, where inactivation of p53 was shown to completely rescue the embryonic lethality caused by loss of Mdm2 function (de Rozieres et al., 2000; Jones et al., 1995; Montes de Oca Luna et al., 1995).
Regulation of Mdm2 Function by Other Cellular Factors
Several mechanisms exist to modulate the p53-Mdm2 pathway during times of cellular stress. These include posttranslational modifications of p53 and Mdm2 and interactions with other cellular factors such as ARF. ARF (known as p14ARF in humans and p19ARF in mouse) was originally identified as an alternative transcript of the Ink4a/ARF tumor suppressor locus, a gene that encodes the p16Ink4a inhibitor of cyclindependent kinases (Sherr, 2001). The importance of p19ARF in the p53-Mdm2 pathway has been well established for quite some time. ARF suppresses aberrant cell growth in response to oncogene activation,at least in part, by inducing the p53 pathway (Sharpless and DePinho, 2004; Sherr, 2001). p53 induction by ARF is mediated through inhibiting the activities of either Mdm2 (Kamijo et al., 1998; Pomerantz et al., 1998; Zhang et al., 1998) or a recently identified ARF binding partner, ARF-BP1 (Chen et al., 2005). In addition, several ribosome proteins are capable of stabilizing p53 and may provide a possible link between the p53 pathway and ribosome biogenesis. Ribosome proteins such as L11 and L23 can all inhibit the activity of Mdm2 on p53 (Dai et al., 2004; Lohrum et al., 2003; Zhang et al., 2003). These proteins may then act as sensors of nucleolar stress that can inhibit Mdm2 activity, allowing for the efficient stabilization of p53. Interestingly, DNA damage seems to also stimulate the binding of L26 to the 5′ untranslated region of p53 mRNA, causing an increase in the translation of p53 protein and shedding new light on p53 regulation at the translational level (Takagi et al., 2005).
MdmX is yet another important protein that has an intricate and poorly understood involvement in p53 regulation (Marine and Jochemsen, 2005). The embryonic lethal phenotype of MdmX null embryos and the rescue of this phenotype when they are crossed with p53 null mice clearly places it as an important negative regulator of p53 during embryonic development (Finch et al., 2002; Migliorini et al., 2002; Parant et al., 2001). Still, the true physiologic function of MdmX remains ambigious. MdmX possesses structural similarities with Mdm2 and, though it has a C-terminal RING domain, does not possess an in vivo ability to ubiquitinate and degrade p53. MdmX can stabilize p53 when overexpressed, as polyubiquitinated forms of p53 readily accumulate within the nucleus (Jackson and Berberich, 2000; Stad et al., 2001). However, when the ratio of MdmX:Mdm2 is low, these proteins cooperatively decrease p53 levels (Gu et al., 2002; Iwakuma and Lozano, 2003). It has been shown that MdmX can act as a transcriptional repressor, suggesting another possible physiologic role for MdmX (Kadakia et al., 2002; Wunderlich et al., 2004; Yam et al., 1999). MdmX also imparts a negative effect on p53 acetylation, possibly through inhibition of p300/CBP (Danovi et al., 2004; Sabbatini and McCormick, 2002). This observation is also supported by an increase in the levels of acetylated p53 in mdmx mutant cells. Regardless of the mechanisms, MdmX may prove to be as important as Mdm2 in tumorigenesis, considering that it is found upregulated in many tumors expressing wild-type p53.
Regulation of p53 Ubiquitination by Protein Modifications
In addition to ubiquitination, phosphorylation and acetylation remain the major posttranslational modifications that occur on p53 and Mdm2 during times of cell stress (Brooks and Gu, 2003), although additional modifications have also been identified under certain conditions, including sumoylation, neddylation, and methylation (Chuikov et al., 2004; Rodriguez et al., 1999; Xirodimas et al., 2004).
Several mechanisms exist that serve to both stabilize and activate p53. One mechanism that has been shown to occur during times of cellular stress is the direct competition between acetylation and ubiquitination for the same C-terminal lysine residues on p53 (Ito et al., 2002; Li et al., 2002b). The major sites for p53 ubiquitination are located at its C terminus, and acetylation of these residues during times of cell stress serves to block protein degradation and stabilize p53. In addition, Mdm2 itself can be acetylated (Wang et al., 2004). The acetylation of residues within the RING domain of Mdm2 inactivates the protein and leads to an increase in p53 transcriptional activity. More importantly, modulations of the p53 and Mdm2 interaction have been proven essential for p53 activation, as shown by the small molecule inhibitor Nutlin (Vassilev et al., 2004). This inhibitor specifically blocks p53-Mdm2 binding and, by doing so, activates p53 and suppresses tumor growth in vivo. The promise of small molecule inhibitors in blocking these pathways has also been shown with another family of inhibitors, HLI98, that specifically blocks the E3 ubiquitin ligase activity of Mdm2 (Yang et al., 2005). In the natural setting of the stressed cell, phosphorylation has been proposed to be a key mechanism for blocking the Mdm2-p53 interaction. Phosphorylation of serine 15 and serine 20 at the N terminus of p53 during times of particular DNA damage events may assist in blocking this interaction (Prives and Hall, 1999). In addition, modifications occurring on Mdm2 can also disrupt their direct interaction during specific DNA damage events, such as ionizing radiation, when Mdm2 is phosphorylated at serine 395 (Maya et al., 2001). Homeostatic phosphorylation at Thr216 of Mdm2 may also be important in p53 regulation, as dephosphorylation of this site enhances the activity of Mdm2 on p53 (Okamoto et al., 2002). Hypophosphorylation of Mdm2 within the acidic conserved region II (aa 237–260) has been shown to augment p53 stability in response to ionizing radiation as well (Blattner et al., 2002).
However, in vivo knockin experiments have demonstrated that the major modification sites on p53 apparently are not essential for p53 stabilization upon stress (Xu, 2003; Feng et al., 2005; Krummel et al., 2005), suggesting that the mechanism of stress-induced p53 stabilization is complex and remains unsolved. Given that almost all of the known modification sites of p53 were identified through either in vitro enzymatic assays or overexpression systems, the possibility remains that yet uncovered modification sites may be even more critical for p53 regulation in vivo.
p53 Ubiquitination: Mono versus Poly
Protein ubiquitination, including both mono- and polyubiquitination, is involved in a broad spectrum of cellular processes (Pickart, 2001). Whereas polyubiquitination can serve to target proteins for degradation by providing a recognition signal for the 26S proteasome, monoubiquitination has been implicated in a number of degradation-independent processes, including endocytosis, virus budding, and transcriptional regulation (Hicke and Dunn, 2003). Mdm2 was recently found to differentially catalyze monoubiquitination and polyubiquitination of p53 in a dosage-dependent manner (Li et al., 2003). As a consequence, low levels of Mdm2 activity induce monoubiquitination and nuclear export of p53, whereas high levels promote polyubiquitination and nuclear degradation of p53. It seems likely that these distinct mechanisms are exploited under different physiological settings. For example, Mdm2-mediated polyubiquitination and nuclear degradation may play a critical role in suppressing p53 function during the later stages of a DNA damage response or when Mdm2 is malignantly overexpressed (Shirangi et al., 2002; Xirodimas et al., 2001). On the other hand, Mdm2-mediated monoubiquitination and subsequent cytoplasmic translocation of p53 may represent an important means of p53 regulation in unstressed cells, where Mdm2 is maintained at low levels (Boyd et al., 2000; Freedman et al., 1999; Geyer et al., 2000; Stommel et al., 1999). Nevertheless, the precise mechanism and the involvement of export machinery such as Crm1 remain elusive. It is possible that ubiquitination may modulate the tetramerization of p53 and thus unmask the nuclear export signal (NES) as proposed previously (Stommel et al., 1999).
Interestingly, in contrast to the cytoplasmic localization of monoubiquitination-mimicking p53-ub protein, fusing Nedd8 or SUMO-1 to the C terminus of p53 failed to alter its subcellular localization (Figure 1). These results indicate that simply modulating the protein conformation is not sufficient to induce nuclear export. Thus, it is possible that ubiquitin may serve as a unique and specific signal in the nucleocytoplasmic shuttling of p53. Moreover, movement of p53 into the cytoplasm may be important for transcription-independent functions of p53 such as interactions with mitochondrial proteins in the apoptosis response (Chipuk et al., 2004; Leu et al., 2004; Mihara et al., 2003). Thus, several important questions remain. Does monoubiquitination modulates other properties of p53 in addition to nuclear export, for example, regulating its interactions with mitochondrial proteins? Is there a specific cellular factor that recognizes monoubiquitinated p53 and facilitates its nuclear export? Is monoubiquitinated p53 hydrolyzed by HAUSP eventually for recycling or further polyubiquitinated by an E4-like enzyme in the cytoplasm?
Figure 1. Ubiquitin Acts as a Specific Signal for the Nuclear Export of p53.
H1299 cells were transfected with HA-p53-Ub, HA-p53-SUMO1, and HA-p53-Nedd8 expression constructs. Twenty-four hours posttransfection, the cells were fixed with 4% paraformaldehyde and immunofluorescence was done with anti-HA monoclonal antibody and DAPI.
Mdm2-Independent Ubiquitination of p53
Recent data suggest that Mdm2-mediated ubiquitination, at least, is not the only important factor for p53 regulation, as in vivo knockin experiments show that a p53 mutant protein, lacking the major ubiquitination sites for Mdm2, has a normal half-life and is stabilized and activated in response to stress (Feng et al., 2005; Krummel et al., 2005). In addition to Mdm2, other E3 ligases have been shown to impart specificity toward p53 and promote its proteasome-mediated degradation. Pirh2, a RING-H2 domain-containing protein, interacts with p53 and promotes Mdm2-independent p53 ubiquitination and degradation (Leng et al., 2003). Similar to Mdm2, Pirh2 is a p53 responsive gene and participates in a similar autoregulatory negative feedback loop. Another E3 ligase, COP1, has also been described recently as a direct ubiquitin ligase for p53 (Dornan et al., 2004). COP1 is also a p53-inducible gene and can ubiquitinate and degrade p53. Further, COP1 depletion by siRNA enhances p53-mediated G1 arrest and can sensitize cells to ionizing radiation. TOPORS has been shown to have both SUMO and Ub E3 ligase activity on p53, though the physiologic implications of these reactions remain ambiguous (Rajendra et al., 2004; Weger et al., 2005). ARF-BP1 was recently identified as a HECT domain-containing E3 ligase that can ubiquitinate and degrade p53 (Chen et al., 2005). ARF-BP1 was purified as a major ARF binding protein from p53 null cells. Interestingly, inactivation of ARF-BP1, in a manner reminiscent of ARF overexpression, induces tumor suppression effects in both p53 null cells and the cells expressing wild-type p53, indicating that ARF-BP1 is involved in both p53-dependent and p53-independent functions of ARF. Indeed, ARF-BP1/Mule/HectH9 has been shown to be important in regulating other proteins such as c-Myc and MCL-1 (Adhikary et al., 2005; Zhong et al., 2005), which mediate p53-independent functions.
Together, Mdm2, COP1, Pirh2, and ARF-BP1 represent an array of E3 ligases that the cell can call upon to regulate and maintain p53 levels. They suggest that both Mdm2-dependent and -independent mechanisms are used cooperatively by the cell for tight p53 regulation. It is yet uncertain exactly how these proteins are specifically regulated and under what situations they may be differentially activated. Moreover, the identification of multiple p53 isoforms in vivo that lack an Mdm2 binding domain (Bourdon et al., 2005) also suggests that these isoforms might be degraded in an Mdm2-independent fashion.
p53 Ubiquitination Is Reversible
Originally, the ubiquitin-proteasome pathway was thought to have a one way direction from substrate ubiquitination to degradation by the 26S proteasome. However, the discovery and emergence of deubiquitination enzymes (DUBs) changed the global view of the enzymatic process and quickly showed the incredible dynamics of this pathway.
HAUSP was originally identified as a cellular factor that bound to the Herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 (Everett et al., 1997). The finding that the herpesvirus-associated ubiquitin-specific protease (HAUSP) could bind to and stabilize p53 added yet another layer of regulation to the p53 ubiquitination pathway and was one of the first indications that DUBs exhibited substrate specificity (Hu et al., 2002; Li et al., 2002a). In the presence of HAUSP, p53 levels were sufficiently stabilized to induce cell growth arrest and apoptosis. This simple linear model was obscured, however, with the finding that transient siRNA-mediated reduction or complete removal of HAUSP by somatic knockout in HCT116 cells led to profound p53 stability, observations that contradicted the proposed role of HAUSP in p53 stabilization (Cummins et al., 2004; Li et al., 2004). Concurrently, it was found that HAUSP interacted with Mdm2 and exhibited strong deubiquitinase activity and stabilization toward the protein. These data suggest that HAUSP-mediated deubiquitination of Mdm2 is required to maintain a sufficient level of the protein to act as an E3 ligase for p53. If HAUSP levels are reduced to a point where Mdm2 becomes destabilized, the pool of Mdm2 available to ubiquitinate p53 is not sufficient to degrade the protein and it becomes stabilized (Li et al., 2004).
A more recent and provocative idea for p53 stabilization, in agreement with the importance of relative Mdm2 levels, lies within Mdm2 function itself. Mdm2, similar to many E3 ligases, possesses autoubiquitination ability and can catalyze its own ubiquitination and degradation. An eloquent set of experiments using the radiomimetic drug neocarzinostatin (NCS) shows that after treatment, the protein half-life of Mdm2 quickly drops from ~30 min to 5 min and destabilization of Mdm2 was apparently necessary for p53 accumulation and transcriptional activation (Stommel and Wahl, 2004). Because HAUSP serves as a deubiquitinase for Mdm2 and p53, a signaling pathway that targets HAUSP could be the “switch” that triggers it to have preference for one substrate or the other. Indeed, the binding affinity between HAUSP and Mdm2 as well as MdmX is greatly reduced after treatment with NCS due to ATM-mediated phosphorylation (Meulmeester et al., 2005). With the increase of Mdm2 self-ubiquitination and destabilization after DNA damage, this mechanism remarkably allows for Mdm2 and p53 to coexist within the nuclear compartment. With enzymatic reaction time and energy expenditures spent almost exclusively on Mdm2 itself, there are not sufficient protein pools available to ubiquitinate p53. The speedy nature in which this occurs would allow p53 to be rapidly stabilized to a level sufficient for transcriptional activation.
Deubiquitination catalysis may provide a more quick and efficient way for stabilizing p53 rapidly in response to stress. Removing ubiquitin moieties (in the case of p53) and simply allowing autoubiquitination to occur (in the case of Mdm2) are much simpler and energetically favorable mechanisms for quickly stabilizing and activating p53 than through other signaling pathways. However, more important questions regarding HAUSP still remain. How is HAUSP regulated? Are there unidentified deubiquitinases involved in this pathway? A growing number of substrate-specific mammalian DUBs involved in tumorigenesis are continually being revealed such as USP1 and USP9X, which deubiquitinate FANCD2 and β-catenin, respectively (Nijman et al., 2005). Considering the enzymatic process of deubiquitination does not require the cascade of enzymes needed for ubiquitination (e.g., E1, E2, and E3), DUBs may be simpler and better targets for therapeutic purpose.
Rethinking the Conventional View of Mdm2 in p53 Turnover
Although this redundancy of E3 ligases underscores the need to keep p53 activity under tight control, it also raises an important question: is Mdm2 absolutely required for p53 turnover? The high levels of mutant p53 proteins in tumor cells have been well accepted as a “trade marker” of tumor-associated p53 mutations by pathologists. The accumulation of mutant p53 proteins is commonly attributed to their inability to induce Mdm2 transcription and the consequent feedback loop for Mdm2-mediated p53 degradation. Recent studies of mouse models that harbor hotspot p53 missense mutations reveal that high levels of the mutant p53 proteins do indeed accumulate in the tumor cells of these animals (Lang et al., 2004; Olive et al., 2004). Interestingly, however, no significant stabilization of the mutant p53 proteins was observed in the adjacent normal tissue. These results challenge the conventional view that Mdm2 is essential for p53 turnover.
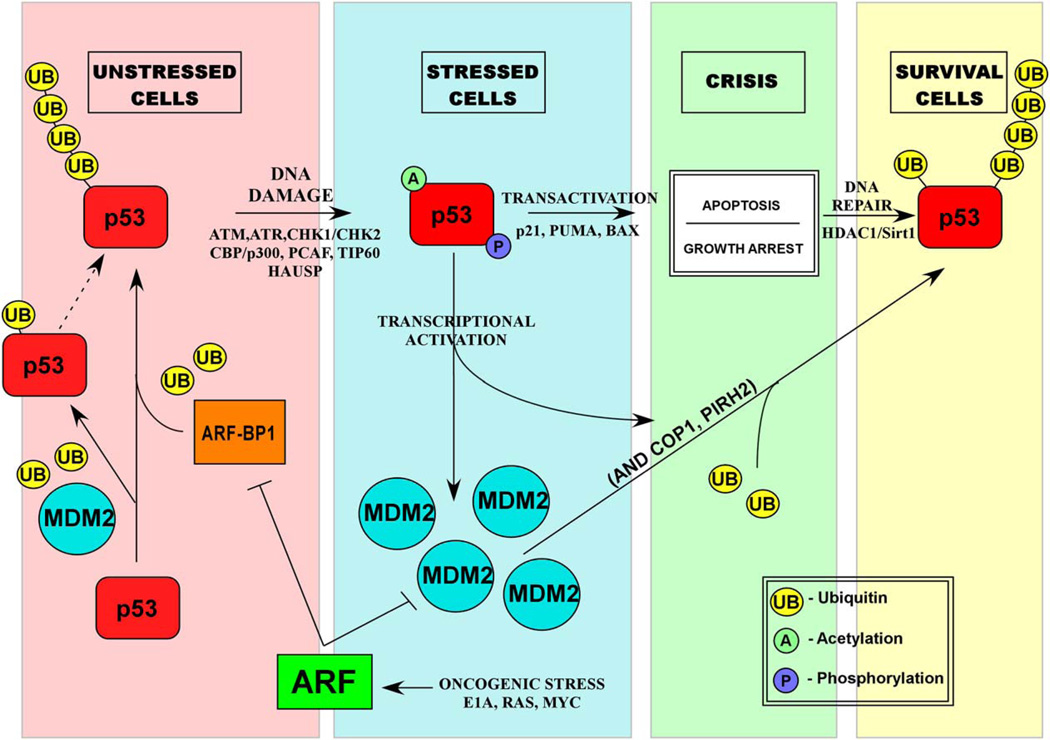
There is no question that Mdm2 plays a pivotal role in repressing p53 activities in numerous cellular settings, including stressed and unstressed conditions. Nevertheless, Mdm2 is maintained at low levels in unstressed cells, and at these levels, it preferentially induces monoubiquitination of p53 (Li et al., 2003). Monoubiquitination may sabotage the transactivation potential of p53 by inducing its nuclear export, but this type of modifications is not sufficient for p53 degradation. Consistent with this notion, p53 protein levels are not increased in normal unstressed tissues of mice bearing a hypomorphic mdm2 allele that only supports low levels of Mdm2 expression (Mendrysa et al., 2003). Nevertheless, stressed cells of these mice display an increased p53-mediated apoptotic response to DNA damage. To rationalize these observations, we propose that the predominant role of Mdm2 is not to mediate p53 degradation in unstressed cells but instead to control p53 levels and activities during the stress response (e.g., Mdm2-mediated degradation of p53 was observed at the late stage of DNA damage response; [Shirangi et al., 2002]) and facilitate possible termination of the stressed cellular state. In this scheme, one or more of the other E3 ligases (see below) would be responsible for p53 degradation and thus maintain low steady-state levels of p53 in unstressed normal cells (Figure 2). Interestingly, early studies showed that mdm2 gene inactivation in mice leads to embryonic lethality between implantation and day E5.5 of development (Montes de Oca Luna et al., 1995; Jones et al., 1995). However, if Mdm2 was the primary factor controlling p53 turnover and activities in normal unstressed cells, it would seem unlikely that mdm2 null embyronic cells would proliferate without any obvious growth arrest for up to 5.5 days (Montes de Oca Luna et al., 1995). Instead, mdm2-null embryos may die because p53 is activated by an undefined stage-specific developmental stress and its proapoptotic functions remain incessant in the absence of Mdm2 feedback inhibition.
Figure 2. A Model for the Role of Mdm2 in Cell Survival.
In unstressed cells, low levels of Mdm2 only induce monoubiquitination of p53, which is not sufficient for degradation, whereas ARF-BP1 may be the major E3 ligase responsible for p53 ubiquitination and degradation. Upon DNA damage, p53 becomes stabilized and activated by posttranslational modifications and other signaling pathways, which lead to induction of hdm2, COP1, and pirh2 genes (see text for details).
This hypothesis might also explain the differential effects of p53 mutations in tumor versus normal tissues. Tumor cells are generally under stress (e.g., due to oncogene activation) or are the survivors of a stress response induced, for example, by DNA damage or genomic instability. As such, p53 mutant proteins, but not p53 wild-type proteins, are stable in tumor cells because they do not mediate transcriptional activation of the mdm2 gene. However, in unstressed normal cells, mutant and wild-type p53 proteins should be equally unstable if they are in fact degraded by Mdm2-independent ubiquitination. Moreover, the observed instability of mutant p53 in unstressed cells offers a potential clue to the identity of the E3 ligase(s) responsible for p53 turnover in these cells; namely, the expression of this ligase should not be dependent on the transcriptional activity of p53. Similar to Mdm2, both COP1 and Pirh2 are p53-responsive target genes that are strongly activated by stress. In contrast, ARF-BP1 is not responsive to p53 transactivation. Thus, it is possible that ARF-BP1 is critical for p53 turnover under unstressed conditions, whereas Mdm2 together with COP1 and Pirh2 play key roles in downregulating p53 function in stressed cells (Figure 2).
The Physiological Role of Mdm2: Promoting Cell Survival Under Stress?
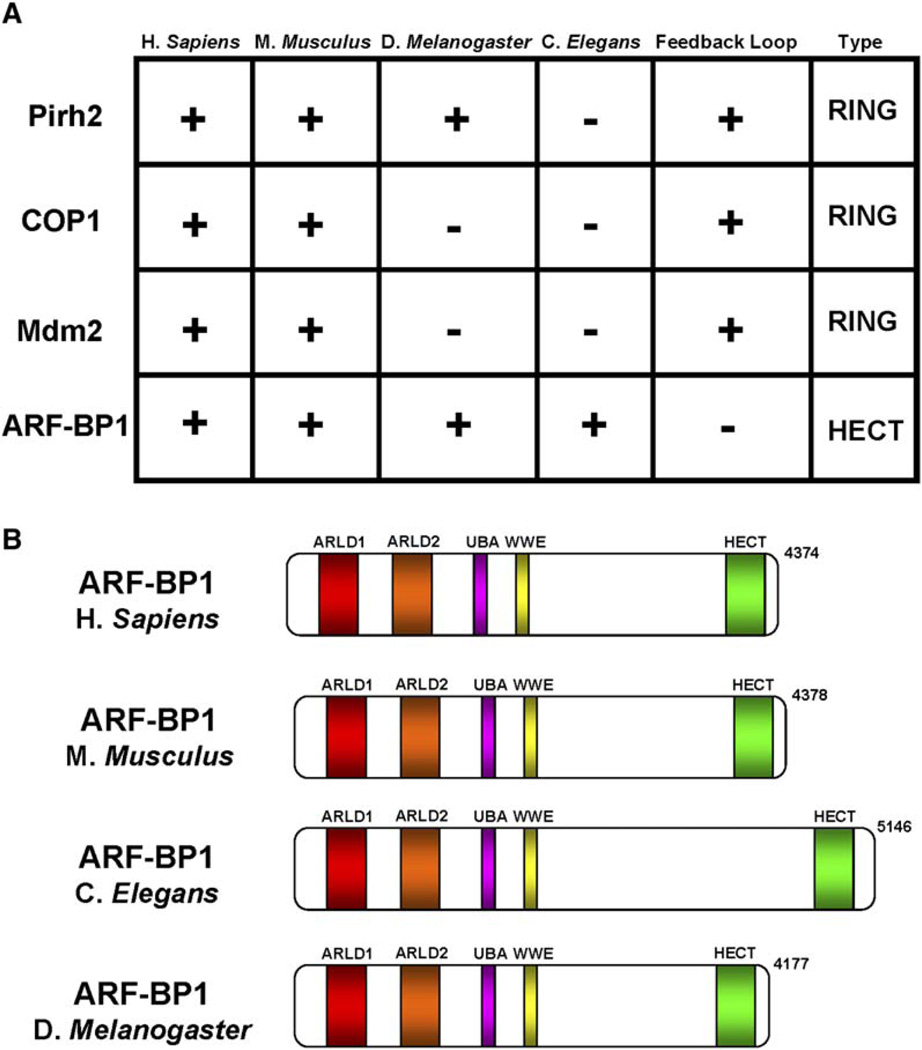
Given the increasing number of E3 ligases found with specificity for p53, the question that immediately comes to mind is can the cell survive without Mdm2. Clearly, it can only in the case of lower organisms such as Caenorhabditis elegans and Drosophila melanogaster, which have a fully functional p53 gene but lack an mdm2 ortholog. Also, counterparts of the COP1 ligase have not been identified in either organism, although Pirh2 has a putative homolog in D. melanogaster only (Yi and Deng, 2005; Bernards and Hariharan, 2001; Leng et al., 2003) (Figure 3A). In contrast, the ARF-BP1 gene is well conserved in both D. melanogaster and C. elegans (Adams et al., 2000) (Figure 3B).
Figure 3. ARF-BP1 Is Evolutionarily Conserved.
(A) A table of E3 ubiquitin ligases specific for p53. The genomes of H. sapiens, M. musculus, D. melanogaster, and C. elegans were screened for putative homologs of the indicated genes with BLASTP using the NCBI genebank. A putative ARF-BP1 gene was found in both D. melanogaster (CG8184-PB; gi 24642255) and C. elegans (Y67D8C.5; gi 71999446) (see text for details).
(B) A schematic of functional domains for the ARF-BP1 genes found in human, mouse, and putative genes found in C. elegans and D. melanogaster. Abbreviations: ARLD1, armadillo-like repeats 1 (also called DUF908, domain of unknown function); ARLD2, armadillo-like repeats 2 (also called DUF913, domain of unknown function); UBA, ubiquitin-associated domain; and WWE, domain occurring in two functional classes of proteins, namely those involved in ubiquitination and those involved in poly-ADP ribosylation. This name is derived from two conserved Trp residues (W) followed by a Glu residue (E); HECT, homologous to E6AP C-terminal domain.
Although a larger phylogenetic spectum will need to be examined, it is possible that ARF-BP1 is present in all species that encode p53 and that the additional p53-specific E3 ligases (e.g., Pirh2, Mdm2, and COP1) emerged in evolution to assume diverse roles in p53 regulation under different cellular conditions. In this regard, it’s interesting to note that the p53 response itself also appears to have evolved. Although p53 can trigger either growth arrest or apoptosis in mammalian cells, cells from C. elegans and D. melanogaster do not exhibit p53-mediated cell growth arrest but only mount an apoptotic response to DNA damage (Brodsky et al., 2000; Derry et al., 2001; Ollmann et al., 2000; Schumacher et al., 2001; Sogame et al., 2003). Thus, in C. elegans, where p53 activation inevitably induces cell death, ARF-BP1 and/or other unidentified factors would be required to suppress p53 function in unstressed cells, but p53-inducible E3 ligases such as Mdm2 would be unnecessary. Instead, Mdm2, and perhaps the other p53-inducible E3 ligases, evolved to rescue stressed cells from the default apoptotic response. Thus, by restraining p53 function in stressed cells, these ligases might provide a cell with the opportunity to choose transient growth arrest before committing itself to a death response. In those arrested cells that successfully repair damaged DNA, the stressed-activated p53 would be proteolyzed by an Mdm2-dependent feedback loop together with other p53-inducible ubiquitin ligases (Pirh2 and COP1) (Figure 2), allowing for restoration of the unstressed cellular state. In support of this, reduction or ablation of Mdm2 expression in mice yields a robust apoptotic response and early embryonic lethality (Mendrysa et al., 2003; de Rozieres et al., 2000; Montes de Oca Luna et al., 1995; Jones et al., 1995).
Although the evolution of feedback loops involving Mdm2 is probably beneficial for development or other physiological purpose, as it allows normal cells to survive a stress response, it may have also rendered mammalian cells more susceptible to tumorigenesis, as manifested in human tumors in which Mdm2 overexpression inhibits the tumor suppressor function of p53.
Acknowledgments
We especially thank Dr. Qing Zhong and Dr. Xiao-Dong Wang from the University of Texas Southwestern Medical Center at Dallas for sharing their independent studies on p53 regulation by ARF-BP1/ Mule. We apologize to all authors whose findings are not cited in this paper because of space limitation. We also thank Drs. Wenhui Zhao, Delin Chen, and Muyang Li in the Gu lab for sharing unpublished data. We thank Dr. Richard Baer for critical comments of the manuscript. This work was supported in part by grants from the Leukemia and Lymphoma Society, the Irma T Hirschl Trust, and the National Institutes of Health/National Cancer Institute to W.G.
References
- Adams MD, Celnkier SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123:409–421. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Bernards A, Hariharan IK. Of flies and men–studying human disease in Drosophila. Curr. Opin. Genet. Dev. 2001;11:274–278. doi: 10.1016/s0959-437x(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Blattner C, Hay T, Meek DW, Lane DP. Hypophos-phorylation of Mdm2 augments p53 stability. Mol. Cell. Biol. 2002;22:6170–6182. doi: 10.1128/MCB.22.17.6170-6182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat. Cell Biol. 2000;2:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Cahilly-Snyder L, Yang-Feng T, Francke U, George DL. Molecular analysis and chromosomal mapping of amplified genes isolated from a transformed mouse 3T3 cell line. Somat. Cell Mol. Genet. 1987;13:235–244. doi: 10.1007/BF01535205. [DOI] [PubMed] [Google Scholar]
- Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, New-meyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, et al. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–360. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428:1–486. doi: 10.1038/nature02501. [DOI] [PubMed] [Google Scholar]
- Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell. Biol. 2004;24:7654–7668. doi: 10.1128/MCB.24.17.7654-7668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R, de Graaf P, Francoz S, Gasparini P, Gobbi A, et al. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol. Cell. Biol. 2004;24:5835–5843. doi: 10.1128/MCB.24.13.5835-5843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rozieres S, Maya R, Oren M, Lozano G. The loss of mdm2 induces p53-mediated apoptosis. Oncogene. 2000;19:1691–1697. doi: 10.1038/sj.onc.1203468. [DOI] [PubMed] [Google Scholar]
- Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O’Rourke K, Koeppen H, Dixit VM. The ubiquitin ligase COP1 is a critcal negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakharzadeh SS, Trusko SP, George DL. Tumori-genic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10:1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Lin T, Uranishi H, Gu W, Xu Y. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol. Cell. Biol. 2005;25:5389–5395. doi: 10.1128/MCB.25.13.5389-5395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch RA, Donoviel DB, Potter D, Shi M, Fan A, Freed DD, Wang CY, Zambrowicz BP, Ramirez-Solis R, Sands AT, Zhang N. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 2002;62:3221–3225. [PubMed] [Google Scholar]
- Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell. Mol. Life Sci. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat. Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- Gu J, Kawai H, Nie L, Kitao H, Wiederschain D, Jochemsen AG, Parant J, Lozano G, Yuan ZM. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J. Biol. Chem. 2002;277:19251–19254. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041–1054. doi: 10.1016/s0092-8674(02)01199-6. [DOI] [PubMed] [Google Scholar]
- Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21:6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakuma T, Lozano G. MDM2, an introduction. Mol. Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- Jackson MW, Berberich SJ. MdmX protects p53 from Mdm2-mediated degradation. Mol. Cell. Biol. 2000;20:1001–1007. doi: 10.1128/mcb.20.3.1001-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Kadakia M, Brown TL, McGorry MM, Berberich SJ. MdmX inhibits Smad transactivation. Oncogene. 2002;21:8776–8785. doi: 10.1038/sj.onc.1205993. [DOI] [PubMed] [Google Scholar]
- Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl. Acad. Sci. USA. 1998;95:8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel KA, Lee CJ, Toledo F, Wahl GM. The C-terminal lysines fine-tune P53 stress responses in a mouse model but are not required for stability control or transactivation. Proc. Natl. Acad. Sci. USA. 2005;102:10188–10193. doi: 10.1073/pnas.0503068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002a;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J. Biol. Chem. 2002b;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell. 2004;13:879–886. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- Marine JC, Jochemsen AG. Mdmx as an essential regulator of p53 activity. Biochem. Biophys. Res. Commun. 2005;331:750–760. doi: 10.1016/j.bbrc.2005.03.151. [DOI] [PubMed] [Google Scholar]
- Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrysa SM, McElwee MK, Michalowski J, O’Leary KA, Young KM, Perry ME. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol. Cell. Biol. 2003;23:462–472. doi: 10.1128/MCB.23.2.462-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E, Maurice MM, Boutell C, Teunisse AF, Ovaa H, Abraham TE, Dirks RW, Jochemsen AG. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol. Cell. 2005;18:565–576. doi: 10.1016/j.molcel.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Migliorini D, Denchi EL, Danovi D, Jochemsen A, Capillo M, Gobbi A, Helin K, Pelicci PG, Marine JC. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 2002;22:5527–5538. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pan-coska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Li H, Jensen MR, Zhang T, Taya Y, Thorgeirsson SS, Prives C. Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol. Cell. 2002;9:761–771. doi: 10.1016/s1097-2765(02)00504-x. [DOI] [PubMed] [Google Scholar]
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S, Whittaker K, Demsky M, Fisher WW, Buchman A, et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101:91–101. doi: 10.1016/S0092-8674(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Joc-hemsen AG, Lozano G. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Al-land L, Chin L, Potes J, Chen K, Orlow I, Lee HW, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2′s inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J. Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, Lutzker S, Saleem A, Rubin EH. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J. Biol. Chem. 2004;279:36440–36444. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP, Hay RT. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 1999;18:6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P, McCormick F. MDMX inhibits the p300/ CBP-mediated acetylation of p53. DNA Cell Biol. 2002;21:519–525. doi: 10.1089/104454902320219077. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Schumacher B, Hofmann K, Boulton S, Gartner A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 2001;11:1722–1727. doi: 10.1016/s0960-9822(01)00534-6. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J. Clin. Invest. 2004;113:160–168. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- Shirangi TR, Zaika A, Moll UM. Nuclear degradation of p53 occurs during down-regulation of the p53 response after DNA damage. FASEB J. 2002;16:420–422. doi: 10.1096/fj.01-0617fje. [DOI] [PubMed] [Google Scholar]
- Sogame N, Kim M, Abrams JM. Drosophila p53 preserves genomic stability by regulating cell death. Proc. Natl. Acad. Sci. USA. 2003;100:4696–4701. doi: 10.1073/pnas.0736384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stad R, Little NA, Xirodimas DP, Frenk R, van der Eb AJ, Lane DP, Saville MK, Jochemsen AG. Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep. 2001;2:1029–1034. doi: 10.1093/embo-reports/kve227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel JM, Wahl GM. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 2004;23:1547–1556. doi: 10.1038/sj.emboj.7600145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Fili-povic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Wang X, Taplick J, Geva N, Oren M. Inhibition of p53 degradation by Mdm2 acetylation. FEBS Lett. 2004;561:195–201. doi: 10.1016/S0014-5793(04)00168-1. [DOI] [PubMed] [Google Scholar]
- Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 2005;579:5007–5012. doi: 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- Wunderlich M, Ghosh M, Weghorst K, Berberich SJ. MdmX represses E2F1 transactivation. Cell Cycle. 2004;3:472–478. [PubMed] [Google Scholar]
- Xirodimas DP, Stephen CW, Lane DP. Cocompart-mentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp. Cell Res. 2001;270:66–77. doi: 10.1006/excr.2001.5314. [DOI] [PubMed] [Google Scholar]
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- Yam CH, Siu WY, Arooz T, Chiu CH, Lau A, Wang XQ, Poon RY. MDM2 and MDMX inhibit the transcriptional activity of ectopically expressed SMAD proteins. Cancer Res. 1999;59:5075–5078. [PubMed] [Google Scholar]
- Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Da-vydov IV, Safiran YJ, Oberoi P, Kenten JH, Phillips AC, et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y. Ribosomal protein L11 negatively regulates on-coprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol. Cell. Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]